Abstract
Organic residues can be a major source of nutrients and are valuable fertilizers. But their benefits with regard to soil quality are undisputed. However, only few studies have focused on emissions of greenhouse gases from soil enriched with organic residues. A microcosom approach was employed to investigate the influence of the origin and composition of various organic residues on mineralization and N2O and CO2 emissions in an arable soil. In total, we set up six treatments: control, poultry manure, bio-waste compost, sheep and wheat straw compost, cow manure (CM) and for further comparison, the mineral fertilizer calcium ammonium nitrate. 500 g of sieved and homogenized soil was mixed with the amendments and packed into microcosms. After a pre-incubation period of 10 days, gas concentrations were measured periodically from the headspace of the microcosm by means of an airtight surgical syringe. The measurement period continued for 32 days. Soil amended with CM showed a significantly (α = 0.05) higher cumulative CO2 emission (914 mg kg−1) followed by bio-waste compost than poultry manure, sheep waste compost, control and calcium ammonium nitrate. Amending soil with cow manure and poultry manure led to the highest N2O-N emissions (110 µg kg−1). However, poultry manure and calcium ammonium nitrate significantly enhanced mineralization and net nitrification. Amendment of sheep and wheat straw compost and cow manure led to C sequestration and reduced N2O emission. Soil pH greatly decreased with poultry manure, sheep and wheat straw compost and bio-waste compost. Summing up, the application of organic residues to soil has some disadvantageous environmental effects calling for further research.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Climatic perturbations have increased due to greenhouse gas emissions from anthropogenic sources during the last decades. The agricultural sector is generally known to release about 50 % of the climate-relevant gas emissions (IPCC 2007). Soils are the major source of CO2, N2O and CH4 emissions (Rastogi et al. 2002). The aerobic decomposition of organic compounds is the main biological process responsible for the release of CO2 from soil to the atmosphere (Alexander 1977). The incorporation of organic residues has caught worldwide attention because it improves the physical and chemical properties of soils. The application of organic residues can enhance N2O and CO2 emissions (Flessa and Beese 1995). Specific practices, such as organic residue management (Pattey et al. 2005) can reduce greenhouse gas emissions from the agricultural sector.
N2O is produced naturally in soils through the microbial processes of nitrification and denitrification. In agricultural soils, these processes depend on environmental factors, heterogeneity of soil conditions and management practices (Philippot et al. 2009), organic C (Boeckx et al. 2011), soil pH (Mørkved et al. 2007), temperature (Saad and Conrad 1993), and water content (Bergsma et al. 2002).
Application of organic fertilizer is not an immediate source of N but the provision of organic C in the form of organic fertilizer enhances denitrifiers’ activity and the denitrification process. Mineral-N fertilizers also activate microbial activity by providing inorganic nitrogen (N) needed for growth (Khan et al. 2007).
Animal wastes are added to the soils as N source and, besides this, for storing organic C in soil (Flessa and Beese 1995). Cow manure contains about 4.5–18.1 kg of N per ton, most of it being in organic form. About half of this N is converted to available N to the plants during the first growing season. Organic matter, N and P contents in soils significantly increased over the control when the soil was amended with poultry manure and cow manure (Rahman 2010). Excess application of manure accumulates N in the soil, resulting in the accumulation of NO3 − in crops and ground water pollution (Harada 1992). Composting is a reasonable solution to re-cycle the animal manure for agricultural purposes (Bernal et al. 2009).
In Germany, the application of bio-waste is regulated by “application and introduction of materials onto or into the soil” (BioAbfV 1998). Organic residues can be a significant source of nutrients and a sound alternative to chemical fertilizers if properly managed. The amount of N converted to N2O depends on the bio-chemical composition of residues, in particular on their C/N ratios (Baggs et al. 2000) and physicochemical properties (Danga et al. 2010). Cumulative emissions of N2O and CO2 were found to be negatively correlated with C/N ratios of the organic residues (Huang et al. 2004).
Applications of manure, compost and bio-solids are the recommended nutrient management practices to enhance C sequestration. Recent findings (Mondini et al. 2010) suggest that the addition of organic wastes can reduce CO2 evolution; however, this increases N2O emissions because organic residues serve as an energy source for the microbes enhancing nitrification and denitrification processes. The organic material is enriched with carbohydrates (e.g. cellulose), lipids, fiber and lignin. These compounds will decompose slowly (Tuomela et al. 2000). The pre-dominant kind of proteins in the residues affects C mineralization (Mondini et al. 2010). It is a promising option to produce compost with different degradability and consequently, different N mineralization (Pal et al. 2010). The magnitude of greenhouse gas reduction affiliated with compost might be a few times higher than that of mineral fertilizers (Enzo and Schleiss 2009).
An incubation experiment was performed with soil amended with two different animal manures (poultry and cow manure) and two composts of different organic composition (sheep and wheat straw and kitchen garbage composts) and studied their biochemical properties in relation to nitrogen concentration and total organic carbon enhancing N2O and CO2 emissions from soil. The study was based on the hypothesis that composts and animal manures similar in composition amended to the soil alter C and N cycling differently.
Materials and methods
Soil sampling and manure collection
The soil was taken from plots at the “Heidfeldhof” research station of Hohenheim University, Stuttgart, Germany, which had been recently harvested for wheat. Samples were taken from 0 to 15 cm depth. Sampled soil was immediately transported to the laboratory, well homogeneized, sieved to 4 mm (soil properties are given in “Physico-chemical properties of soil” section).
