Abstract
The Doce River Basin (DRB) is located in the southeastern region of Brazil and is home to the largest steel complex in Latin America, including the largest open pit mining industry in the world. Several economic activities are performed along the DRB, including mining, agriculture, and food industry activities. The mining activities involve the exploration of a range of minerals and gold on a smaller scale. The quality of water in the basin has been monitored since 1997 by the state agency for environmental control; however, there are no reports about metals in sediments of the basin. There is insufficient information for an assessment of metal distribution in the DRB, especially for the most toxic metals, such as mercury. Therefore, the aim of this work is to quantify Al, Cr, Co, Cu, Fe, Hg, Mn, Pb, and Zn levels and the physico-chemical parameters in sediment from the basin and to statistically analyze these variables. The results are also discussed using the geoaccumulation index (I geo) and enrichment factor (EF), which contribute to the knowledge of the metal geochemistry in this basin. The results for Cr, Cu, Hg, Pb, and Zn indicate that 41 % of the sediment samples analyzed were above the sediment quality guideline value (SQG), and the use of geochemical parameters indicated that eight points of the basin were considered the most contaminated. Therefore, this study revealed that an implementation of a sediment monitoring program is of great value.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The trace metals in sediments are derived from the weathering of rocks and soils, atmospheric deposition, and runoff of anthropogenic origin. They can accumulate in the sediment and remobilize through a complex cycle of systems involving chemical, biological and hydrodynamic factors (Ramiro Pastorinho et al. 2012). These metals, adsorbed or precipitated, can be immobilized in aquatic environments, causing different impacts depending on the chronicity of the adverse effects generated by prolonged exposure, even at low concentrations (Poleto 2008). When an accumulation occurs in any compartment of the environment, these trace metals may cause adverse effects on biota, mainly depending on the chemical species present, pH and redox potential of the medium, and the presence of ligands (Poleto 2008; Mulholland et al. 2012).
High concentrations of metals in sediments are not necessarily associated with contamination processes and may simply reflect the natural mineralogical composition of the region or background (Silva et al. 2012). Because of adverse hydrodynamic and geomorphological conditions, the establishment of appropriate natural background values for metals in sediments is difficult (Qi et al. 2010). The differentiation between the natural background and anthropogenic contamination is critical for evaluating the potential impact that can be generated in specific ecosystems (Ramiro Pastorinho et al. 2012; Mil-Homens et al. 2013a).
Because it is an environmental compartment where trace metal enrichment can cause risks to biota, the establishment of comparative standards for sediment, in terms of relative concentrations, has been demonstrated to be very useful. Thus, geochemical patterns that relate the concentrations of contaminants and natural background values have been established (Turekian and Wedepohl 1961). These standards are based on the normalization methods of the concentrations of metals, involving mathematical correlation between the concentration of the metal and a reference concentration for various compounds, including Al, Fe, and Li, among others (Ramiro Pastorinho et al. 2012; Silva et al. 2012).
Several studies evaluating anthropogenic metal contamination in sediments have recently been conducted using geochemical normalization methods for estimating the degree of contamination in sediments (Nasrabadi et al. 2010; Duplay et al. 2012; Franz et al. 2013; Mil-Homens et al. 2013b). These methods include the geoaccumulation index and enrichment factor (Zhang and Liu 2002; Buccolieri et al. 2006; Abrahim and Parker 2008; Hortellani et al. 2008; Devesa-Rey et al. 2011; Silva et al. 2011). For both indices, the establishment of background values is necessary to be able to discern the origin of the metal as natural or anthropogenic.
Spanish researchers (Rodriguez-Barroso et al. 2010) evaluated the heavy metal contamination in surface sediments from Bahia de Cadiz, Spain, by determining the geoaccumulation index (I geo) in combination with multivariate analyses and classified the sediments as non-polluted in relation to metals studied. The Bay of Liaodong, China, was also evaluated for the degree of anthropogenic pollution of metals by determining the I geo and Enrichment Factor (EF), which verified the high concentrations of Pb and Hg in the region (Hu et al. 2013). Nasrabadi et al. (2010) evaluated the efficiency of the use of methods that determine values of indices of pollution, such as I geo and EF in sediments of the Haraz River, belonging to the Caspian basin. The authors noted the importance of using indexes with caution, as comparisons to background values not related to the study area may lead to biased conclusions about the degree of contamination of the region.
