Abstract
Groundwater from the shallow aquifers of the Vientiane Plain, Laos is used for domestic needs including to some extent for drinking and for household gardening. The objective of this study is to assess the groundwater quality for drinking and irrigation activities and to determine the processes that lead to the presence of major ions in groundwater. Twenty groundwater samples were collected from a village on the Plain in December 2014, January 2015, and May 2015, and analysed for major ions and selected suite of minor ions and heavy metals. Groundwater is largely acidic, fresh and soft in nature. Geochemistry showed dominant Ca–Mg–HCO3 and mixed Ca–Na–HCO3 groundwater. Sodium impacts the suitability of water for irrigation to some extent. Hydrogeochemical processes identified and verified through factor analysis indicate weathering, carbonate dissolution, ion exchange, and anthropogenic sources including salinisation, due to irrigation and use of fertilizers as sources for the occurrence of major ions at such concentrations in this area. Only concentrations of lead and iron were above the permissible limits with arsenic, copper, zinc, mercury, and uranium found to be within safe limits. Background sample (groundwater) collected 5 km from the study area and the bottled water sample were all within suitable limits for drinking. This study is the first to provide a local-level assessment of geochemical processes in groundwater of this area indicating that the groundwater does not pose any threat to human health if used for drinking based on major ions, minor ions and a suite heavy metals except for iron and lead.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The Lao People’s Democratic Republic (PDR) or simply ‘Laos’, located in Southeast Asia with a population of 6.68 million (in 2014) (World Bank 2016) is one of the least developed countries in the world. The economy of this land locked country is largely driven by agriculture, hydropower, and mining. Area under agriculture has increased from 9.9 % in 2001–2005 to 10.1 % from 2011–2015 (World Bank 2016) and is a source to reduce poverty in rural areas. Rice dominates 72 % of the cultivated area (FAO 2016) and other crops, including maize, coffee, vegetables, etc., are also grown. Rice production had already increased by 75 % from 1.4 million tons in 1986 to 2.5 million tons in 2004 to meet the local demands as well as for exporting the surplus produce (Bestari et al. 2006). This shift from subsistence to commercialized agriculture witnessed in recent years is evident by the plans of the government to further increase the irrigated cropping area in lowlands largely in the central and southern parts of the country (FAO et al. 2012). Hydropower and mining (gold, tin, zinc, coal, barite, gypsum, sapphire, limestone, etc.) sectors are also fast-growing contributing to one-third of the country’s economic growth between 2005 and 2013 (UNDAF 2015). With the Mekong River bordering most of the western part of the country, 35 % of the water flows in the Mekong River is contributed from the watersheds in Lao PDR (MoNRE 2012) and has over 60 dams, planned, or under construction (Grumbine et al. 2012). Other sectors that show increasing growth are the manufacturing (garments, motorcycles, and its assembly etc.), tourism, transportation, and forestry (timber). These sectors are not comparatively labour intensive, so boasting the agriculture productivity is also seen as a key to reduce poverty and improve livelihoods (World Bank 2014; United Nations 2015). With decline in forest cover from the estimated 70 % in 1940s to 41 % in 2002, it is targeted to achieve back the 70 % by 2020 by the Ministry of Agriculture and Forestry (STEA and UNEP RRCAP 2012). Urbanization has increased the population growth rate in cities by 5.3 % during the 2005–2010 period, and is more concentrated in the Vientiane capital city (two-third) compared to the other urban areas (United Nations 2015).
Water needs for these development activities as well as household supply are met from surface and groundwater. Lao PDR is a water rich country with average annual rainfall ranging from 1300 mm in the north to 3700 mm in the south (World Bank 2007). Having the highest per capita annual renewable water resource of about 53,747 m3/person in the region, it is also the fastest declining in the per-capita volume of internal renewable water resources having decreased by 34 % from 1990 to 2014 (FAO 2016). Annual freshwater withdrawal was 3.5 BCM in 2014 with 91 % used by the agriculture sector (World Bank 2016). Surface water is the main source for urban water supply, while groundwater meets the needs of the rural areas. In lowlands, where there is shallow groundwater, it is easy to access, whereas in uplands of the northern and eastern parts of the country, gravity flow systems, i.e., streams and springs, aid in water access (STEA and UNEP RRCAP 2012). Improved drinking water supply to the total population through piped supply, public taps, tube/dug/bore wells, protected springs, etc. is estimated to have improved from 70 to 76 % between 2011 and 2015, with more coverage in urban (86 %) than in rural (69 %) areas (WHO/UNICEF 2015). Tube wells are the important source of water supply in rural Laos, as there is no piped water distribution network like in urban areas.
