Abstract
Spatial variability and geochemical factors controlling fluoride (F−) concentrations were evaluated in five villages of subdistrict Gujrat in Pakistan. Groundwater samples (n = 70) were collected along the River Chenab and examined for F− concentrations. The average F− concentrations in groundwater of village Chhani Nikoani (1.8 mg/L) and Dhool Khurd (2.2 mg/L) were crossing the World Health Organization (WHO) permissible limit of 1.5 mg/L, while village Kalra Khasa, Ghazi Chak and Kula Chor were below the WHO guidelines. About 64 % of the samples contain fluoride concentrations that exceed the 1.5 mg/L drinking water standard set by WHO. Spatially F− concentrations in groundwater increased as moving away from the River Chenab because more residence time for water–rock interaction was available. High F− concentrations were associated with high electric conductivity (EC), total dissolved solids (TDS), pH and Na+ values. The overall groundwater quality is controlled by silicate mineral weathering and less by evaporation. Fluoride originates from anion exchange (OH− for F−) on clays and weathered micas under high pH conditions. High fluoride levels are associated with NaCl- and NaHCO3-type water produced by calcite precipitation and/or base ion exchange. The groundwater with higher fluoride concentrations is accompanied by lower Ca+2 levels. This study enhances our understanding on geochemical behavior of F and highlights the importance of spatial variability on F release in aquifers of Punjab in Pakistan, as well as in other countries of Asia region.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Fluoride (F−) is an important nourishing constituent for health which is present in groundwater as free fluoride ions. It begins to affect human health as its concentration exceeds World Health Organization (WHO) guideline for F− in drinking water (Saxena and Ahmed 2003). When F− exceeds value of 1.5 mg/L WHO guideline for F− in drinking water, it results in dental and skeletal fluorosis, crippled limbs, rheumatic pain, the calcification of ligaments stiffness, and the rigidity of the joints (Ayoob and Gupta 2006). It can adversely affect the kidneys, liver, gastrointestinal tract as well as the nervous, immune, and reproductive systems disorders (Ibrahim 2011).
The problem of fluorosis develops extensively in many areas of the world, such as in Argentina, Bangladesh, China (Guo et al. 2007), India (Jacks et al. 2005), Japan, Morocco, New Zealand, South African Countries, Thailand and Middle East countries, and USA (Rafique et al. 2009). In Pakistan, the high F− concentrations in groundwater have been reported from Karachi (Siddique et al. 2006), Lahore (Farooqi et al. 2007), Peshawar (Shah and Danishwar 2003), and Thar Desert (Rafique et al. 2008).
Fluoride has affinities for rocks and occurs in F−-bearing minerals that lead to transportation of substantial amount of F− into the groundwater (Lunshan et al. 2007). Hence, groundwater with high F− concentrations is probable in areas where F−-bearing minerals are abundant (Shaji et al. 2007). Common natural sources of F− in groundwater are the dissolution of F−-bearing minerals like fluorspar and fluorapatite and some micas weathered from silicates (Guo et al. 2007). The dissolution of F−-bearing minerals not only produces kaolinite but also discharges cations, such as Ca2+, Mg2+, Na+ and K+, causing an increase in TDS which indirectly affect F− concentration in groundwater (Li et al. 2015). Fluoride-bearing minerals occur in deep soils, when soluble F− concentration is higher in deep soil than in surface soils, and it follows the geological source of F− contamination (Jayawardana et al. 2012).
Higher concentrations of F− usually occur due to water–rock interaction of fluorite, fluorapatite, and cryolite or any F−-bearing mineral which results in Ca2+ depletion of groundwater due to common ion effect (Chae et al. 2007). The levels of dissolved F− are generally linked with the pH value and water type, although the depth and temperature of groundwater and climatic factors like evaporation/precipitation also play significant roles (Rafique et al. 2015). Ion exchange in which OH− replaces F− in groundwater on certain clay minerals or weathered micas (Rafique et al. 2009). Higher F− concentrations are associated with both NaCl- and NaHCO3-type waters, and lower F− concentrations are linked with CaHCO3- and CaMgCl-type waters, where greater Ca2+ levels lead to precipitation of fluorite (Rafique et al. 2015). The clay proportion of soil has positive relation with F− concentrations in soil than silt and negative relation with sand (Farooqi et al. 2009).
The groundwater flow rate is higher in areas nearby river, residence time is lower, and there is a less time of contact between water and F−-bearing rocks. This led to lower concentrations of F− in the groundwater. However, in the central basin areas, groundwater had moved a longer distance and thus had a higher residence time (Li et al. 2015). This indicates that groundwater is more enriched in F− along the flow path away from river. As a result, F− concentrations got increased (Basharat 2012).
Gujrat has been famous for the production of low-temperature pottery and ceramic goods. Gujrat is also known in the world for its clay which is used to produce quality pottery since ages. The clay content of soil of Gujrat varied along with increasing distance from River Chenab as subdistrict Gujrat is located near River Chenab. Sandy loams are more abundant near river that has negative relation with F− concentrations (Farooqi et al. 2009), while moving away from river, silty loams and clayey soil are evident that has capacity to hold higher F− concentrations (Ashraf and Ahmad 2008).