In addition to geochemical standards of comparison, the sediment quality guideline (SQG) values are also important tools for predicting contamination. Although they do not provide definitive toxicity values, when associated with other requirements, they can be used to identify areas that may have potential adverse effects on biota (Hübner et al. 2009).
The threshold effect limit (TEL) and probable effect limit (PEL) are values established by empirical observations transformed into time series and form a database of contamination levels in sediments and biological responses (CCME 1999). The background values for sediments, toxicity tests associated with spiked-test of metals in sediments, and quality criteria for other matrices, such as water, tissue of living organisms, soils and benthic communities, were also considered for the calculations involved in the establishment of the TEL and PEL values. Given the uncertainties regarding the bioavailability of contaminants in sediment and possible synergistic effects of mixtures of pollutants, the SQG should be used with caution (Hübner et al. 2009).
The Doce River Basin (DRB) is located in southeastern Brazil, and various economic activities occur throughout its length, such as steel complex, mining, farming, pig farming, and industrial food activities. Soil contamination due to the developed activities associated with the disposal of industrial and sanitary sewage waters has produced major negative impacts to the DRB, and one of the most important is the introduction of heavy metals in the aquatic environment. The quality of water in the basin has been monitored since 1997 by the state agency for environmental control; however, studies on the environmental impacts all these activities on soils, sediments and basin atmosphere are still incipient.
In the case of sediments, there are no published studies evaluating this matrix in Doce River Basin. Thus, the goals of this study were to determine the total content of the metals Al, Cr, Co, Cu, Fe, Hg, Mn, Pb, and Zn in sediments from different parts of the basin, to use the SQG to compare them, to evaluate them for contamination using I geo and EF, and to contribute to environmental assessment and geochemical knowledge of this important basin.
Experimental
Study area
The drainage area of the Doce River Basin (DRB) has a significant area of approximately 83,400 km2 in southeastern Brazil. It covers areas of 228 municipalities and has a total population of approximately 3.1 million inhabitants (MMA 2005). The Doce River, with a length of 853 km, has the Piranga and Carmo rivers as its major contributing rivers. Several economic activities are extensively developed in the basin, with the major activities being related to mining, farming, pig farming, and the food industry. Mining activity includes the exploitation of iron, aluminum, beryl, chrysoberyl, mica, and feldspar. In addition, there are small-scale gold mining activities, called “garimpos”. These activities use Hg to improve the gold extraction efficiency. The Au–Hg amalgam can be separated easily from the other sediment mineral components, following which it is heated to release Hg and obtain the gold. In general, these activities are illegal and Hg is released to the water systems and directly to the atmosphere. Metallurgical industry and forestry activities are also present in the basin (IGAM 2010a, b). These activities affect the quality of water in the basin in different ways. The main forms of contamination are domestic and industrial wastes, runoff from agriculture, and also atmospheric emissions from the mining and metallurgical industries.
Sample collection and pretreatment
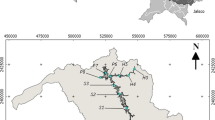
Sediment samples were collected from 19 sites selected in the basin in October 2011 by the State Institute of Water Management (IGAM), as shown in Fig. 1. The points were chosen because of their classification as having poor water quality according to water monitoring reports (IGAM 2010a). The collection was performed in areas of low fluvial energy where the deposition of finer material is favored and therefore that of most interest for the environmental assessment of the behavior of chemical elements (Pereira et al. 2007). Approximately 1 kg samples of surface sediments were collected from each point, according to the recommendations of the United States Geological Survey (USGS), using a sampler with the shell manufactured form stainless steel (Shelton and Capel 1994). The samples were placed in plastic vials and kept at 4 °C until arrival at the laboratory, where they were dried at room temperature, disaggregated and sieved, and the fraction <0.062 mm (silt–clay) was used for the determinations.