Importance of preserving the water quantity and quality has only recently been realized. Surface water, the largest supply for urban areas, is generally considered good with high dissolved oxygen levels and low nutrients, but, are at increased threat due to pollution from sewage disposal, runoff from agriculture, wastewater, and effluents from industries, which is commonly disposed into surface drains and water bodies (World Bank 2007). High sediment loads also affect several tributaries of the Mekong. On the other hand, groundwater, major source for rural population, is subject to pollution largely due to lack of proper sanitation access, poor construction, and unmaintained sewage pits leading to bacterial contamination (Ribolzi et al. 2011). High levels of arsenic in groundwater is a serious problem, especially in central and southern Lao PDR apart from few reports on heavy-metal contamination in groundwater resulting from mining and geogenic sources (Fengthong et al. 2002; Chanpiwat et al. 2011, 2014). One of the drawbacks is that there is only little information on the groundwater quality in the country. Nam Saat (Ministry of Health) and Nam Papa (Ministry of Public Works and Transport) are responsible for monitoring the water quality and ensuring its compliance with the Lao Drinking Water Quality Standards (United Nations 2015). However, this is not carried out as a systematic monitoring (World Bank 2007) at regular intervals for comparison of water quality deterioration or to maintain a database. Though water from different sources and forms have been extracted and used in irrigation, industry, households, etc., it is generally not used for drinking as such in most parts of the country. It is a large-scale trend in urban as well as in rural areas to purchase packaged drinking water available in bottles for drinking, because of risks associated with water-borne diseases. There are exceptions with the poor/uneducated households depending on groundwater for their drinking source. As there are chances that groundwater is likely to grow as a source of drinking water, especially in rural areas, it makes it essential to know the status of water quality. Pressure due to urbanization, increasing population, agriculture production, etc., also emphasizes the need for increased focus on water safety and water quality. Realising the importance of groundwater and owing to the limited knowledge on the water quality in the country, this study was designed to assess the quality of groundwater at a village scale by detailed analysis of geochemical processes that control the presence of chemical ions in groundwater and the suitability of water for drinking and irrigation use. Thus, the present work is aimed to evaluate the general status of water quality at a village level based on major ions, minor ions and a suite of heavy metals, their sources, variations, and the potential risk to human health in using groundwater as a source of drinking water.
Methodology
Study Area Description
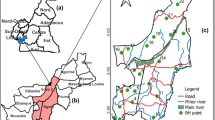
Ekxang village selected for study is situated on the Vientiane Plain in Phonhong district, Vientiane Province (Fig. 1). There are three distinguishable seasons in Laos, the summer season with high humidity, rainy season, and the dry winter season. The average temperature ranges between 24 and 34 °C during the summer months of March to May and during winter the temperature can drop to 16 °C (WMO 2014). Being located in a tropical region, this village experiences an average annual rainfall of 2254 mm, most of which falls from July to October (Pavelic et al. 2014). The western part of the village is more elevated than the eastern part (Fig. 1). Ekxang village has 236 households with a total of 580 women and 648 men. Larger part of the population, i.e., 85 % are farmers, and the remaining 15 % gain their livelihoods off farm (Serre 2013). This village extends to about 17 km2 with a small residential area (0.48 km2), forests (2.3 km2), paddy field (5.46 km2), and other crops (8 km2) with remaining area occupied with water bodies, streams, and roads (Fig. 2). Rice in the major crop grown during wet season and vegetables/fruits is grown during both dry season and wet season. Groundwater being the major source for all the needs in this village is pumped from private (n = 7) and NGO-drilled tube wells (n = 3), concrete-lined large diameter wells of 6–10 m deep (n = minimum 233), and unlined large diameter wells of 4–5m depth (n = 40) (Serre 2013; Suhardiman et al. 2015). About 20 % of households use groundwater for drinking without prior treatment (n = 20). The remaining households purchase packaged drinking water in bottles from private suppliers for drinking and cooking, as there is no piped water distribution network in the village. Tube wells installed in the village as a water supply system are used for non-consuming domestic needs.
According to Perttu et al. (2011a), due to the sea level rise during the Cretaceous period, the plateau in Vientiane, Laos underwent marine influx and was sporadically isolated from the ocean. The lower cretaceous group, also known locally as the Champa formation, of about 400-m thickness consisting of quartzite–sandstone–siltstone and the Jurrasic–Cretaceous formation (locally known as Phu Phanang formation) of about 300-m thickness consisting of mica interbedded with conglomerates occur in this area (Perttu et al. 2011a). Champa formation (K2cp) comprised of sandstone, siltstone, brownish sandstone, white arkose, and quartz-feldspar; Vientiane formation (N2-Q1vc) with gravel, shingle, sandy kaolinite, and laterite; Quaternary sediments consisting of clay, shale, gravel, shingle, kaolinite and peat (QII-III) and sand, gravel, shingle, clay, and peat (QIV) are seen at the surface (Fig. 3) (Perttu et al. 2011a). The Tha Ngon formation is present below the Quaternary sediments (QII-IV) in the south of the Vientiane Plain. Fence diagram prepared from the borehole lithologs (locations shown in Fig. 3) drilled in the village is presented in Fig. 4 (Department of Irrigation 2014). These wells drilled up to a maximum depth of 40 m bgl predominantly shows sandy top soil followed largely by clayey soil up to 20 m in L1 and L2, while in L3, it was up to 26 m. The top few meters up to 10 m mainly consist of sand and clay. The regional geology indicates the occurrence of unconsolidated sediments at the bottom and the upper parts consists of clay and sand (Fig. 4). The minimum yield of these wells is estimated to be around 5 l/s (Viossanges et al. forthcoming).