Groundwater in semiarid areas surrounding Gujrat is known to have F− concentrations that go above the guideline for drinking water 1.5 mg/L set by WHO (Rafique et al. 2008). The percentile F− contribution in lowering the water quality of areas around Gujrat, i.e., Sargodha (7 %), Faisalabad (16 %), Lahore (18 %) and Kasur (18 %) is comparatively high than Gujrat (2 %) which indicate certain geological factors that are controlling F− enrichment in groundwater of Punjab (Pakistan Council of Research in Water Resources 2010).
The present study evaluates groundwater quality in a region where screening by Pakistan Council of Research in Water Resources (PCRWR) reported fluoride in groundwater, and the area is known for its high class of clay minerals and was undertaken to define the sources of dissolved fluoride and the geochemical processes that influence its occurrence. The aim is to identify geochemical relationships that influence in understanding the origin of high fluoride established on measures of the overall water quality. This study was the first attempt to analyze F− and to predict its geochemistry controlling the F−concentrations.
Materials and methods
Study area
Location and hydrometeorology
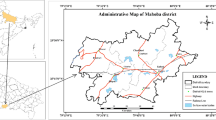
District Gujrat (Fig. 1) including an area of approximately 1254 km2 was selected as the study area for this study. It is located in the semiarid zone of Punjab, Pakistan between 32.5738N and 74.0789E. For political and administrative purposes, the whole district is divided into three subdistricts (Kharian, Sarai Alamgir, and Gujrat); subdistrict Gujrat was selected for study. Climate of the study area is tropical with an average annual rainfall of 670 mm, 70 % of which occurs between June and September. The wet season starts from end June and lasts up to the start of November, whereas the rest of the months are generally dry. The temperature rises to maximum of 48.2 °C in summer and drops to a minimum of 2 °C during winter (Gujrat city profile). The main rivers of the study area are River Jhelum and River Chenab. The study area falls within the area of upper Chaj Doab and sandwiched between these two rivers but more influenced by River Chenab, having a distance of 12 km. Gujrat is populated with 270,000 people and majorly drained by River Chenab (Ashraf and Ahmad 2008).
Geological and hydrogeological setting
The soil loams of the Gujrat are composed of silty and sandy alluvial substantial (Ashraf and Ahmad 2008). Punjab Province is drained by five major rivers that flow down from high range mountains of Himalaya. The sediments carried along with these rivers water from the massive alluvial basin of the aquifer that contains material washed down from the Himalaya Mountains were set down. Study area contains more than 500-m-thick Quaternary deposition (Ashraf and Ahmad 2008). The alluvium material consists of sand and silt and minute amounts of gravel and clay, set down by the Indus River by its present and ancestral offshoots. In harmony with their mode of deposition by large continually flowing rivers, the alluvial depositions are heterogeneous by nature, and individual layers have restricted horizontal and vertical continuity (Greenman et al. 1967).
In general, unconfined aquifers and some confined aquifers are present in the study area (Basharat 2012). Groundwater table is declining with increasing population and over abstraction (81 % annual abstraction) of groundwater than recharge (Mahmood et al. 2011). Aesthetically acceptable water starts below the depth of 50 feet (Ashraf and Ahmad 2008). The direction of groundwater flow is from recharge zone, foothills of Himalaya toward discharge plain area of Gujrat. Flow direction of groundwater in the study area also got affected by River Chenab’s fluctuating flow during rainy and dry season (Mahmood et al. 2011).
Sampling
Groundwater samples were collected from five villages of subdistrict Gujrat during October 2014. To cover entire subdistrict Gujrat, five random villages were selected (Fig. 1). From these villages, in total 70 groundwater samples were collected. In Dhool Khurd (DK), Kalra Khasa (KK), Ghazi Chak (GC), and Kula Chor (KC), 15 groundwater samples were collected from each village at the depth of 14–105, 7–105, 8–122, and 11–75 m, respectively. From village Chhani Nikoani (CN), 10 groundwater samples were collected at the depth of 20–90 m because the area is sparsely populated compared to other villages. From each village Dhool Khurd (DK), Kalra Khasa (KK), Ghazi Chak (GC), and Kula Chor (KC), total 10 soil samples (each composite of 3) were collected in polyethylene zipper bags (five from surface and five from subsurface), while from village Chhani Nikoani (CN), four soil samples were collected (two from surface and two from subsurface). Soil sampler (hand Augur) was used for surface (0–5 cm) and subsurface (35–40 cm) soil samples collection.
Groundwater samples were collected in 300-mL polyethylene bottles that had been pre-washed with dilute (1 %) nitric acid according to standard methods (Greenberg et al. 1998). Prior to sample collection the pump was flushed for 5 min. The bottles were first rinsed with groundwater and then completely filled and sealed with caps to avoid the oxidation of redox-sensitive constituents. At each sampling point, two samples were collected. One was preserved by acidifying to the pH value of <2 with ultra-pure HNO3 for the later analysis of cations, while the other was stored without acid for the later analysis of anions in the environmental geochemistry laboratory. The exact sampling coordinates were identified using a GPS unit.