Reagents
All reagents used in preparing solutions were of the highest analytical grade, and purified Milli-Q (18.2 MΩcm resistivity, Millipore Direct-Q3, Molsheim, France) water system was also used. The glassware and plastics used were decontaminated in an HNO3 acid bath containing 10 % v/v for at least 24 h and subsequently washed with distilled and deionized water. The calibration curves for the quantification of the elements were prepared following appropriate dilution of stock solutions of 1,000 mg L−1 of each analyte. Two certified reference materials, NIST SRM 2711 (Montana Soil) and GBW 08301 (River Sediment), were analyzed to assess the accuracy of the methods.
Instrumentation
A digital pH meter Mars Model MB-10 and combined glass electrode EPC-70 were used for pH measurements and calibrated with buffer solutions of pH 4.0 and 7.0. The organic carbon was determined by a Perkin Elmer Elemental Analyzer CHNS/O 2400 Series II.
A microwave oven closed with a cavity (Milestone, ETHOS model 1) was used for sediment sample digestion. The measurements were performed employing a fast sequential flame atomic absorption spectrometer (FS-FAAS) Varian AA240FS, with the conditions specified for each element. Cobalt and Pb were quantified by inductively coupled plasma optical emission spectrometry (ICP-OES), using a Perkin Elmer Optima 8000 instrument and the following instrumental parameters: power of 1,300 W, gas flow into the plasma of 15 L min−1, flow auxiliary gas 0.2, 0.8 L min−1 of the nebulizer gas flow rate, and time to obtain the analytical signal from 1 to 10 s. All analyses were performed in triplicate. The determination of total Hg was performed using a direct mercury analyzer (DMA-80, Milestone).
Total metal digestion procedure
The digestion methodology of sediment samples was based on the work of Vieira and coworkers (Vieira et al. 2005). The metals were extracted by digesting a 200 mg sample in a closed TFA vessel with 6.0 mL of aqua regia (HCl/HNO3, 3/1), 2.0 mL of H2O2, and 2.0 mL of HF. The heating program was performed in two steps: 10-min ramp to 120 °C, held for 5, and 8-min ramp to 200 °C, held for 15 min. Both steps were carried out with 800 W of power, with venting for 30 min. After cooling, the vessels were opened, and the solutions were transferred to previously decontaminated PTFE vials. Then, 1.0 g of boric acid was added, followed by resting for 30 min to complete solubilization. The vials were completed to 50 mL and subjected to centrifugation for the separation of possible solid waste. The solutions were subjected to the determination of the metals Al, Cd, Cr, Cu, Fe, Mn, and Zn by FS-F AAS and Co and Pb by ICP-OES.
The detection and quantification limits were calculated for the FS-F AAS and ICP-OES methods, according to a national chart that provides guidance on validation procedures (DOQ-CGCRE-008 2010). The sample blanks obtained in microwave extractions were considered. Each round of digestion of sediment samples generated a sample blank, for a total of 7 independent blanks at the end of each batch analyzed.
Hg determination by DMA
The samples were analyzed in triplicate masses of 80–200 mg, depending on an initial test to check the signal obtained by the equipment. In the direct analysis equipment (DMA), the samples were subjected to a heating program that involves a heating ramp of 30 s to reach 200 °C (drying step) and then another ramp of 30 s to reach 750 °C and 120 s at 750 °C (pyrolysis step). The calibration curve was obtained in aqueous medium with standard solutions of Hg prepared from a stock solution of 1,000 μg L−1 Merck (Darmstardt, Germany) in HNO3 2 % (v/v), with absorption measurements at 257 nm. To evaluate the accuracy of the measurements, a reference material, GBW-08301 (River Sediment), was analyzed.
Organic carbon and pH determination
The organic matter or organic carbon content was determined by weighing 2 mg of sample, which was brought to combustion in an O2 atmosphere, separated by column chromatography and detected using a thermal conductivity gas detector. The percentage of organic matter was obtained by multiplying the organic carbon result by a factor of 1,724 (EMBRAPA 1997).