Soil organic amendments used were fresh poultry manure (PM), fresh cow manure (CM), sheep and wheat straw compost (SWC) and bio-waste compost (BWC). Fresh poultry and cow manures were provided by the research stations of Farm Animal Ethology and Poultry Production and of Husbandry and Organic Farming affiliated with Hohenheim University. The research station of Husbandry and Organic Farming prepared sheep and wheat straw compost using 15 % chopped wheat straw and pure sheep excreta. Institute of Plant Nutrition, Hohenheim University, prepared bio-waste compost that included bio-degradable fraction (Andersen 2010) of kitchen and garden waste (European Commission 2010).
Both composts under investigation were prepared by Indian Bangalore method (FAO 1980). This method does not require a particular structure. Consequently, recommended dimensions for a heap are 5 feet wide by 3 feet high (Illinois 2013). The chemical properties are mentioned in Table 1. The major advantages of this method are: frequent turning that provides aeration, reduces composting period and higher temperatures are produced as a result of turning (32.2–60° F) which will kill major pathogens and weed seeds (Illinois 2013). This method has also some disadvantages (FAO 2003), which are direct exposure to weather conditions, nutrient losses due to high wind, frequent turnings, time and labor consumptions.
For comparison we applied commercial mineral-N fertilizer calcium ammonium nitrate (CAN; 27 % total N).
Experimental setup
Organic residues were oven dried at 60 °C, mashed in a shredder and passed through a 1 mm stainless steel sieve. The experimental setup included six treatments: control, soil + PM, soil + SWC, soil + BWC, soil + CM, and soil + CAN with ten replicate each. In total, 60 microcosms were prepared. 500 g soil (gravimetric water content: 28 %, on dry basis) was filled into each microcosm (0.82 l). Amendments were mixed homogenously into the soil. All amendments were applied as 50 mg N kg− 1 soil comparable to the field applications. Likewise we added C as 600, 666, 416.5 and 116.64 as mg/kg through PM, SWC, BWC and CM to each treatment.
Microcosms were pre-equilibrated for 10 days at room temperature (23 °C) in the dark. Temperature was recorded with two probes in the room. The water content of the cores was regularly controlled by weighting. Evaporation losses were compensated by adding deionized water to achieve the target gravimetric water content of 28 %. Except for the addition of water, no changes were made during the entire period of incubation, that is, there was no further mixing and no extra addition of fertilizer.
Prior to incubation, soil and manures were analyzed for their properties. Mineral-N (NH4 + and NO3 −) and pH were measured before amendment as well as on the 8th, 16th, 24th, and 32nd day (termination of experiment). Total organic carbon % (TOC) was measured at the beginning and at the termination of experiment.
N2O and CO2 flows measurement
The whole system was airproof with rubber seals and butyl rubber septa in plastic lids of the microcosms allowing for the sampling of headspace gas. The plastic lids were fitted with a two way Luer-Lock valve. Samples were taken at 0, 30 and 60 min by connecting the headspace with a vacutainer (25 ml) through a mounted septum using a 100 cm3 multi sample syringe (Smith et al. 1995). To ensure the prior absence of N2O, all vacutainers were evacuated and rinsed with N2 thrice, shortly before sampling. N2O and CO2 concentrations were analyzed by gas chromatography (AutoSystem XL Perkin Elmer) equipped with an N63 electron capture detector (ECD) and a flame ionization detector (FID), respectively, coupled to an auto sampler. The instrumental conditions were as follows: oven temperature 65 °C, ECD operation temperature 100–350 °C, carrier gas for ECD and FID CH4/Ar (10 %/90) and He (95 %), respectively. Calibration was done with three external standards of 700, 900 and 1500 ppb for N2O and 400, 1500 and 3000 ppm for CO2. Gas flow rates were calculated from the change of concentration in the headspace of the microcosm using linear regression (Livingston and Hutchinson 1995). Cumulative emission was calculated by linear interpolation thereafter.
Analytical methods used for organic fertilizers
The dry matter content of the organic fertilizers was determined by drying samples to constant weight at 60° C. Total N and total organic carbon (C) contents were analyzed using an automatic elemental micro-analyser (NA 1500 Carlo Erba). Remaining analyses were performed at the State Institute of Agricultural Chemistry (LA Chemie), Stuttgart. Crude protein, total sugar, acid detergent fiber (ADF), acid detergent lignin (ADL) and cellulose were determined according to EU and VDLUFA guidelines [VO (EG) Nr.152/2009 III C, VO (EG) Nr.152/2009 III M, VO (EG) Nr.152/2009 III J, see http://eur-lex.europa.eu; VDLUFA MB III 6.5.2, VDLUFA MB III 6.5.3, see http://www.vdlufa.de/Methodenbuch/]. The cellulose content was determined by subtracting ADF (cellulose+lignin) from ADL.
Soil analysis
TOC % was detected by a LECO 2000 CN analyzer. The particle size distribution was determined by the Pipette method (Gee and Bauder 1986). Soil pH was measured in 1:2.5 (soil/0.01 M CaCl2) using glass electrode pH meter. Soil mineral-N (NH4 + and NO3 −) was extracted in a 1 mol L−1 KCl solution (soil/liquid ratio 1:5 w/w) as referred by Keeney and Nelson (1982). The filtrates were then analyzed on an automated flow injection analysis (Brann en Luebbe TrAAcs 800 Auto analyzer).