Analytical techniques
Temperature and pH were measured by digital pH meter, oxidation reduction potential (Eh) by Eh meter, conductivity, and total dissolved solids (TDS) were measured in the field using digital meter. In laboratory, water samples were analyzed for alkalinity as HCO3 − by acid–base titration, sulfates by titration with barium chloride method, chlorides by silver nitrate titrimetric method, nitrates by phenol disulphonic method, and phosphates by ammonium molybdate method (APHA 1995). Similarly, acidified samples were analyzed for major cations (Na+, K+, Ca2+, Mg2+) using Sequential Flame Atomic Absorption Spectrophotometer (Varian Spectra AA-240) under standard operating conditions having r > 0.99.
Fluoride was analyzed by using fluoride ion-selective electrode Orion 9609 with ion analyzer EA 940 by diluting water sample with total ionic strength adjustment buffer (TISAB) in ratio 1:1 to avoid dissociation of fluoride complexes with other metals present in samples that can hinder the F analysis (Greenberg et al. 1998). High levels of precision and accuracy were maintained during all phases of laboratory analysis, which included blank and spiked samples to ensure the reliability of each method. The ionic balance error (IBE) values were within an acceptable limit of ±5 % (Domenico 1990; Mandel 1981) for all samples.
Soil samples were grounded followed by sieving through 200-mm mesh. The pH value of soil samples was determined with pH meter in ratio of 1:5 soil to water, while the soluble fluoride concentrations were determined in same ratio with ion-selective meter same as that of water. Minerals were identified by X-ray diffraction spectroscopy (XRD).
All scientific and statistical computations were made by using IBM SPSS statistics version 20, XLSTAT Pro v7 5.2, PHREEQC, and Arc GIS 10 software. Multivariate statistical methods were used for interpretation and classification of large environmental datasets. The normality of data under variables was checked by Kolmogorov–Smirnov test. Principal component analysis (PCA) was employed on the dataset to reduce the analytical data collected from each village. For mineral speciation, saturation indices were used to analyze the degree of equilibrium between mineral species and water.
Reagents, procedural blanks, and samples were measured three times, and the average of three values was used. For QC/QA, Standard Reference Materials (JSL-1 & JSD-1) was used and the recovery was found 98 % using the same procedure and reagents. Arsenic was measured by using the atomic absorption graphite technique at Geoscience Advanced Research Laboratories.
Results and discussion
Overall groundwater quality
Table 1 shows the chemical parameters measured in the groundwater samples collected at the CN, DK, KK, GC and KC. The pH of the samples varied from 7.8 to 8.7, 7.1 to 8.9, 7.2 to 8.5, 7.0 to 8.0, and 7.1 to 8.6 at CN, DK, KK, GC, and KC, respectively. Since pH usually has no direct impact on consumers, no health-based guideline value has been proposed for pH by WHO (WHO 2011). The TDS of the samples varied from 342 to 1528, 107 to 1152, 127 to 1152, 87 to 1246, and 134 to 2238 at CN, DK, KK, GC, and KC, respectively. The palatability of water with a total TDS level of less than about 600 mg/L is generally considered to be good; drinking water becomes significantly and increasingly unpalatable at TDS levels greater than about 1000 mg/L (WHO 2011).
In almost all of the groundwater samples, the EC and TDS measurements were higher than the WHO guidelines. The EC measurements ranged from 0.13 to 3.34 mS/cm, and the TDS measurements ranged from 87 to 2238 mg/L. Soluble electrolytes and high salinity are generally the main cause of elevated EC and TDS in groundwater (Brahman et al. 2013). It is clear that much of the groundwater in this region is unacceptable for drinking in terms of high EC and TDS.
Sodium was the major cation, with concentration ranging from 73.9 to 529 mg/L. The average concentration of Na+ in groundwater samples from Chhani Nikoani (398 mg/L) and Kula Chor (291 mg/L) was higher than WHO guideline (200 mg/L), while the average concentration of Na+ in samples of other villages was within the WHO guideline. The Na+ naturally occurs in water; sometimes, mineral deposits enhance its concentration in groundwater to unacceptable levels (Rafique et al. 2015). Potassium concentration varied from 0.04 to 52.9 mg/L with 95 % of the samples were below the WHO guideline for K+ in drinking water (12 mg/L) and 5 % of samples contained K+ higher than the WHO guideline. The Ca2+ concentration ranged from 1.12 to 256 mg/L in groundwater of present study area. Most of the samples (90 %) had Ca2+ concentrations under the WHO guideline (100–150 mg/L), while few (10 %) had Ca2+ values above the WHO guideline. Magnesium concentration ranged from 0.38 to 98.4 mg/L. Around 8 % of the samples had Mg2+ concentration above WHO guideline, while 90 % had Mg2+ concentration below WHO guideline. Ca2+ and Mg2+ are relatively lower in the groundwater of the study area. Chloride (Cl−) and HCO −3 were the dominant anions in groundwater samples. Cl− ranged from 200 to 950 mg/L and HCO −3 ranged from 17.8–958 mg/L. Fifty percent of the groundwater samples contained Cl− above the WHO guideline. The HCO −3 concentrations were more than WHO guideline (500 mg/L) in 33 % of samples. Higher values of Cl− might be due to the presence of geologic deposits containing halite in the groundwater of study area (Srinivasamoorthy et al. 2008). And high concentrations of HCO −3 in few samples are linked to pH and weathering of carbonaceous minerals (Kumar 2014). Sulfate (SO 2−4 ) concentration ranged from 33.3 to 133 mg/L.