The pH of the samples was measured according to the methodology of EMBRAPA (1997), in water and 1 mol L−1 KCl solution, at a ratio of 10 g of sediment to 25 mL of solution.
Geoaccumulation index (I geo)
The I geo index was originally defined by Müller (1979) and established as a ratio between the local metal concentrations in sediments and a reference value taken as the standard or a background value (Eq. 1), originally defined by the work of Turekian and Wedephol (1961):
where C m is the measured concentration of metal M in the sediment; C bg concentration taken as background to the metal M; and 1,5 is the factor that minimizes the possible variations of the background values determined for the metal in the environment, as well as small anthropogenic influences.
Seven classes make up the I geo, ranging from unpolluted to extremely polluted, which corresponds to a 100-fold enrichment relative to the value of the postulated background.
Enrichment factor (EF)
The EF is the element concentration ratio in the sediment and its natural background concentration (Eq. 2) (Zhang and Liu 2002; Feng et al. 2004; Qi et al. 2010). In the case of EF, a reference element concentration, treated as the normalizer (element whose source must be natural and whose concentration should not vary greatly) is inserted in the calculation to balance the mineralogical variations in sediment (Qi et al. 2010).
where M sed is the measured concentration of metal M in the sediment; R sed concentration of R normalizing element in the sediment; M bg concentration taken as background to the metal M; and R bg is the background concentration assumed as the R normalizing element.
The normalization technique is used to distinguish the natural variability of metals from the metals that are associated with the accumulation in the sediments due to anthropogenic activities. Normalization of sediment for trace metals can use the concentration of elements such as Al, Fe, Li, and Y, among others, in addition to parameters such as organic matter and particle size (Chen and Selvaraj 2008).
The metal/Fe and metal/Al ratios are very constant in crustal materials, particularly in rocks associated with river basins. Thus, variations in these ratios allow the identification of anthropogenic metal contributions. Despite the similarities, the use of Al as a normalizer element has advantages over Fe. Aluminosilicates are a dominant fraction in the binding phase of the sediments (clay), whereas Fe often is present in fractions that may contain iron oxides and sulfides of anthropogenic origin. Aluminum is a conservative element widely used to compensate for variations in textural and in composition of sediments, and their accumulation in sediments by anthropogenic action is rarely observed (Chen and Selvaraj 2008). Thus, Al content was used as a normalizer in the EF calculations.
Statistical analysis
The calculations for principal component analysis (PCA), hierarchical cluster analysis (HCA), and Pearson correlation were performed using Statistica for Windows version 7.0 (StatSoft Inc., USA) program. Data were autoscaled for PCA and HCA analysis and Ward’s linkage and Euclidean distance for HCA.
Results and discussion
Metal, pH, and organic carbon distributions
The results of total metals concentrations and physico-chemical parameters are shown in Table 1. The analysis of certified reference materials showed good accuracy and precision, with recoveries ranging from 80.4 to 112.8 % and RSD up to 15.2 % for the metals determined. The observed concentration ranges were, in μg g−1, 96–1,371 for Cr, 14.5–57.0 for Co, 11.2–75.6 for Cu, 505–9,568 for Mn, 10.2–69.3 for Pb; 60.3–240.7 for Zn, and in %, 6.0–16.2 for Al, 4.8–31.1 for Fe, and 82 to 364 ng g−1 for Hg. The highest values for metals were found at sites RD001, RD004, RD009, RD025, RD026, RD029, and RD034.
The pH is directly influenced by many parameters, such as cation exchange capacity (CEC), clay content, redox conditions and others, being responsible for the solubility and mobility of metals and metalloids in soils and sediments. The pH in water ranged from 5.31 to 8.53. In the case of sediments, a pH value less than 5.5 is considered acidic, and such a level was verified at site RD018 (Meurer 2008). Values above 7.0 are considered common in arid or semi-arid regions. The sampled region is not arid or semi-arid, but 7 points displayed pH values above 7.0, suggesting the influence of domestic waste.