Cross section of few wells (Department of Irrigation 2014)
Field Sampling and Analysis
In December 2014, a well survey was carried out in the village and the EC was measured in groundwater of many wells as an exercise to understand the general groundwater quality. These groundwater samples (n = 20) were tested for total arsenic (as it is reported to be present in southern Laos by Fengthong et al. 2002; Chanpiwat et al. 2011, 2014) and few other heavy metals. This was followed by another sampling in January 2015, wherein major ions, minor ions (fluoride, bromide, nitrate, nitrite, ammonium, and phosphate), and few heavy metals were analysed. Based on the results obtained in December 2014 and January 2015, the chemical analysis was restricted to the determination of major ions and a limited number of heavy metals in May 2015. Groundwater samples were collected from twenty wells that were used for household and agricultural uses. One surface water sample from a stream in the village was also collected. The sampling included a background groundwater sample collected in January and May 2015 at a distance of about 5 km from the village having surface geology QII-III and situated in a settlement area. Based on the surface geology, large part of the study area consists of Quaternary formation, and most of the sampling wells are located in this formation. There were not many existing tube wells or dug wells in the Champa Formation and hence, the only well in this formation was sampled (Fig. 3). This was used to compare the groundwater chemistry in the other geological formations (Fig. 3). Rainwater was collected twice from Vientiane city (19th and 28th August 2014), one sample from the piped drinking water supplied by Water Supply Authority (called as ‘Nam Papa’ in Lao) from Naneo village and one bottled drinking water were also analysed.
Care was taken to collect the samples from various land uses, including the settlement areas, rice fields, and other crops cultivated areas, such as fruits and vegetables. The dug wells in this area are shallow and extend to a maximum depth of less than 10 m bgl. Water level in these wells was recorded using a water level meter. Tube wells were pumped for at least 10 min before collecting the sample in order to collect the groundwater from the formation. EC and pH were measured using a Eutech (model: Testr 35) portable digital meter which was previously calibrated using 84 and 1413 µS/cm conductivity solution and 4.01, 7 and 10.1 buffer solution for pH. Pre-cleaned sample bottles were rinsed thrice with the sample before collection. Alkalinity of the water samples was determined by titration (APHA 1998). Other major ions and minor ions were analysed by ion chromatography after filtration (0.45 µm). Precision of the chemical analysis was determined by calculating the ion balance error for major and minor ions, which was within ±5 %. Heavy metals were determined using atomic absorption spectrophotometer (AAS GBC 932 Plus, detection limit for arsenic, zinc, copper, cadmium, and mercury is 0.001 µg/l, iron and lead is 0.02 µg/l) and uranium was analysed using an LED-based laser fluorimeter (Quantalase, detection limit 0.05 µg/l). To ensure precision of the chemical analysis, blanks and standards were run regularly. In addition, for every five samples, one sample was analysed in triplicate by varying the concentration of the standard and a calibration curve was obtained to cross-check the accuracy of the instrument and to avoid handling errors. Statistical analysis was performed using IBM SPSS version 22.0 (IBM SPSS 2013).
Heavy-metal pollution index was used to calculate the cumulative toxicity of metals using the following formula (Mohan et al. 1996):
where Wi is the unit weightage of the ‘i’th parameter, n is the number of parameters considered, and Qi is the sub-index of the ‘i’th parameter.
where K is the proportionality constant, S n is the standard permissible limit in water for the nth parameter.
where Mi is the monitored value of heavy metal of the ‘i’th parameter, Ii is the ideal value of the ‘i’th parameter, and Si is the standard value of ‘i’th parameter. The standard value for these heavy metals is the maximum permissible limit prescribed by many organisations. Since the presence of these ions in drinking water is not desired, the ideal values for these ions are given as 0 µg/l.
Results and Discussion
Depth to groundwater level in the village was at a maximum of 5.3, 5.7, and 7.2 m in December 2014, January 2015, and May 2015, respectively. These measurements represent the post-monsoon period and continuous decrease in water table depth due to local-pumping activities. As an example, spatial variation of the measured depth to groundwater level shown in Fig. 5a and b indicates deeper water levels in the residential part of the village due to increased pumping for daily needs. This is also associated with the high density of wells and less recharge in the central part of the village.