Data were plotted on diagrams developed by Gibbs (1970) for the purpose of understanding the dominant control(s) on water quality (Fig. 2a, b; Table 1). These plots suggest 60 % contribution of rock weathering and 40 % evaporation in controlling groundwater chemistry for an area which is semiarid. It signifies the water–rock interaction phenomena which got affected by residence time. The dominance of NaCl-type groundwater is the resultant of evaporation, which assists to increase the ionic strength of the groundwater. Primarily, it also increases the minerals solubility in groundwater, including those that release fluoride into groundwater. However, as the dissolved species concentrations continue to increase, the groundwater becomes supersaturated with respect to minerals that are less soluble, which ultimately precipitate out from groundwater (Rafique et al. 2015).
Gibbs plot which illustrate the dominant processes that control the chemistry of groundwater samples, a for cations, b for anions. Symbols are as described in Fig. 3
Chadha (1999) proposed a hydrogeochemical classification scheme for water by plotting the difference between (CO3 + HCO3) and (Cl + SO4) concentrations in meq/L expressed as percent versus (Ca + Mg) and (Na + K) concentrations in meq/L also expressed as percent (Chadha 1999). This classification is a modified version of the Piper diagram (Piper 1944). The resulting diagram has four fields, representing four types of hydrogeochemical processes (Fig. 3). Nearly, 43 % (N = 30) of the samples collected from the study area belong to NaCl-type water affected by evaporation. Rest 21 % (N = 15) belong to CaMgCl-type waters, 14 % (N = 20) with are NaHCO3-type water, and 16 % (N = 11) classified as CaHCO3-type waters (Fig. 3). Water quality produced by either base ion exchange (NaHCO3) or reverse ion exchange (CaMgCl) type waters would only occur where the aquifer includes the proper clay minerals. Carbonate minerals are prevalent within the study area (Naseem et al. 2010). Moreover, high Na/Ca ratio for Field 4 samples means that Na increased and calcium decreased through base ion exchange or calcite precipitation. The relatively low Ca/(Ca + SO4) ratios and high sodium for Field 3 and Field 4 samples support the conclusion that ion exchange is influencing groundwater chemistry at the locations where these samples were collected (Edmunds and Smedley 2005; Chae et al. 2006; Guo et al. 2007). The NaHCO3-type water that is undersaturated with respect to calcite which is due to the exchange of calcium and magnesium ions in solution for sodium on clays or other secondary minerals.
Chadha diagram showing groundwater samples classification taken from the study area (after Chadha 1999). Field 1: CaHCO3-type waters, reflecting recharge and weathering. Field 2: CaMgCl-type waters, reflecting reverse ion exchange. Field 3: NaCl-type waters, reflecting evaporation or mixing with seawater. Field 4: NaHCO3-type waters, reflecting base ion exchange (Symbols same as used in Fig. 2)
Spatial distribution of fluoride and role of geochemical control
The F− concentrations from study area Gujrat varied from 0.3 to 6.4 mg/L. The mean F− concentration in groundwater samples from village Chhani Nikoani (1.8 mg/L) and Dhool Khurd (2.2 mg/L) was exceeding WHO drinking water standard of 1.5 mg/L. According to classification used by (Nagendra Rao 2003), about 48 % of groundwater samples from village Chhani Nikoani and Dhool Khurd had F− concentrations below WHO standard, while 32 % of samples were within range of 1.6–3.0 mg/L which can cause dental fluorosis, and remaining 20 % of samples had F− concentrations of 3.1–7.0 mg/L which can cause skeletal fluorosis in humans (Nagendra Rao 2003). The mean F− concentration in groundwater samples from village Kalra Khasa (1.3 mg/L), Ghazi Chak (0.9 mg/L), and Kula Chor (1.45 mg/L) was mostly below WHO standard. Overall, about 73 % of samples had F− concentrations below WHO standards, while remaining 22 % of samples from these villages were within range of 1.6–3.0 mg/L which can cause dental fluorosis, and remaining 5 % of samples were within the range of 3.1–7.0 mg/L, which lead to skeletal fluorosis (Nagendra Rao 2003).
A slight positive correlation (R 2 = 0.30) between F− concentration and TDS was observed (Fig. 4a). In the study area, high TDS values were observed in few samples from village Chhani Nikoani, Dhool Khurd, and Kula Chor with comparatively high F− concentrations, while village Kalra Khasa and Ghazi Chak had low TDS values with comparatively lower concentrations of F− to other villages. Similar results of correlation between F− and TDS were reported from Nagar Parkar (Rafique et al. 2009).
Relationship of F− with a TDS, b with pH+ in groundwater of study area Gujrat. Symbol for different villages is as described in Fig. 2
Figure 3 shows that higher F− concentrations are associated with both NaCl (Field 3) and NaHCO3 (Field 4), and the samples plotted in Fields 3 and 4 of Fig. 3 are relatively depleted in calcium and show evidence of base ion exchange (as described in “Overall groundwater quality” section), and lower F− concentrations are linked with CaHCO3- and CaMgCl-type waters, where greater Ca2+ levels lead to precipitation of fluorite (Rafique et al. 2015).