High levels of organic matter (OM) in sediments generally result in increasing the buffering capacity of the medium, as well as its reactivity. This implies a greater retention of ions, mainly by complexation mechanisms, and also interactions with organic molecules (Meurer 2008). Sediments with OM content lower than 10 % are called mineral (Esteves 2011), and such OM contents were observed at all sampling sites, with results ranging from 1.93 to 8.15 %. The main sources of organic matter in the sediments are domestic and industrial waste, in addition to runoff from agriculture.
The specific area of the particles is directly related to increased reactivity thereof. Because of this, the particle size analysis by textural classification, it is so important. The granulometric analysis show that all samples had sandy characteristic, since the percentage of this fraction (>0.063 mm) was larger than the finer one. However, the results of silt–clay fraction (FSA, <0.063 mm) showed a large range, from 0.6 to 30.7 %.
For the element Cr, all samples displayed results above the limit SQG PEL, ranging from 96 ± 3 to 1,371 ± 7 μg g−1 (RD034), which is nearly 15 times greater than the threshold (90 μg g−1). In the case of Cu, 10 samples with concentrations greater than the limit SQG TEL, 35.7 μg g−1, were observed, with values ranging from 11.2 ± 0.3 to 75.6 ± 0.4 μg g−1. The results for Zn varied from 60.3 ± 1.4 to 240.7 ± 3.4 μg g−1, and 5 samples were above the limit SQG TEL of 123 μg g−1 (RD021, RD026, RD029, RD034, and RD035).
In the case of Hg, 8 samples displayed values greater than the concentration SQG TEL: RD009, RD034, and RD029, with values of 364 ± 2, 320 ± 12, and 243 ± 24 μg kg−1, respectively, followed by RD019, RD027, RD013, RD026, and RD035, with values ranging from 185 ± 1 to 189 ± 1 μg kg−1. The RD029 site is located between the cities of João Monlevade and Nova Era. The RD0034 site is located between the Coronel Fabriciano and Ipatinga. These cities are quite industrialized, and their industry mainly includes mining and steelmaking activities. The RD009 site is associated with illegal gold mining activities developed in the region of Ouro Preto and Mariana. Previous studies have reported high levels of Hg in this region (Costa et al. 2003; Palmieri et al. 2006; Windmöller et al. 2007).
Comparing the results with similar studies performed in Brazil and worldwide (Table 2), it was observed that some of the concentrations ranges found for the metals determined (Cu, Fe, Hg, Pb, and Zn) were similar to those obtained for Brazilian rivers, such as the Betari and Paracatu and the estuarine region of Bahia de Santos. The concentration ranges observed for Cr, Co, and Mn were higher than those observed in the cited studies. For Al, the values were similar to those observed for the Louro River in Spain and Nestos River in Greece (Filgueiras et al. 2004; Papastergios et al. 2009). It is noteworthy that such comparisons are limited by the type and number of samples collected, as well as different methods of treatment and digestion of the same (Table 2), and mainly, the region studied.
PCA/HCA
HCA and PCA chemometric analysis were performed to identify correlations between the metal concentrations and physico-chemical characteristics of the sediment samples. It was used as a matrix basis for the analyses of the sediment samples. The variables were the concentrations of Al, Cr, Co, Cu, Fe, Hg, Mn, Pb, Zn, organic matter (OM%), pH and silt–clay percentage (FSA%) obtained by texture classification.
Two main groups (A and B) were observed in the dendrogram of variables (Fig. 2a) and several smaller clusters. Group A indicates the Pb, Zn, and Hg concentrations, Fe and Mn (oxides) concentrations, and organic matter. Group B indicates the association of the metals Cr, Co, and Cu in clay phases containing Al, as the Al concentration and FSA% were also grouped. In the dendrogram of samples, three major groups were formed (Fig. 2b). The first two, A and B, are characterized generally by higher concentrations of Cr, Hg, Zn, and Pb compared with the SQG.