Drinking and Irrigation Quality Based on Major Constituents
Statistical details of the chemical composition of groundwater analysed during different sampling periods are given in Table 1. Maximum EC was 463 µS/cm, i.e., within drinking water standards proposed by Water Resources and Environment Administration (WREA 2009), now under the Ministry of Natural Resources and Environment (MoNRE). Low EC wells are located in the outer fringe of the village, i.e., away from the settlements (Fig. 5c, d). This is an indication that anthropogenic activities, such as increased pumping, local use of agrochemicals for household irrigation, or return flows from household water use practises can contribute to the chemistry of shallow groundwater. During the drilling of boreholes by the Department of Irrigation (2014), EC from 1600 to 1800 µS/cm was measured at depths greater than 35 m. Background sample had EC ranging from 16 to 78 µS/cm, rainwater had 3.8 and 7.1 µS/cm, urban water supply system had 148.5 µS/cm and bottled water had 34 µS/cm. Total dissolved solids (TDS) calculated from EC and total hardness (TH) calculated from calcium and magnesium indicates that groundwater is soft fresh water type (Fig. 6) except for two groundwater samples in January 2015. Surface water collected in January and May fall in soft type and hard type, respectively (Fig. 6).
All major and minor ions in groundwater were within the drinking water guidelines (WHO 1993; WREA 2009) and do not pose any health risk. Compared among the major ions, chloride was the most widely ranging (Table 1) and carbonates were absent in groundwater. Acidic pH was noticed in few parts of the village. Background sample, urban water supply system, and bottled water were also within the permissible limits for drinking for all measured parameters. Xayyavong and Babel (2010) found that the conductivity of water source used for manufacture of bottled water (from seven factories in Vientiane) was above WREA (2009) limit, i.e., 1000 µS/cm, but the final product as sold had conductivity suitable for human consumption (maximum 451 µS/cm).
Several indices were used to ensure the suitability of groundwater for agriculture, as most of the times chemical ions as a standalone are suitable for intended use, but, as a ratio or in combination with other ions, they may not be in the preferred range suitable for plant growth, and this may also reduce soil quality. Residual sodium carbonate (RSC) (Eaton 1950), sodium adsorption ratio (SAR) (Richards 1954), and EC (Fig. 7a) indicated that the use of groundwater does not pose an irrigation hazard (Table 2). Magnesium hazard (MH) based on the ratio of magnesium to the total concentration of calcium and magnesium (Szabolcs and Darab 1964) and Kelly’s ratio based on the concentration of sodium to the total concentration of calcium and magnesium (Kelly 1957) were not suitable in 37 and 43 % of the groundwater samples and surface water collected at both times were also unsuitable for irrigation (Table 2). Permeability index (PI) considers the cations and bicarbonates for determining the irrigation suitability (Doneen 1964). Due to the dominance of bicarbonates, permeability was low in 81 % of the groundwater samples apart from the surface water which was also found unsuitable for irrigation (Fig. 7b) owing to the high concentration of certain ions. Sodium percent (Na %) that considers all major cations in water (Wilcox 1955) was doubtful and unsuitable in most locations (Table 2). However, when compared with the EC, they are still good for agricultural use (Fig. 8). Sodium, to a certain extent, impacts the irrigation water quality, because of its dominance in the cations, though currently, it is not a major threat. This suggests the importance of monitoring sodium levels regularly to avoid serious issues, such as soil salinity in future.
Heavy Metals Variation and Risk
Arsenic is the most widely studied chemical constituent present in groundwater (Chanpiwat et al. 2011, 2014). Earlier studies in other parts of Lao PDR have recorded arsenic and barium pollution, whereas the levels of chromium, copper, manganese, nickel, and lead in groundwater are within safe limits (Chanpiwat et al. 2014). Chanpiwat et al. (2011) reported uranium concentration in groundwater ranging from 0.11 to 0.12 µg/l in Vientiane city, which is the closest location (about 50 km) to the present study area and highest uranium concentration of 38.19 µg/l in the southern provinces of Laos. Mercury levels in soils, sediments, and food stuffs of Vientiane city have also indicated low health risk to the population (Guédron et al. 2014). However, many of the other constituents in groundwater which may be important have not been studied. The Lao government does not have a maximum standard prescribed for uranium in drinking water, though limits are proposed for other heavy metals (WREA 2009). International organisations, such as WHO have recommended 15 µg/l (WHO 2004) and USEPA, have recommended 30 µg/l (USEPA 2003) of uranium in drinking water as a safe limit. The concentration of uranium in the study area is well below these limits. Heavy metals, including arsenic, zinc, copper, cadmium, and mercury, did not pose any threat to human health according to WREA (2009). Iron was above the proposed guidelines (1 mg/l of WREA 2009) in eight cases in December 2014 and one sample in January 2015. Lead pollution (above 10 µg/l) in eight samples from December 2014 was also recorded.