Net nitrogen nitrification (NNN) was calculated as final concentration of NO3 − minus initial concentration of NO3 −. Likewise, net nitrogen mineralization (NNM) was assumed as final concentration of NO3 − plus NH4 +, minus initial concentration of NO3 − plus NH4 +.
Physico-chemical properties of soil
The soil was classified as Haplic Luvisol (FAO-UNESCO 1997). Soil pH was nearly neutral (pH 6.8). Bulk density was 1.23 g/cm3. Particle size distribution was 19 % sand, 52 % silt and 29 % clay (silty loam). TOC was 1.2 % by mass. NO3 − and NH4 + contents of the soil before incubation and addition of amendments were 10.8 and 5.6 mg kg−1, respectively.
Statistical analysis
Measured data were statistically evaluated by one way analyses of variances (ANOVA) using the software IBM SPSS Statistics Version 19. The least significant difference (LSD) test (α = 0.05) was used to identify significant differences between treatments. Correlation was calculated as Pearson’s correlation coefficient using Sigma plot 12.
Results
Chemical and bio-chemical properties of organic fertilizer added
Bio-waste and sheep and wheat straw composts had pH values of 7.3 (Table 1). Animal manures were somewhat more alkaline. All amendments had low C/N ratios (Table 1). CM contained high crude protein. Total sugar of PM was slightly higher than 1 %, but higher than that of the other organic fertilizers. In contrast, BWC had the highest cellulose content followed by CM, SWC and PM. ADF and ADL were pre-dominantly higher in BWC and SWC compared to the other organic fertilizers tested (Table 1). The only inorganic fertilizer used was CAN, which is a compound fertilizer (NH4NO3 + CaCO3) with neutral pH (pH 7.0). Ca and N contents of CAN were 8 and 27 % by mass, respectively.
Cumulative N2O flow
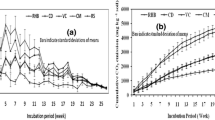
Figure 3 shows the release of N2O of the different treatments, measured over a period of 32 days. The amendment with animal manures resulted in significantly (α = 0.05) higher N2O emissions compared to the other amendments (Fig. 1). N2O flows decreased in the following sequence: PM < CM < CAN < BWC < SWC < control.
Cumulative CO2 flow
Soils amended with CM and BWC showed a significantly higher release of CO2 than the other soils (Fig. 2). CO2 emission decreased in the following order CM < BWC < CAN < PM < SWC < control. The cumulative flows of PM, CAN and SWC were similar to that of the control.
Course of N2O flow from soil
The highest flow of N2O was observed during the first 3 days after the addition of CM (Fig. 3). With PM, the N2O flow was low in the first week of incubation, but started to rise between the 10th and the 15th day. SWC treatment showed continuously low N2O flows from the beginning to the end of experiment (<2 µg kg−1 h−1). BWC and CAN also emitted low flows (<4 µg kg−1 h−1).
Dynamics of CO2 evolution from soil
Soil respiration (Fig. 4) showed the largest reaction to CM and BWC amendment, where it sharply increased at the early stages of the experiment, while it declined after a few days. CAN showed a similar increase, which, however, remained smaller. CO2 emissions of PM, SWC and also the control fluctuated at relatively low levels.
Total organic carbon
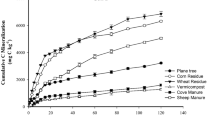
Only the amendment of SWC significantly increased the TOC over the time of incubation (Fig. 5). It was significantly higher than the other treatments.
The application of PM and CAN led to a significant increase of N concentration after 32 days (Fig. 6). In the PM treatment, concentration of NH4 + shot up to 110 mg kg−1. From the fifth day on, nitrification started and NH4 + decreased. Soil amended with CAN reached 50 mg kg−1 NH4 + and then, like PM, dropped down from the fifth day on. In the other treatments, the NH4 + concentration increased slightly and remained more or less constant at values of around 20 mg kg−1 for most of the incubation period until it declined towards the end. The NO3 − concentration was higher during the experiment. Throughout the experiment, the net mineralization and net nitrification of PM were 52.8 62.0 mg kg−1, while those of CAN 57.7 and 56.4 mg kg−1, respectively. These values were significantly higher than the respective values of SWC, BWC and the control. The net nitrification of CM and SWC was only 5.6 and 6.7 mg kg−1 and in case of BWC even less 2.53 mg kg−1 (Fig. 6).
Soil pH
The soil pH data are presented in Fig. 7. During the incubation soil pH of the SWC, BWC and PM amended treatments decreased by about one unit. After addition of CAN soil pH slightly increased, while after CM amendment it slightly decreased.
Correlation
No other factors were correlated with each other. Only NO3 − concentration was negatively correlated (r = −0.61) with NH4 + in PM amended soil.