The first 4 principal components variables, totaling 77.8 % of the explained variance, reflected mainly the geochemical relationships of samples (Table 3). The variables that appear in more than one principal component (PC), with moderate to strongly positive loadings, appear to reflect a geochemical process or distinct sources associated with the concentrations of these metals that affect their spatial distribution in the DRB.
PC1 indicated positive loadings for Mn, Fe, Hg, Zn, and Pb and OM%, reflecting the tendency of positively charged metal ions bind to negatively charged sites present in organic matter. The latter has good correlations with metals in sediments, as was also observed for Pb and Zn by other researchers (Ramiro Pastorinho et al. 2012) and also Hg (Zheng et al. 2012), among others. This observation corroborates the grouping verified by the HCA analysis of the variables, where OM%, Zn, Pb, Hg, Fe, and Mn formed a distinct group, and is indicative of sediment contamination due to most likely domestic and/or industrial waste in the RDB region due to its association with organic matter (Sundaray et al. 2011; Mil-Homens et al. 2013a). These results are in agreement with the water quality reports of State Institute of Water Manegement (IGAM) in 2009 and 2010, which identified the discharge of effluents “as a major cause of water contamination” and such material may be deposited in the sediments (IGAM 2010a, b). Considering the above observations, it is clear that for most samples, PC1 is related to anthropogenic influence.
PC2 indicated high-negative loadings for Al, Cr, Co, and Cu, with a strong correlation. For PC3, positive loadings for Co, Cu, Hg and FSA% were found; the first two lithogenic elements associated with Fe and Mn, may indicate that their origin is weathering of rocks and soils in the basin (Sundaray et al. 2011; Mil-Homens et al. 2013a). It is noteworthy that for Hg, natural sources are not known in the region, and the presence of the metal is due to illegal gold mining activities still present in the area. The value observed for Cr loading in PC4, high and significant, suggests that this source is industrial activity, as the other positive values found (Hg, Al and FSA%) were small. This observation corroborates what was found in the HCA, where Cr is grouped with Al and FSA%.
When examining a graphical projection of the samples, it is seen that the sites RD034 and RD001 are separated from the other groups by their high concentration of Cr, 1,371 ± 8 and 308 ± 4 μg g−1, respectively, thus behaving as outliers (Fig. 3). Sites RD009, RD026, and RD029 again appear grouped, confirming the HCA observations. The remaining sites form a large group, indicating similarity between them.
Pearson correlation was also performed and expressed using correlation coefficients. As the correlation coefficient is a measure of the degree of relationship between two variables, significant correlation coefficients are ≥0.5000 (Santos et al. 2008). Significant positive correlations were observed between Fe and Mn (0.52), Fe and Pb (0.54), Hg and Mn (0.78), Pb and Zn (0.74), Co and Cu (0.68), Cr and Hg (0.50), Hg and OM% (0.55), OM% and Pb (0.72), and OM% and Zn (0.70). Negative significant correlations were observed for Al and Fe (−0.77), Al and Mn (−0.61), and FSA% and Pb (−0.51). These observations are consistent with the observed HCA and PCA, especially for Hg, Pb, Zn, pH, and OM%.
Geoaccumulation index
I geo is one of the parameters used to define the metal contamination in sediment by comparing the current concentration with levels considered pre-industrial or natural, known as background. The use of background values determined for each study area is an alternative to the use of global averages. These values take into account the natural variability of geochemical region in accordance with different compositions and characteristics of rocks and sediments. The use of global backgrounds can result in a wrong interpretation of geochemical data quality standards for a given location (Silva et al. 2012). For this research, the background values used were that defined by Costa et al. (2003), which was a study of a sub-basin of the North Gualaxo River, belonging to the Doce River Basin.