Sometimes comparing the determined concentration of an ion in groundwater with the standards to ascertain its drinking suitability alone is insufficient to establish the risk of drinking groundwater. In addition, a groundwater sampling location may possess all ions within permissible limits except for one, still making the water unsuitable for ingestion. At such times, heavy-metal pollution index (HPI), a cumulative index that helps to represent the combined influence of all the heavy metals in the water is used to indicate the water quality. Though in this village, the majority (80 %) of people do not drink the local groundwater and depend mostly on packaged drinking water, as an extreme case, the risk of human exposure to metals was calculated considering that groundwater is used by the entire population for drinking purposes. This will determine if groundwater in this area can serve as a source of drinking water in the absence of an alternate source and the effects on human health that it might bring.
HPI was determined for each sample, and the min, max, and average during the study period were 24, 286, and 65, respectively. This index is usually divided into five categories as excellent (0–25), good (26–50), poor (51–75), very poor (76–100) and unsuitable (>100). In December 2014, 35 % of the groundwater samples were found to be unsuitable. The percentage of groundwater samples in the village under different classes of HPI are given in Table 3. Water quality was better in January and May compared to December. Spatial variation in HPI during the two sampling periods is shown in Fig. 9. Most of the wells in the central part of the village are unsuitable in December 2014. Surface water sample had maximum HPI of 42 and the background sample located at about 5 km from the village had maximum HPI of 113. Overall, while the groundwater samples were unsuitable in December 2014, it was good during the later period. Lack of seasonal pattern in these ions may be an indication that these may be contributed by local activities, such as changes in pumping patterns, land use practises, i.e., application of fertilizers, and the quantity used during the different time periods and rainfall recharge. This study is limited to three time sampling which may not be sufficient to decipher the reasons for the variation and complicated processes governing the metal migration in groundwater and additional data collection on the soil and rock chemistry can help in the process.
Geochemical Processes
Most of the groundwater samples (n = 16) were collected from the Quaternary sediments, three from the Vientiane formation and one from the Champa formation (Fig. 3). A clear variation in the concentration of major or minor ions or heavy metals or in the groundwater types from these different geological areas was not observed. It is notable that the depth of the wells sampled is only in the top few meters of the formation compared to the total depth of these deposits which extend several hundreds of meters below the ground surface. Thus, the chemical composition of groundwater may depend on the ease of its dissolution from these deposits. To determine the various processes that control the groundwater chemistry, several comparisons were made between the ions. Ca–Mg–HCO3 and mixed Ca–Na–HCO3 were the dominant groundwater types (Fig. 10). This is similar to the Ca–HCO3, mixed Ca–Mg–Cl, and Na–Cl dominant groundwater reported in the Vientiane Plain by Perttu et al. (2011b). The order of dominance of cations was sodium > calcium > potassium > magnesium and that of anions was bicarbonate > chloride > sulphate.
Of the various processes that play an important role in the composition of groundwater and are responsible for spatial and temporal variation in its quality, rainfall and rock–water interaction have dominant roles in determining the chemistry of groundwater in Ekxang (Fig. 11). This is a broad classification that includes a number of lithological processes. Weathering and dissolution processes are further refined by plots of Ca/Na versus HCO3/Na and Ca/Na versus Mg/Na (Fig. 12). Groundwater has been largely influenced by silicate weathering and partially by carbonate dissolution. General reaction for weathering of silicate rocks with carbonic acid (Das and Kaur 2001) is given as:
Champa formation, Vientiane formation, and Quaternary sediments making up the surface geology of this area (Perttu et al. 2011a) are made of sandstones mostly composed of quartz (SiO2) and feldspar (KAlSi3O8–NaAlSi3O8–CaAl2Si2O8), kaolinite (Al2Si2O5(OH)4), and laterite (Fe2O3, Al2O3·3H2O) that are the abundant source for silicate minerals in this area. Most common cementing materials in such rock types that hold the sand particles are silica, calcium carbonate, or iron oxide (Bailey et al. 2002). Thus, sodium, potassium, calcium, and magnesium are likely to be contributed through silicate weathering. Similar observations have been made in silica rich lithogenic areas (Rajesh et al. 2012; Kumar 2014).
Dominance of bicarbonates in groundwater is due to significant contribution from carbonic acid formed from the carbon dioxide in the soil. Reaction involving the carbon dioxide in the unsaturated zone which reacts with rainwater to form bicarbonate is given by Drever (1988).
Apart from rainwater, feldspar minerals also react with carbonic acid and releases bicarbonates (Rogers 1989; Elango et al. 2003; Rajmohan and Elango 2004) which explain the presence of bicarbonates as the dominant cation in this area. Weathering of calcite (calcium carbonate) is given by Sami (1992).