Discussion
CO2 flows and C sequestration
The soils amended with organic fertilizers (composts, manures) behaved quite differently in the first 10 days of incubation (Fig. 4). BWC and CM showed CO2 emission peaks while PM and SWC did not. The peaks may be attributed to the low C/N ratios of BWC and CM rather than to other factors (Table 1). The stability of SWC (cf. Fig. 5), the compost with the higher C/N ratio, may be attributed to the fact that SWC is made up to 15 % of wheat straw, which contains a high fraction of lignin (Table 1). It is, therefore, quite resistant to mineralization due to strong chemical bonds (McCrady 1991). Addition of such high lignocellulosic material to a compost leads to slower decomposition, so that less CO2 is emitted. Comparing sheep and wheat straw compost to cow and wheat straw compost, Jianming et al. (2008) pointed out that sheep and wheat straw compost is more resistant to rapid degradation, so that its nutrients are released rather according to the changing demand of the growing crop (melon). Fabrizio et al. (2009) found a linear response of C sequestration to the application dose of sheep and wheat straw compost. Roberson et al. (2008) suggested that no tillage is an effective way to mitigate CO2 emission from soil amended with PM.
The respiration peak of BWC indicates that, even after 3 months of storage, BWC still contains a considerable fraction of easily degradable material. CM shows a similar CO2 emission pattern as BWC (Fig. 4), but showed the different trend for N2O (Fig. 3) the N2O flows were lower in BWC treatment, probably because the easily minerlizable N containing substances have already been mineralized during the composting process. This is also indicated by the C/N ratios of these amendments (Table 1). However, CAN produced a priming effect, it enhanced soil respiration by providing N to accelerate the decomposition of indigenous organic matter by soil microorganisms. The stimulation of soil respiration by the addition of mineral-N fertilizer has already been reported many times (e.g. Khan et al. 2007; Wang et al. 2010).
With SWC amended soil, the maximum emission of CO2 (Fig. 4) was observed in the second phase of the experiment. It was accompanied by a decline in NH4 +-N content (Fig. 6). The coincidence mirrors the interconnections of microbial utilization of C compounds as energy source and of mineral-N for their cellular synthesis (Deenik 2006). Over all, bio-waste compost amended soil respired 80 % of the C added within first 5 days. Approximetly 46 % of added C respired till third day, added as cow manure. Conversely, poultry manure and sheep and wheat straw compost amendment only emitted 19 and 2.5 % of C added (Fig. 4).
N2O flow and N concentration
N2O emission from soil amended with manures is well documented in the literature (Baggs et al. 2003; Huang et al. 2004; Millar and Baggs 2004; Millar et al. 2004). In case of the CM amendment, the main N2O emission occurred within the first 5 days and it was accompanied by a CO2 peak, reflecting rapid mineralization. It seems that the mineral-N released in the course of mineralization is readily denitrified and released as an N2O peak. Despite a similarly high mineralization, BWC showed no such peak. However, because of the low sampling resolution (7 days) a mineral-N peak may have remained undetected. The late appearance of the N2O peak after the PM amendment (compared to CM amendment) is a consequence of the higher C/N ratio and the higher crude protein content (Table 1). Because of the slower mineralization, nitrification of PM amended soil started later than that of CM amended soil (Fig. 6). Dutta and Stehouwer (2010) reported immediate heterotrophic activity after the application of organic amendment, but low levels of NO3.
To ensure homogeneity, all manures were finely ground and sieved before mixing with soil. This may have resulted NO3 − content and N2O emission. Cabrera et al. (1994) noticed higher N2O emissions when PM was applied as a powder rather than as pellets.
CAN amendment generated a moderate N2O peak shortly after the beginning of incubation, which was considerably lower than the N2O peak of the soil amended with animal manures. Upendra et al. (2010) reported that N2O emission after PM amendment was higher than after the application of mineral fertilizer. They explained their finding with the enhancement of C and N cycling by the addition of extra C. In contrast, Cayuela et al. (2010) and Velthof et al. (2005) observed similar N2O emissions after the application of CM and CAN, respectively.
NH4 + and NO3 − contents of soil after CAN application were similar in the first half of the experiment, reflecting the equal proportion of NH4 + and NO3 − in CAN. The solubility of a mineral fertilizer depends on its type (Millar et al. 2010). CAN is one of the readily soluble N fertilizers. NH4 + stimulates the activity of nitrifiers in soils (Khan et al. 2007). Nitrifiers are obligate chemolithoautotrophs as they use NH4 + as an energy source instead of organic material. The divergence of the NH4 + and NO3 − contents in the second half of the experiment indicates the onset and ongoing of nitrification.
The amendment of composts, be it SWC or BWC, resulted in considerably lower N2O flows due to the slow release of N from composts. This finding is in an agreement with the data of Dennis and Burke (2001). It is mainly due to high contents of cellulose and fiber (Pal et al. 2010), as well as of lignin (Tuomela et al. 2000) (Table 1). Organic N mineralization of SWC stopped at the 15th day of incubation. N concentration in SWC amended soil is low and with low N2O emission. Mixing of straw is an effective strategy to avoid excess NO3 − and to reduce greenhouse gas emission (Shaojun et al. 2009) and nitrate leaching (Eghball et al. 1997).
Because of the small proportion of readily mineralizable N in composted manures, organic N is less likely to be released quickly. The developing microorganisms require more N than the substrate provides. In compost amended soil, the mineral-N pool was constant until the end of the incubation, reflecting the gradual mineralization and utilization of mineral-N by microbes (Deenik 2006).
The TOC contents of the CAN amended soil (0.81 %) were similar to that of the manure amended soil in the beginning. CAN application has an impact on the activity of nitrifying bacteria and subsequent N transformations (Watson et al. 1995). The course of mineralization of PM amended soil resembled that of the CAN amended soil, but that of CM did not.