The NaHCO3-type water is result of the removal of Ca2+ and Mg2+ from groundwater by Na+ ion exchange with F− on clay minerals. This shows cation exchange reaction is important in controlling F− chemistry in groundwater (Chae et al. 2007; Rafique et al. 2015) where Ca2+ has been replaced with Na+ in waters containing high F−. The dominance of NaCl-type groundwater (Fig. 3) is due to evaporation, which may increase the ionic strength of the water. The concentration of dissolved mineral got increased because of evaporation, and groundwater becomes saturated with respect to the least soluble minerals, which ultimately precipitate out from groundwater. Hence NaCl water type can affect F− concentration in groundwater due to minerals precipitation (Rafique et al. 2015). In the current study, NaCl type of water contains the highest concentration of F− as indicated before in Fig. 3.
Fluoride-rich groundwater in many regions of the world is often of NaHCO3 type with low concentrations of Ca2+ and alkaline pH values (Li et al. 2011). The present study shows positive correlation with pH A positive correlation (R 2 = 0.85) between F− concentration and pH was observed (Fig. 4b). Similar positive correlation of pH with F− concentration was observed in different regions of the world (Valenzuela-Vasquez et al. 2006; Farooqi et al. 2007; Rafique et al. 2009; Wang et al. 2009; Mamatha and Rao 2010; Li et al. 2011). Groundwater samples from present study area have pH range from 7.1 to 8.6. Water with pH value <7 rarely have detectable F− concentration (Genxu and Guodong 2001). The F− and OH− have similar sizes of their ionic radii and could play substitute role for each other in minerals. Clay particles have ability to adsorb F− ions, with increasing pH the F− ions got displaced by OH− ions, ultimately releasing F− ions into groundwater (Genxu and Guodong 2001). This relationship might explain the high F− concentrations in samples from village Chhani Nikoani and Dhool Khurd with pH value from 7.8 to 8.9.
High pH would also favor calcite precipitation (thereby increasing fluorite solubility). The solubility of calcite (CaCO3) is of particular importance, because it shares a common ion with fluorite (CaF2), which normally controls the upper limit of dissolved fluoride. Figure 5a–d plots the calcite and fluorite saturation indices. This plot shows that groundwater in the study area has evolved to the point where all samples with high fluoride are supersaturated with respect to calcite, which should favor the removal of calcium from solution and allow fluoride concentrations to increase (Kundu et al. 2001). Assuming that NaHCO3-type waters were produced by base ion exchange (the removal of Ca and Mg from solution in exchange for Na on clay minerals), the net result is the same as described above for NaCl-type waters. As Ca2+ concentrations are decreased, the groundwater is able to contain higher levels of fluoride before saturation with respect to fluorite is reached. This interpretation is supported by the fact that fluoride concentrations are generally lower in the CaHCO3- and CaMgCl-type waters, where higher Ca2+ levels lead to fluorite precipitation.
Statistical verification of correlation among physicochemical parameters
The correlation matrix for physicochemical parameters is given in Table 2. For statistical verification of these correlations, PCA was applied on groundwater dataset. The PCA plot is powerful recognition technique that explains characteristics of samples and distribution of intercorrelated parameters (Brahman et al. 2013). PCA plot was drawn for dataset of present study to verify the intercorrelation with physicochemical parameters. The PCA plot with two component axes explained 71.4 % of variance in data set. The first component axes explained 50.86 % of variance in data, while second component axes explained 20.54 % of variance in data. Parameters distribution in upper right side of plot was more enriched with Na+, K+, F−, pH, EC, and TDS which indicate geological sources of these components (Fig. 6). High F− groundwater is generally characterized by Na+ rich and Ca2+ poor with comparatively high pH values and HCO −3 concentrations (Li et al. 2015), as a result of natural processes of evaporation, dissolution of minerals, and cation exchange. The results of present study show high values for Na+, TDS, EC, pH and HCO −3 which might lead to F−-rich groundwater. The first component axes showed negative loadings for Ca2+, Mg2+, and Cl− which indicate climatic effects. The current study was observed to have lower values for Ca2+ and Mg2+ which lead to F−-rich groundwater (Li et al. 2011). Water type is very important in controlling F− enrichment; NaHCO3 type support high concentrations of F− in groundwater (Li et al. 2011). Figure 6 illustrates the Na+ value was more inclined toward Cl− than toward HCO −3 , indicating dominance of NaCl water type that can limit F− concentrations in groundwater. NaCl water type can affect F− concentrations in groundwater due to minerals precipitation (Rafique et al. 2015). The second component axes of PCA showed positive loadings for all parameters which indicate natural source, i.e., mineral weathering as source of contamination (Mamatha and Rao 2010).
Spatial distribution of fluoride and role of local geology
The spatial distribution of categorized F− concentrations along with River Chenab is presented in Fig. 7. The F− concentrations in groundwater of study area were decreased while moving away from the River Chenab. Northwestern villages Chhani Nikoani and Dhool Khurd had more F− concentrations, located away from River Chenab. While southwestern villages Kalra Khasa and Ghazi Chak were observed to have low F− concentrations, located nearer to River Chenab. A northwestern village also observed to have comparatively more F− concentrations, which was located away from River Chenab. The higher F− concentrations in groundwater were analyzed in villages away from River Chenab, while villages nearer to River Chenab had lower concentrations. This obvious spatial distribution patterns reflect the geochemical controls on its occurrence and are clearly linked to the interpolation of groundwater flow regime, high rate of groundwater flow made harder to lose F− from its mineral rock (Kausar et al. 2003; Chae et al. 2007; Currell et al. 2011; Dey et al. 2012; Li et al. 2015). Sites nearer to River Chenab had high groundwater flow rate, lower residence time, and less time for contact between water- and F− bearing minerals. Hence, decreased concentrations of F− were observed in nearby sites of river. However, when moved away from River, residence time was increased which led to increased F− concentrations in groundwater (Li et al. 2015). Similar trend of F− concentrations with flow regime of groundwater was reported from many regions of the world (Suthar et al. 2008; Rango et al. 2009; Naseem et al. 2010; Yitbarek et al. 2012; Su et al. 2013; Li et al. 2015).