Ion exchange indices occurring in water can be identified by two chloro-alkaline indices (CAI) proposed by Schoeller (1965).
These indices are positive indicating reverse ion exchange when sodium and potassium in groundwater exchange with calcium and magnesium minerals in the aquifer. Indices are negative when calcium and magnesium replace sodium and potassium. Most of the groundwater had positive CAI, but there exist few wells with ion exchange reactions. The relationship between Ca + Mg and SO4 + HCO3 shows that most samples plot close to the 1:1 line indicating the dissolution of calcite and reverse ion exchange (Fig. 13). Samples that plot close to the line with slope of −1 in a plot between Na–Cl and Ca + Mg–HCO3–SO4 also indicate significant reverse ion exchange, but samples that plot close to 0 on the x-axis are not influenced by this process (Jankowski et al. 1998). Slopes of −1 and −0.98 in January and May 2015 support ion exchange process (Fig. 13). Calcium, magnesium, and chloride can be contributed to groundwater by reverse ion exchange. Rainwater and background samples also plot similar to the groundwater showing that it is not highly polluted.
The potential for surface water–groundwater interactions was assessed using Scholler diagram plotted with the concentration of ions in stream water and the groundwater sample locations nearby (Fig. 14). Similar variation in major ion concentration is observed for both surface and groundwater indicating an influence of recharge of surface water on groundwater. Though high concentration of sodium and chloride was recorded in the surface water in May 2015, this appears to be a one-time phenomenon with the probable source being from upstream.
Factor analysis was used to differentiate and support the different hydrogeochemical processes identified. Four significant factors had eigenvalues above 1, and the cumulative variance was 85.8 %. Calcium and bicarbonate had the highest loadings in factor 1 (Table 4). Common source of these ions is silicate weathering, especially feldspar and carbonate dissolution. Second factor showed positive loadings between sodium and potassium with weak positive correlation with chloride and weak negative correlation with calcium and magnesium suggesting the source from reverse ion exchange process. These two factors have clear differentiation of the source compared to the other two factors. In factor 3, magnesium and chloride have positive correlation (Table 4). Typically, aquifers-containing halite (rock salt, composed of sodium chloride) or saline groundwater is the major source for chloride. Perttu et al. (2011a, b) had reported halite dissolution in other parts of the Vientiane Plain for Na–Cl type groundwater. However, weak correlation was observed between chloride and sodium, which omits any contribution from halite. In addition, lack of clear relations of chloride with nitrates and sulphates ignores the possibility from fertilizers. Salinisation of the soil through irrigation return flows, and domestic sewage may be a possible source for chloride. Magnesium with weak positive loadings with calcium and sodium may have been contributed by rock–water interaction. Factor 4 has positive loading only for sulphate indicating that this ion has a distinctive source different from all other ions (Table 4). Sulphate does not comply with any of the processes discussed and may be from human-induced sources. Evaluation of sulphate with other ions indicates that it may be contributed from anthropogenic activities, such as fertilizers, as the other ions are not from this source.
Conclusion
Major ions, minor ions and a selected number of heavy metals in groundwater were studied in a village on the Vientiane Plain, Laos, where groundwater is the major source of domestic and agricultural supplies. Ekxang village has shallow water table with maximum depth at 7 m and settlement areas having deeper water levels due to pumping for daily needs. Local-pumping patterns influence the spatio-temporal variation in water levels of this village. Groundwater meets all the measured criteria for drinking purposes except for iron and lead. Groundwater at certain locations is also unsuitable for drinking according to the HPI. Sodium in a few cases may slightly impact the irrigation water quality. Ca–Mg–HCO3 and mixed Ca–Na–HCO3 were the dominant water types. Geological processes that control the groundwater chemistry include feldspar weathering, carbonate dissolution, and ion exchange. Anthropogenic sources include salinisation and fertilizers used in agriculture. From this study, it would appear that the groundwater in this area is mostly suited for human consumption for the constituents considered, however, the reasons for the presence of lead and iron should be further studied.