Nitrogen content of manures
Nitrogen content of poultry manure was found to be lower than that of cow manure (Table 1). It is a general finding (Kaur et al. 2005) that the total N content of poultry manure is higher than that of cow manure. The poultry manure used in this study was collected from layer poultry cages. Layer poultry manure contains lower N content than that from broilers and turkeys (Menzi et al. 1998). The cow manure was collected directly in cow houses. Within the dairy cow group, calves, heifers, lactating cows, dry cows and veal calves will show variations with regard to their N content of manure. The nutrient content of manures varies with type of animal, bedding material, storage, and processing (Rosen and Bierman 2005).
There are still some gaps in the scientific understanding of the factors controlling nutrient composition (especially N), handling of manure and its long term efficiency in northern and central Europe (Menzi et al. 1998). The C/N ratio of the cow manure was much lower (Table 1) than previously calculated by Rynk (1992) and Hills (1979), i.e. 19 and 8. It was lower than the C/N ratio of the decomposers. Soil microorganisms have a C/N ratio of approximately 8. They must acquire enough C and N from the environment in which they live to maintain the C/N of their bodies (USDA 2011). A high C/N ratio means that N will be exhausted before the C is digested by the bacteria as an energy source. Conversely, a low C/N ratio results in high NH4 + concentrations which may become toxic to the anaerobic bacteria (Homan et al. 2013).
Soil pH as an influencing factor of denitrification
The application of compost leads to a decrease of soil pH. Ano and Ubochi (2007) found a significant decrease of soil pH (by one unit) after compost application because of the release of fulvic and humic acids (Tuomela et al. 2000). The decrease of pH with PM was similar (Fig. 7). In CM, it declined negligibly due to uric acid (Nahm 2003). This effect of cow manure is known to be due to its buffering agents, such as carbonates and organic matter. Risse et al. (2006) found that soil pH of plots receiving mineral fertilizer increased by half a unit (5.2–5.6). This supports the results of the current study, which showed a similar increase of pH in the CAN treatment. N2O emission from CAN treated soils decreased regardless of the increase in pH insinuates that the decrease in soil pH in the treatments with organic amendments was not the limiting factor of N2O emission. Denitrification is not only positively correlated with soil pH, but several other factors exert an influence, such as abundance and kind of denitrifiers (Lakha et al. 2009). The ultimate low N2O emission rate can be credited to the decline or declined activity of the denitrifier population with the passage of time (Nemeth 2012). Although the trends of pH are different, PM and CAN amendment leads to a comparable reaction regarding the process of nitrification and the course of NO3 − concentrations. Tarre et al. (2004) also observed a high nitrification rate despite a low pH.
Efficiency of manures and composts in sequestering C
The slow degradability of sheep and wheat straw compost is the result of high lignin content (Table 1) which becomes even higher in the composting process (Leifeld et al. 2002). Co-composting with wheat straw, which is a relatively slow releasing material has been suggested as a mitigation practice for N2O (Kariyapperuma et al. 2012). Organic residues, either as manures (green or animal) or composts are added to the soil as a source of organic matter (Bot and Benites 2005). Organic matter with low C/N ratio provides larger amount of readily mineralizable N substrate for microbial N2O production (Kawabata et al. 2010). In the end of the experiment, only sheep manure and wheat straw compost had sequestered C significantly. It can be concluded that the bio-degradable portion of organic C of the mentioned compost was not high enough. An other explanation is in the maturity of compost (Biala 2011). The sheep and wheat straw compost was more mature (1 year) than the bio-waste compost (3 months). On the other hand, the application of sheep manure alone has also been reported to increase organic matter and cation exchange capacity of soil (Dong and Shu 2004). The sheep manure exchanges NH4 + with other cations and retains nutrients in soil. For soil C sequestration strategies to be effective in the long-term, it is likely to increase the slow and passive pools of soil organic matter (Franzluebbers and Stuedemann 2002). Further research is recommended to compare sheep manure application with composted sheep manure regarding CEC efficiency and reduction of CO2 and N2O emissions.
The results obtained were in line with the hypothesis suggesting that manures contain different easily soluble C and N fractions. The small proportion of readily mineralizable N in some manures suggests that the organic N in these manures may be more strongly bound and is less likely to be released quickly. In our investigation, all organic residues had C/N ratios less than 20. The higher net NH4 + mineralization of the poultry manure treatments might be explained by a higher specific mineralization rate, probably related to a higher decomposability and low C/N ratio of poultry manure. Organic residues are applied to the soil as a source of N and require a reasonable understanding to predict the mineralization of organic N compounds.
Conclusion
The use of composts has decelerated not only N mineralization and N2O flow, but it also decreased soil pH, which could slow down nitrification. The practice of applying composts with such low C/N ratios could be advantageous for soils with moderate to high pH, but it is not recommended for soils with pH below 7. Application of PM has emitted significantly more N2O, but still less than 1 % of applied N. PM showed fast mineralization, but it considerably reduces soil pH which again is not suitable for acidic or neutral soils. CM and BWC emitted significantly more CO2 indicating higher mineralization, which, in principle, could enhance denitrification.
Not only composts but also poultry manure reduced soil pH; this will slow down the nitrification process and can reduce rapid NO3 − losses through leaching. Cow manure stabilized soil pH. Whalen et al. (2000) reported that application of cow manure increased pH of acid soils. Soils with low organic matter and N content and high pH can be improved by cow manure application lowering soil pH and increasing N content to fulfill crop requirement. Cow manure application to soil as a fertiliser and organic matter source is the most adopted agricultural technology by developing countries.