XRD and thin sections were used to analyze minerals present in the soils of the study area (Fig. 8a, b). The results inferred that F−-bearing minerals were not identified by XRD pattern. The main mineral was quartz, with muscovite and biotite identified in three villages with relatively higher F− concentrations and not detected in Ghazi Chak and Kula Chor. Muscovite and biotite might be the source of F− in these villages by the following reaction (1 and 2). High alkalinity values and Na+ in groundwater have more OH− ions which can replace with F− content of F−-bearing minerals, increasing its concentrations in groundwater (Guo et al. 2007).
Relationship between a F− and pH, b F− and sand %, c F− and silt %, d F− and clay % of soil samples. Symbols are same as used in Fig. 3
Weathering of muscovite and biotite was major source of F− content in groundwater other than geochemical factors (Kumar et al. 2015). The muscovite and biotite which is considered as the major source of F− in groundwater is detected by the petrographic thin sections (Fig. 8a) and by XRD (Fig. 8b).
Although the F− concentrations exceeded the WHO guideline in 23 % of samples, the concentrations are lower as compared to other reported areas in Pakistan (Table 3). Water-soluble F− concentration was measured in soil samples. The descriptive statistical summary of surface and subsurface soil samples from each village is given in Table 4. The mean value of F− in surface soil (0–5 cm) samples from all villages was 1.29 mg/Kg, while in subsurface soil (35–40 cm) samples was 1.53 mg/Kg. Water-soluble F− from all villages also showed a positive correlation with pH (R 2 = 0.64, Fig. 9a) as that in groundwater. Soil pH is an important factor in controlling F− concentrations (Loganathan et al. 2003) The F− enrichment of groundwater occurs with increasing alkalinity of soil and releases F− into water as result of ion exchange reaction (Sheikhy et al. 2014). Increasing alkalinity of soil causes desorption of Ca2+, and data points shifted to right after ion exchange reaction with Na+.
Mostly, sandy loams were found in all villages except Chhani Nikoani which is silty loam. Different soil fractions have different effects on F− concentrations. Fluoride showed a strong negative correlation with sand (R 2 = −0.58, Fig. 9b), weak correlation with silt (R 2 = 0.08, Fig. 9c), and clay fraction was positively correlated (R 2 = 0.35) with F− concentrations (Fig. 9d). This positive correlation indicates that clay particles, which occur at low concentrations in sandy loam soils, have great capability to bound F− on their surface. The clay content was evenly distributed in all villages except Kula Chor which have less percentage of clay particles in its soil samples.
A negative correlation was observed between groundwater F− concentrations and soil F− concentrations from same study area (Fig. 10). More F− concentration in soil samples as compared to water samples from village Chhani Nikoani, Dhool Khurd, and Ghazi Chak was observed. This is due to more percentage of clay content in soil of these villages, which have capability to adsorb F− on surface (Guo et al. 2007). Less F− concentration in soil sample as compared to water samples from village Kalra Khasa and Kula Chor was observed. The presence of more percentage of sand particles as compared to silt and clay particles have less holding capability and might result in F− release from soil into groundwater (Li et al. 2011).
Conclusions
The groundwater occurring in district Gujrat has been found unsuitable (23 %) for drinking as a result of high total dissolved solids and the occurrence of elevated fluoride concentrations; 23 % of the samples collected in this study contain fluoride concentrations that exceed the WHO permissible limit of 1.5 mg/L. This study reveals the fact that fluoride originates from the weathering of minerals in the aquifer matrix and possibly the exchange of OH− for F− on clay minerals and weathered micas under high pH conditions. Rock weathering is the dominant process affecting groundwater quality, of 57 % of the samples, and second process is evaporation. As the ionic strength of groundwater increases through evaporation, minerals are initially more soluble, but finally the solubility limits for non-evaporite minerals are reached, causing precipitation which is best shown by the saturation indices of the samples from the study area which are supersaturated with respect to calcite containing high fluoride, and it is likely that the removal of calcium from solution and permits fluoride concentrations to increase. Some calcium is removed from solution through base ion exchange, thus increasing the fluoride concentrations associated with NaCl- and NaHCO3-type water. Spatial distribution and relatively lower concentrations in the area and presence of clay minerals also suggest the role of local geology and rock–water interaction in controlling the fluoride concentrations.