References
APHA (American Public Health Association) (1998) Standard methods for the examination of water and wastewater, 20th edn. American Public Health Association/American Water Works Association/Water Environment Federation, Washington DC
Bailey RA, Clark HM, Ferris JP, Krause S, Strong RL (2002) Chemistry of the environment, 2nd edn. Academic Press, USA
Bestari NG, Shrestha S, Mongcopa CJ (2006) Lao PDR: an evaluation synthesis on rice. A case study from the 2005 Sector Assistance Program Evaluation for the Agriculture and Natural Resources Sector in the Lao People’s Democratic Republic, Asian Development Bank, p 17
Chanpiwat P, Sthiannopkao S, Cho KH, Kim K-W, San V, Suvanthong B, Vongthavady C (2011) Contamination by arsenic and other trace elements of tube-well water along the Mekong River in Lao PDR. Environ Pollut 159:567–576
Chanpiwat P, Lee B-T, Kim K-W, Sthiannopkao S (2014) Human health risk assessment for ingestion exposure to groundwater contaminated by naturally occurring mixtures of toxic heavy metals in the Lao PDR. Environ Monit Assess 186:4905–4923
Das BK, Kaur P (2001) Major ion chemistry of Renuka Lake and weathering processes, Sirmaur district, Himachal Pradesh, India. Environ Geol 40:908–917
Department of Irrigation (2014) Results of drilling well numbers 1/14, 2/14 and 3/14 at Ekxang Village, Phonhong District, Vientiane Province, Lao PDR. Unpublished Report Dated 26 September, 2014 (In Lao)
Doneen LD (1964) Water quality for agriculture. Department of Irrigation, University of California, Davis
Drever JI (1988) The geochemistry of natural waters. Prentice Hall, Englewood Cliffs
Eaton EM (1950) Significance of carbonates in irrigation waters. Soil Sci 69:123–133
Elango L, Kannan R, Senthilkumar M (2003) Major ion chemistry and identification of hydrogeochemical processes of groundwater in a part of Kancheepuram district, Tamil Nadu, India. J Environ Geosci 10(4):157–166
FAO (Food and Agriculture Organization of the United Nations) (2016) AQUASTAT website. Accessed 18 May 2016
FAO, World Bank, and IRRI (International Rice Research Institute) (2012) Lao’s People Democratic Republic Rice Policy Study, p 175
Fengthong T, Dethoudom S, Keosavanh O (2002) Drinking water quality in the Lao People’s Democratic Republic. ESCAP—IWMI Seminar on Environmental and Public Health risks due to contamination of soils, crops, surface and groundwater from urban, industrial and natural sources in South East Asia. Hanoi, Vietnam, pp 1–3
Grumbine R, Dore J, Xu K (2012) Mekong hydropower: drivers of change and governance challenges. Front Ecol Environ 10(2):91–98
Guédron S, Tisserand D, Garambois S, Spadini L, Molton F, Bounvilay B, Charlet L, Polya DA (2014) Baseline investigation of (methyl)mercury in waters, soils, sediments and key foodstuffs in the Lower Mekong Basin: the rapidly developing city of Vientiane (Lao PDR). J Geochem Explor 143:96–102
IBM SPSS (International Business Machines Corporation Statistical Package for the Social Sciences) (2013) IBM SPSS Statistics for Windows, Version 22.0. IBM Corp, Armonk
Jankowski J, Acworth RI, Shekarforoush S (1998) Reverse ion exchange in a deeply weathered porphyritic dacite fractured aquifer system, Yass, New South Wales, Australia. In: GB Arehart, JR Hulston (eds) Proceedings of the 9th international symposium, water–rock interaction. Balkema, Rotterdam, pp. 243– 246
Kelly WP (1957) Adsorbed sodium cation exchange capacity and percentage sodium sorption in alkali soils. Science 84:473–477
Kumar PJS (2014) Evolution of groundwater chemistry in and around Vaniyambadi Industrial Area: differentiating the natural and anthropogenic sources of contamination. Chem Erde 74(4):641–651
Mohan SV, Nithila P, Reddy SJ (1996) Estimation of heavy metal in drinking water and development of heavy metal pollution index. J Environ Sci Health A 31:283–289
MoNRE (Ministry of Natural Resources and Environment) (2012) Lao Environment Outlook 2012. Lao PDR, Ministry of Natural Resources and Environment, p 71
Pavelic P, Xayviliya O, Ongkeo O (2014) Pathways for effective groundwater governance in the least-developed-country context of the Lao PDR. Water Int 39(4):469–485
Perttu N, Wattanasen K, Phommasone K, Elming S-Å (2011a) Characterization of aquifers in the Vientiane Basin, Laos, using magnetic resonance sounding and vertical electrical sounding. J Appl Geophys 73:207–220
Perttu N, Wattanasen K, Phommasone K, Elming S-Å (2011b) Determining water quality parameters of aquifers in the Vientiane Basin, Laos, using geophysical and water chemistry data. Near Surf Geophys 9:381–395
Rajesh R, Brindha K, Murugan R, Elango L (2012) Influence of hydrogeochemical processes on temporal changes in groundwater quality in a part of Nalgonda district, Andhra Pradesh India. Environ Earth Sci 65:1203–1213