Animal manure applications are anticipated to supplement soil organic matter and augment total N content, which was not evident in our study (Fig. 5). Total C increased after amendment with SWC, but not BWC. There is little specific research on N2O and CO2 flows after compost application in farming systems. Further studies with a wide variety of C/N ratios are required to evaluate composts and their application rates under laboratory and field conditions.
Recommendations
-
It is recommended to avoid direct application of animal manure to the soil.
-
Co-composting of cow and poultry manures with wheat straw or rice-husk (with high lignin) are advantageous to reduce N losses from soil.
-
Co-composting of bio-waste with wheat straw reduces N2O and CO2 losses.
-
Residual effects of manures and composts (Ginting et al. 2003) should be evaluated under laboratory and field conditions.
-
Applying composts with low C/N ratios to the field is more effective than applying manures with low C/N ratios.
References
Alexander M (1977) Introduction to soil microbiology. Wiley, New York
Andersen JK (2010) Composting of organic waste: quantification and assessment of greenhouse gas emissions. Dissertation, Environmental Engineering Department, Technical University of Denmark (DTU)
Ano AO, Ubochi CI (2007) Neutralization of soil acidity by animal manures mechanism of reaction. Afr J Biotechnnol 6:364–368
Baggs EM, Rees RM, Smith KA, Vinten AJA (2000) Nitrous oxide emission from soils after incorporating crop residues. Soil Use Manag 16:82–87
Baggs EM, Stevenson M, Pihlatie M, Regar A, Cook H, Cadisch G (2003) Nitrous oxide emissions following application of residues and fertilizer under zero and conventional tillage. Plant Soil 254:361–370
Bergsma TT, Robertson GP, Ostrom NE (2002) Influence of soil moisture and land use history on denitrification end-products. Environ Qual 31:711–717
Bernal MP, Alburquerque JA, Moral R (2009) Composting of animal manures and chemical criteria for compost maturity assessment. A review. Bioresour Technol 100:5444–5453
Biala J (2011) The benefits of using compost for mitigating climate change. A short report. Department of Environment, Climate Change and Water NSW
BioAbfV (1998) Ordinance on the utilisation of bio-wastes on land used for agricultural and horticultural purposes 21 Sep 1998. (Federal Law Gazette BGBl. I p. 2955), amended by Article 5 of the Ordinance on the Implementation of the European Waste Catalogue of 10 December 2001 (Federal Law Gazette BGBl. I, p 3379)
Boeckx P, Nieuland KV, Cleemput OV (2011) Short-term effect of tillage intensity on N2O and CO2 emissions. Agron Sustain Dev 31:453–461
Bot A, Benites J (2005) The importance of soil organic matter: key to drought-resistant soil and sustained food production. FAO SOILS BULLETIN, 80. Food and Agriculture Organization of the United Nations, Rome
Cabrera ML, Chiang SC, Merka WC, Pancorbo OC, Thompson SA (1994) Nitrous oxide and carbon dioxide emissions from pelletized and non pelletized poultry litter incorporated into soil. Plant Soil 163:189–196
Cayuela ML, Kuikman PJ, Oenema OR, Bakker R, Groenige JW (2010) Bioenergy residues as soil amendments: climate-relevant C dynamics during decomposition. 19th world congress of soil science, soil solutions for a changing world, Brisbane, Australia. Published on DVD
Danga BO, Mochoge B, Mugwe J, Getenga Z (2010) Soil properties effects and management of organic residues to improve C sequestration, reduce N losses and improve crop yields. Second RUFORUM Biennial Meeting 20–24 September, Entebbe, Uganda
Deenik J (2006) Nitrogen mineralization potential in important agricultural soils of Hawai’i. Honolulu (HI): University of Hawaii. p 5 (Soil and Crop Management; SCM-15)
Dennis A, Burke PE (2001) Dairy waste anaerobic digestion handbook. Environmental energy company. p 57
Dong S, Shu H (2004) Sheep manure improves the nutrient retention capacity of apple orchard soils. ISHS Acta Horticulturae 638: XXVI international horticultural congress: sustainability of horticultural systems in the 21st century
Dutta T, Stehouwer R (2010) N2O and CO2 emission from mine soil reclaimed with organic amendments (Incubation study). In: Proceedings of 19th world congress of soil science (WCSS) Aug 1–5, Brisbane, Australia
Eghball B, Power JF, Gilley JE, Doran JW (1997) Nutrient, carbon, and mass loss of beef cattle feedlot manure during composting. J Environ Qual 26:189–193
Enzo F, Schleiss K (2009) The potential contribution of bio-waste to tackle climate change: issues related to life cycle benefits and relevance to policy-making. Summary of oral presentation for the DAKOFA/ISWA waste and climate conference 3–4 December
European Commission (2010) Communication from the commission to the council and the European Parliament: On future steps in bio-waste management in the European Union. Brussels, May 18. COM. 235 (final)