References
Anjum MN, Shah MT, Ali F, Hussain E, Ali L (2013) Geochemical studies of fluoride in drinking water of union council ganderi, district nowshera, Khyber Pakhtunkhwa. Pakistan. World Appl Sci J 27(5):632–636
Ashraf A, Ahmad Z (2008) Regional groundwater flow modelling of Upper Chaj Doab of Indus Basin, Pakistan using finite element model (Feflow) and geoinformatics. Geophys J Int 173:17–24
Ayoob S, Gupta AK (2006) Fluoride in drinking water: a review on the status and stress effects. Crit Rev Environ Sci Technol 36:433–487
Basharat M (2012) Spatial and temporal appraisal of groundwater depth and quality in LBDC command-issues and options. Pak J Eng Appl Sci 11:14–29
Brahman KD, Kazi TG, Afridi HI, Naseem S, Arain SS, Ullah N (2013) Evaluation of high levels of fluoride, arsenic species and other physicochemical parameters in underground water of two sub districts of Tharparkar, Pakistan: a multivariate study. Water Res 47:1005–1020
Chadha DK (1999) A proposed new diagram for geochemical classification of natural waters and interpretation of chemical data. Hydrogeol J 7(5):431–439
Chae GT, Yun ST, Kim K, Mayer B (2006) Hydrogeochemistry of sodium-bicarbonate type bedrock groundwater in the Pocheon spa area, South Korea. J Hydrol 321:326–343
Chae G-T, Yun S-T, Mayer B, Kim K-H, Kim S-Y, Kwon J-S, Kim K, Koh Y-K (2007) Fluorine geochemistry in bedrock groundwater of South Korea. Sci Total Environ 385:272–283
Currell M, Cartwright I, Raveggi M, Han D (2011) Controls on elevated fluoride and arsenic concentrations in groundwater from the Yuncheng Basin, China. Appl Geochem 26:540–552
Dey R, Swain S, Mishra S, Sharma P, Patnaik T, Singh V, Dehury B, Jha U, Patel R (2012) Hydrogeochemical processes controlling the high fluoride concentration in groundwater: a case study at the Boden block area, Orissa, India. Environ Monit Assess 184:3279–3291
Domenico PA (1990) Physical and chemical hydrogeology. Wiley, NewYork
Edmunds WM, Smedley P (2005) Fluoride in natural waters. Essentials of medical geology. Elsevier, Amsterdam, pp 301–329
Farooqi A, Masuda H, Firdous N (2007) Toxic fluoride and arsenic contaminated groundwater in the Lahore and Kasur districts, Punjab, Pakistan and possible contaminant sources. Environ Pollut 145:839–849
Farooqi A, Masuda H, Siddiqui R, Naseem M (2009) Sources of arsenic and fluoride in highly contaminated soils causing groundwater contamination in Punjab, Pakistan. Arch Environ Contam Toxicol 56:693–706
Genxu W, Guodong C (2001) Fluoride distribution in water and the governing factors of environment in arid north-west China. J Arid Environ 49(3):601–614
Gibbs RJ (1970) Mechanisms controlling world water chemistry. Science 170(3962):1088–1090
Greenberg E, Connors J, David J (1998) Standard methods for the examination of water and wastewater. American Public Health Association, Washington, DC
Greenman DW, Bennett GD, Swarzenski WV (1967) Ground-water hydrology of the Punjab, West Pakistan, with emphasis on problems caused by canal irrigation. US Government Printing Office
Guo Q, Wang Y, Ma T, Ma R (2007) Geochemical processes controlling the elevated fluoride concentrations in groundwaters of the Taiyuan Basin, Northern China. J Geochem Explor 93:1–12
Ibrahim M (2011) Effects of fluoride contents in ground water: a review. Int J Pharm Appl 2:128–134
Jacks G, Bhattacharya P, Chaudhary V, Singh K (2005) Controls on the genesis of some high-fluoride groundwaters in India. Appl Geochem 20:221–228
Jayawardana D, Pitawala H, Ishiga H (2012) Geochemical assessment of soils in districts of fluoride-rich and fluoride-poor groundwater, north-central Sri Lanka. J Geochem Explor 114:118–125
Kausar R, Ahmad S, Rehman K, Ahmad R (2003) Fluoride status of underground water of Faisalabad city area. Int J Agric Biol 5:536–539
Kumar PS (2014) Evolution of groundwater chemistry in and around Vaniyambadi industrial area: differentiating the natural and anthropogenic sources of contamination. Chemie der Erde-Geochem 74:641–651
Kumar PS, Jegathambal P, Nair S, James E (2015) Temperature and pH dependent geochemical modeling of fluoride mobilization in the groundwater of a crystalline aquifer in southern India. J Geochem Explor 156:1–9
Kundu N, Panigrahi M, Tripathy S, Munshi S, Powell MA, Hart B (2001) Geochemical appraisal of fluoride contamination of groundwater in the Nayagarh District of Orissa, India. Environ Geol 41:451–460
Li X, Hou X, Zhou Z, Liu L (2011) Geochemical provenance and spatial distribution of fluoride in groundwater of Taiyuan basin, China. Environ Earth Sci 62:1635–1642
Li C, Gao X, Wang Y (2015) Hydrogeochemistry of high-fluoride groundwater at Yuncheng Basin, northern China. Sci Total Environ 508:155–165