Rajmohan N, Elango L (2004) Identification and evolution of hydrogeochemical processes of groundwater environment in an area of the Palar and Cheyyar River Basins, Southern India. J Environ Geogr 46:47–61
Ribolzi O, Cuny J, Sengsoulichanh P, Mousquès C, Soulileuth B, Pierret A, Huon S, Sengtaheuanghoung O (2011) Land use and water quality along a Mekong Tributary in Northern Lao P.D.R. Environ Manag 47:291–302
Richards LA (1954) Diagnosis and improvement of saline and alkali soils. USDA Handbook, vol 60
Rogers RJ (1989) Geochemical comparison of groundwater in areas of New England, New York, and Pennsylvania. Groundwater 27(5):690–712
Sami K (1992) Recharge mechanisms and geochemical processes in a semi-arid sedimentary basin, Eastern Cape, South Africa. J Hydrol 139:27–48
Schoeller H (1965) Qualitative evaluation of groundwater resources. In: Methods and techniques of groundwater investigations and development, UNESCO, vol 99, pp 54–63
Serre L-A (2013) Etude d’impact des systèmes d’irrigation informels sur l’environnement: cas de la ressource en eau à l’échelle d’un village laotien. ISTOM, Ecole Supérieure d’Agro-Développement International, Mémoire de fin d’études
STEA and UNEP RRCAP (Science Technology and Environmental Agency and United Nations Environment Programme- Regional Resources Center for Asia and the Pacific) (2012) Lao PDR National Environmental Performance Assessment Report, p 209
Suhardiman D, Giordano M, Leebouapao L, Keovilignavong O (2015) Farmers’ strategies as building block for rethinking sustainable intensification. Agric Hum Values. doi:10.1007/s10460-015-9638-3
Szabolcs I, Darab C (1964) The influence of irrigation water of high sodium carbonate content of soils. In: Proceedings of 8th ISSS, Trans vol II, pp 802–812
UNDAF (United Nations Development Assistance Framework) (2015) Lao PDR evaluation report, United Nations Development Assistance Framework 2012–2016, p 283
United Nations (2015) Country analysis report: Lao PDR, analysis to inform the Lao People’s Democratic Republic—United Nations Partnership Framework (2017–2021), p 113
USEPA (US Environmental Protection Agency) (2003) Current drinking water standards; Ground water and drinking water protection agency: Report prepared by Wade Miller Associates, pp 1–12
Viossanges M et al. (forthcoming) Hydrogeological assessment in upper Vientiane Plain, Lao PDR: implications for sustainable groundwater development in data-scarce regions. (In preparation)
WHO (World Health Organisation) (1993) Guidelines for drinking water quality, vol 1, 2nd edn, Recommendations, WHO, Geneva, p 130
WHO (2004) Uranium in drinking-water, background document for development of WHO Guidelines for drinking-water quality, WHO/SDE/WSH/03.04/118, p 15
WHO/UNICEF (United Nations Children’s Emergency Fund) (2015) Joint monitoring programme for water supply and sanitation, estimates on the use of water sources and sanitation facilities. http://www.wssinfo.org/documents/?tx_displaycontroller[type=country_files]. Accessed 20 May 2016
Wilcox LV (1955) Classification and use of irrigation waters. USDA, Washington, DC Circular 969
WMO (World Meteorological Organisation) (2014) Department of Meteorology and Hydrology, WMO. http://worldweather.wmo.int/en/city.html?cityId=235. Accessed 15 May 2015
World Bank (2007) Lao PDR—environment monitor. World Bank, Washington, DC, p 67
World Bank (2014) Lao PDR development report 2014: expanding productive employment for broad-based growth, Report No. ACS9577, p 74
World Bank (2016) Online database, http://data.worldbank.org/country/lao-pdr?display=default. Accessed 20 May 2016
WREA (Water Resources and Environment Administration) (2009) Agreement on the National Environmental Standards, Lao People’s Democratic Republic, p 30
Xayyavong S, Babel S (2010) Quality of bottled drinking water: case study in Vientiane, Laos and Thailand, 4th International conference on bioinformatics and biomedical engineering (iCBBE), Chengdu, pp 1–4
Acknowledgments
Funds for this study was made available through ACIAR Project titled “Enhancing the Resilience and Productivity of Rainfed Dominated Systems in Lao PDR through Sustainable Groundwater Use” (Project No. LWR/2010/081) and contributes to the CGIAR research program on Water, Land, and Ecosystems. From the IWMI, Vientiane Office, the authors wish to thank Mr. Hinsila Phouthavong for his assistance in sample collection, and Mr. Chris Harris Pascal and Mr. Mathieu Viossanges for their constructive comments on the manuscript. Mr. G. Gowrisankar, Research Scholar, Anna University, India, is acknowledged for his assistance in carrying out the chemical analyses.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Brindha, K., Pavelic, P., Sotoukee, T. et al. Geochemical Characteristics and Groundwater Quality in the Vientiane Plain, Laos. Expo Health 9, 89–104 (2017). https://doi.org/10.1007/s12403-016-0224-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12403-016-0224-8