Fabrizio A, Tambone F, Genevini P (2009) Effect of compost application rate on carbon degradation and retention in soils. Waste Manag 29:174–179
FAO (1980) A manual of rural composting. FAO/UNDP Regional Project RAS/75/004 Field Document 15
FAO (2003) On-farm composting methods. Land and water discussion paper. In: Misra RV, Roy RN, Hiraka H (eds). Food and Agriculture Organization of the United Nations, Rome. Retrieved from http://www.fao.org/organicag/doc/on_farm_comp_methods.pdf
FAO-UNESCO (1997) Soil map of the world: revised legend. IRSIC, Wageningen
Flessa H, Beese F (1995) Effect of sugar beet residues on soil redox potential and nitrous oxide emission. Soil Sci Soc Am J 59:1044–1051
Franzluebbers AJ, Stuedemann JA (2002) Particulate and non-particulate fractions of soil organic carbon under pastures in the Southern Piedmont USA. Environ Poll 116:53–62
Gee GW, Bauder JW (1986) Particle-size Analysis. P. 383-411. In Page AL (ed). Methods of soil analysis, Part1, Physical and mineralogical methods. Second Edition, Agronomy Monograph 9, American Society of Agronomy, Madison, WI
Ginting D, Kessavalou A, Eghball B, Doran JW (2003) Greenhouse gas emissions and soil indicators 4 years after manure and compost applications. J Environ Qual 32:23–32
Harada Y (1992) Composting and land application of animal waste. Asian Australas J Anim Sci 5:113–121
Hills DJ (1979) Effects of carbon: nitrogen ratio on anaerobic digestion of dairy manure. Agric Wastes 1:267–278
Homan E, Shaw MD, Bartlett H, Persson S (2013) Biogas from manure. Penn State Extension, Pennsylvania Department of Agriculture, The Governor’s Energy Council, and the Department of Energy. Special Circular 260
Huang Y, Zou J, Zheng X, Wang Y, Xu X (2004) Nitrous oxide emissions as influenced by amendment of plant residues with different C/N ratios. Soil Biol Biochem 36:973–981
IllINOIS ACES. University of Illinois Extention. http://web.extension.illinois.edu. Retrieved Jan 01, 2013
IPCC [Metz B, Davidson, OR, Bosch PR, Dave R, Meyer LA (eds)] (2007) Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA
Jianming L, Pute W, Behboudian MH, Zhirong WZZ, Morton A (2008) Responses of muskmelon to cattle or sheep manure compost mixed with sandy soil. J Org Sys 3(2):1177–4258
Kariyapperuma KA, Furon A, Wager-Riddle C (2012) Non-growing season nirous oxide fluxes from an agricultural soil as affected by application of liquid and composted swine manure. Can J Soil Sci 92:315–327
Kaur K, Kapoor KK, Gupta AP (2005) Impact of organic manures with and without mineral fertilizers on soil chemical and biological properties under tropical conditions. J Plant Nutr Soil SC 168:117–122
Kawabata C, Ohta S, Ishizuka S, Wicaksono A, Heriyanto J (2010) Effect of DOM (dissolved organic matter) derived from litter of Acacia mangium and Eucalyptus pellita on soil N2O emissions. 19th world congress of soil science, soil solutions for a changing world, 1–6 August. Brisbane, Australia. Published on DVD
Keeney DR, Nelson DW (1982) Nitrogen-inorganic forms. In: Page AL (eds) Methods of soil analysis. Part II. Chemical and microbiological properties. 2. ASA and SSSA; Madison, WI: pp 595–624. Agron Monogr 9
Khan SA, Mulvaney RL, Ellsworth TR, Boast CW (2007) The myth of nitrogen fertilization for soil carbon sequestration. J Environ Qual 36:1821–1832
Lakha SS, Shannon KE, Henderson SL, Zebarth BJ, Burton DL, Goyer C, Trevors JT (2009) Effect of Nitrate and Acetylene on nirS, cnorB, and nosZ expression and denitrification activity in Pseudomonas mandelii. J Appl Environ Microbiol 75:5082–5087
Leifeld J, Siebert S, Kogel-Knabner I (2002) Changes in the chemical composition of soil organic matter after application of compost. Eur J Soil Sci 53:299–309
Livingston GP, Hutchinson GL (1995) Enclosure-based measurement of trace gas exchange: applications and sources of error. In: Matson PA, Harrison RC (eds) Biogenic trace gases: measuring emissions from soil and water. Blackwell Science Ltd., Oxford
McCrady E (1991) The nature of lignin. Alkaline Pap Advocate 4:4
Menzi H, Pain B, Smith K (1998) Solid manure in Europe: Results of the survey by the working group on solid manure of RAMIRAN. In: Proceedings of 8th international conference on the FAO ESCORENA network on recycling of agricultural, municipal and industrial residues in agriculture (RAMIRAN 98). [Martinez J, Maudet MN (eds)] vol. 2, Formerly animal waste management, Rennes, 26–29 May 1998
Millar N, Baggs EM (2004) The chemical composition, or quality, of agroforestry residues influences N2O emissions after their addition to soil. Soil Biol Biochem 36:935–943
Millar N, Ndufa JK, Cadisch G, Baggs EM (2004) Nitrous oxide emissions following incorporation of improved-fallow residues in the humid tropics. Global Biogeochem Cycle 18:GB1032. doi:10.1029/2003GB002114
Millar J, Robertson GP, Grace PR, Gehl RJ, Hoben JP (2010) Nitrogen fertilizer management for nitrous oxide (N2O) mitigation in intensive corn (Maize) production: an emissions reduction protocol for US Midwest agriculture. Mitig Adapt Strateg Glob Change 15:185–204