Loganathan P, Hedley MJ, Grace N, Lee J, Cronin S, Bolan NS, Zanders J (2003) Fertiliser contaminants in New Zealand grazed pasture with special reference to cadmium and fluorine—a review. Soil Res 41:501–532
Lunshan Z, Sheng W, Jihua Z, Jinghua W, Jianwu W (2007) School of Earth Sciences and Resources, China University of Geosciences, Beijing 100083, China2. Shanxi Institute of Geological Survey, Taiyuan 030001, China; Eco-geochemical investigation on the endemic As and F poisoning in Datong Basin [J]. Earth Science Frontiers 2
Mahmood K, Rana AD, Tariq S, Kanwal S, Ali R, Haidar A (2011) Groundwater levels susceptibility to degradation in Lahore metropolitan. Depression 150:8-01
Mamatha P, Rao SM (2010) Geochemistry of fluoride rich groundwater in Kolar and Tumkur districts of Karnataka. Environ Earth Sci 61:131–142
Mandel S (1981) Groundwater resources: investigation and development. Academic Press, New York
Nagendra Rao C (2003) Fluoride and environment—a review. Bunch MJ, Suresh VM and Kumaran TV (eds) Paper presented at the proceedings of the third international conference on environment and health, Chennai, India, 15–17 Dec 2003. York University, pp 386–399
Naseem S, Rafique T, Bashir E, Bhanger MI, Laghari A, Usmani TH (2010) Lithological influences on occurrence of high-fluoride groundwater in Nagar Parkar area, Thar Desert, Pakistan. Chemosphere 78:1313–1321
Pakistan Council of Research in Water Resources (2010) Water quality status in rural area of Pakistan, Publication no. 143-2010
Piper AM (1944) A graphic procedure in the geochemical interpretation of water-analyses. Trans Am Geophys Union 25(6):914
Rafique T, Naseem S, Bhanger MI, Usmani TH (2008) Fluoride ion contamination in the groundwater of Mithi sub-district, the Thar Desert, Pakistan. Environ Geol 56:317–326
Rafique T, Naseem S, Usmani TH, Bashir E, Khan FA, Bhanger MI (2009) Geochemical factors controlling the occurrence of high fluoride groundwater in the Nagar Parkar area, Sindh, Pakistan. J Hazard Mater 171:424–430
Rafique T, Naseem S, Ozsvath D, Hussain R, Bhanger MI, Usmani TH (2015) Geochemical controls of high fluoride groundwater in Umarkot sub-district, Thar Desert, Pakistan. Sci Total Environ 530:271–278
Rango T, Bianchini G, Beccaluva L, Ayenew T, Colombani N (2009) Hydrogeochemical study in the Main Ethiopian Rift: new insights to the source and enrichment mechanism of fluoride. Environ Geol 58:109–118
Saxena V, Ahmed S (2003) Inferring the chemical parameters for the dissolution of fluoride in groundwater. Environ Geol 43:731–736
Shah MT, Danishwar S (2003) Potential fluoride contamination in the drinking water of Naranji area, northwest frontier province, Pakistan. Environ Geochem Health 25:475–481
Shaji E, Bindu JV, Thambi D (2007) High fluoride in groundwater of Palghat district, Kerala. Curr Sci 92:240
Sheikhy NT, Ramli MF, Aris AZ, Sulaiman WNA, Juahir H, Fakharian K (2014) Identification of the hydrogeochemical processes in groundwater using classic integrated geochemical methods and geostatistical techniques, in Amol-Babol Plain, Iran. Sci World J
Siddique A, Mumtaz M, Saied S, Karim Z, Zaigham NA (2006) Fluoride concentration in drinking water of Karachi city (Pakistan). Environ Monit Assess 120:177–185
Srinivasamoorthy K, Chidambaram S, Prasanna M, Vasanthavihar M, Peter J, Anandhan P (2008) Identification of major sources controlling groundwater chemistry from a hard rock terrain-a case study from Mettur taluk, Salem district, Tamil Nadu, India. J Earth Syst Sci 117:49–58
Su C, Wang Y, Xie X, Li J (2013) Aqueous geochemistry of high-fluoride groundwater in Datong Basin, Northern China. J Geochem Explor 135:79–92
Suthar S, Garg VK, Jangir S, Kaur S, Goswami N, Singh S (2008) Fluoride contamination in drinking water in rural habitations of Northern Rajasthan, India. Environ Monit Assess 145:1–6
Valenzuela-Vasquez L, Ramirez-Hernandez J, Reyes-Lopez J, Sol-Uribe A, Lazaro-Mancilla O (2006) The origin of fluoride in groundwater supply to Hermosillo City, Sonora, México. Environ Geol 51(1):17–27
Wang Y, Shvartsev S, Su C (2009) Genesis of arsenic/fluoride enriched soda waters: a case study at Datong basin. Appl Geochem 24:641–649
Yitbarek A, Razack M, Ayenew T, Zemedagegnehu E, Azagegn T (2012) Hydrogeological and hydrochemical framework of Upper Awash River basin, Ethiopia: with special emphasis on inter-basins groundwater transfer between Blue Nile and Awash Rivers. J Afr Earth Sci 65:46–60
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Raza, M., Farooqi, A., Niazi, N.K. et al. Geochemical control on spatial variability of fluoride concentrations in groundwater from rural areas of Gujrat in Punjab, Pakistan. Environ Earth Sci 75, 1364 (2016). https://doi.org/10.1007/s12665-016-6155-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12665-016-6155-7