Mondini C, Tania A, Sinicco A, Cayuela ML (2010) Organic residues application is an effective strategy for sustainable management of soil organic matter. 19th world congress of soil science, soil solutions for a changing world 1–6 August. Brisbane, Australia. Published on DVD
Mørkved PIT, Dörsch P, Bakken LR (2007) The N2O product ratio of nitrification and its dependence on long-term changes in soil pH. Soil Biol Biochem 39:2048–2057
Nahm KH (2003) Evaluation of the nitrogen content in poultry manure. World Poultry Sci J 59:77–88
Nemeth DD (2012) Nitrous oxide emission and abundance of N-cycling microorganisms in corn-based biofuel cropping systems. Masteral Thesis, Guleph, Ontario, Canada
Pal P, Clough TJ, Kelliher FM, Sherlock RR (2010) N2O and CO2 emissions following clover and cellulose incorporation into a New Zealand pastoral soil. 19th world congress of soil science, soil solutions for a changing world 1–6 August. Brisbane, Australia. Published on DVD
Pattey E, Trzcinski MK, Desjardins RL (2005) Quantifying the reduction of greenhouse gas emissions as a result of composting dairy and beef cattle manure. Nutr Cycl Agroecosys 72:173–187
Philippot L, Hallin S, Börjesson G, Baggs EM (2009) Bio-chemical cycling in the rhizosphere having an impact on global change. J Plant Soil 321:61–81
Rahman MM (2010) Assessment of household waste, poultry manure and cow dung in rice cultivation. Agriculturists 8:117–125
Rastogi M, Singh S, Pathak H (2002) Emission of carbon dioxide from soil. Curr Sci 82:510–517
Risse JM, DF, Well C, Humenik FJ (eds) (2006) 283–316 in animal agriculture and environment: National center for manure and animal waste management white papers. St. Joseph, Michigan: ASABE, 913c0306
Roberson T, Reddy KC, Reddy SS, Nyakatawa EZ, Raper RL, Reeves DW, Lemunyon J (2008) Carbon dioxide efflux from soil with poultry litter applications in conventional and conservation tillage systems in northern Alabama. J Environ Qual 37:535–541
Rosen CJ, Bierman PM (2005) Using manure and compost as nutrient sources for vegetable crops. University of Minnesota Extension Services
Rynk R (1992) On-farm composting handbook (NRAES-54). Cooperative Extension, Ithaca
Saad OAL, Conrad L (1993) Temperature dependence of nitrification, denitrification, and turnover of nitric oxide in different soils. Biol Fertil Soil 15:21–27
Shaojun Q, Xiaotang J, Ingwersen J, Guo Z, Stange CF, Bisharat R, Streck T, Christie P, Zhang F (2009) Role of added Carbon in the transformation of surplus soil nitrate-nitrogen to organic forms in an intensively managed calcareous soil. The proceedings of International Plant Nutrition Colloquium XVI UC Davis
Smith KA, Clayton H, McTaggart IP, Thomson PE, Arah JRM, Scott A (1995) The measurement of nitrous oxide emission from soil by using chambers. Phil Trans R Soc Lond Ser A 351:327–338
Tarre S, Beliavski M, Denekamp N, Gieseke A, de Beer D, Green M (2004) High nitrification rate at low pH in a fluidized bed reactor with chalk as the biofilm carrier. Water Sci Technol 49:99–105
Tuomela M, Vikman M, Hatakka A, Itävaara M (2000) Biodegradation of lignin in a compost environment: a review. Bioresour Technol 72:169–183
Upendra M, Sainju Z, Senwo N, Nyakatawa ZE, Tazisong A, Reddy KC (2010) Poultry litter application increases nitrogen cycling compared with inorganic nitrogen fertilization. Agron J 102:917–925
USDA (2011) Carbon to nitrogen ratios in cropping systems. East National Technology Support Center, Greensboro, NC, in cooperation with North Dakota NRCS
Velthof GL, Nelemansb JA, Oenemaa O, Kuikman PJ (2005) Gaseous nitrogen and carbon losses from pig manure derived from different diets. J Environ Qual 34:698–706
Wang X, Li X, Hu Y, Lv J, Sun J, Li Z, Wu Z (2010) Effect of temperature and moisture on soil organic carbon mineralization of pre-dominantly permafrost peatland in the Great Hing’an Mountains, northeastern China. J Environ Sci (China) 22:1057–1066
Watson CJ, Kilpatrick DJ, Cooper JE (1995) The effect of increasing application rate of granular calcium ammonium nitrate on net nitrification in a laboratory study of grassland soils. Nutr Cycle Agroecosys 40:155–161
Whalen JK, Chang C, Clayton GW, Carefoot JP (2000) Cattle Manure Amendments Can Increase the pH of Acid Soils. Soil Sci Soc Am J 64:962–966
Acknowledgments
The study was supported by Agriculture Research Sindh, Pakistan and Hohenheim University, Stuttgart, Germany. The authors are highly thankful to Dr. Joachim Ingwersen Senior researcher and lecturer, University of Hohenheim, for his valuable suggestions to improve the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shah, A., Lamers, M. & Streck, T. N2O and CO2 emissions from South German arable soil after amendment of manures and composts. Environ Earth Sci 75, 427 (2016). https://doi.org/10.1007/s12665-015-5126-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12665-015-5126-8