The calculations for the I geo of Hg indicated that 17 sediment sampling sites were classified as practically non-polluted, Class 0. Two sites, RD009 and RD034, were listed as Class 1, unpolluted or weakly polluted (Fig. 4). There was a discrepancy between the SQG and what the I geo indicates for Hg. For the former, the value of TEL is defined as the threshold level of adverse effects to biota. At these two sites, the TEL was exceeded. For Co, 12 samples were classified as heavily polluted, Class 4, and 6 samples were classified as Class 3. Site RD001 was classified as strongly to extremely polluted, Class 5. This may be due to the background value used in the calculation being low, 2.1 μg g−1.
Site RD034 was rated as extremely polluted for Cr, which was expected because of its high concentration, and it was indeed proven to be an outlier value, as demonstrated by the PCA analysis. The remaining samples were distributed among Class 2 (11 samples) and Class 3 (7 samples), including the RD009 and RD029 sites. In agreement with that found by evaluating SQG, all sites that exceeded the PEL (probable level adverse effect on biota), in general, were characterized as moderately to heavily polluted by I geo.
Similar to the Cr behavior, for Cu, 14 samples were designated as Class 2, RD009 and RD029 included, and 3 other sites were classified as Class 3, including RD034. Two samples were considered virtually unpolluted, those from sites RD025 and RD053. Silva et al. (2012), studying the Rio Sergipe Basin, located in the Brazilian Northeast, obtained similar results for Cu, with only one point in the basin considered Class 2. The remainder belonged to Classes 0 and 1.
The values found for Pb were divided the samples into four different classes. The RD029 site was classified as heavily polluted, which is in agreement with that found for SQG. Class 1 included 4 samples, Class 2 six samples, including RD009, and Class 3 the rest, including the last site RD034. For Zn, there are three main classes: Class 2, 3, and 4, the latter including RD026 and RD029. The RD009 and RD034 sites had equal classification values as observed for Pb. The similar behavior observed for the classification of Pb and Zn may indicate that these metals are subject to the same geochemical process of accumulation in the sediment, as both elements displayed strong correlations in the Pearson analysis, HCA and PCA.
In general, the I geo analysis indicated that most of the sites examined in the RDB were not polluted or moderately polluted for the metals analyzed, as most of the values calculated were classified as Class 2 and 3.
Enrichment factor
As with the index I geo, calculating the enrichment factor is a tool that assists in the investigation of environmental contamination in water systems and analyzing the sediment compartment. The relationship between the concentration of the metals investigated and the concentration of Al in sediments is widely used, as it is assumed that its concentration, as a product of weathering, is generally comparable (Zhang and Liu 2002). For the calculation of EF, Al was used as the normalizing factor. The same values used to calculate the background I geo were considered for the determination of EF.
By convention, values between 0.5 < EF < 1.5 are considered as an indication that these metals can be fully analyzed as being the result of the geological composition of the material or the influence of weathering. EF values >1.5 are interpreted as possibly coming from other sources, such as biota or anthropogenic contamination (Zhang and Liu 2002; Feng et al. 2004; Hortellani et al. 2008).
The results revealed that the EF of sediment samples for Co was higher than 1.5 (Fig. 5). Site RD009 had an EF >1.5 for Cr, Pb and Zn. In addition, such EF values for Pb and Zn were observed for site RD029 and for Pb and Cr for site RD034. Again, it appears that the impacted DRB sites are in accordance with the results obtained in the evaluation of SQG, PCA/HCA, and I geo. Silva et al. (2012) found enrichment for Cu, Cr, and Pb at some points of the analyzed Sergipe Basin. Unlike the behavior of Co, Cr, Pb, and Zn, Hg did not display an EF that is classified as anthropogenic in origin, but three sampling sites exceeded their SQG TEL values (RD009, RD029 and RD034). For Cu, Mn, and Fe, all samples were counted as having geologic composition concentrations.
In summary, the RD001, RD009, RD025, RD029, RD031, RD034, and RD035 sites displayed higher values of I geo, FE, and SQG, which classify them in general, as the sites most impacted in the DRB (Table 4).
Conclusion
The sediment analyses revealed that 15 % of DRB samples were above the SQG TEL for Hg, 100 % for Cr, 53 % for Cu, 21 % for Zn, and 21 % for Pb, which is a violation of the quality guide values. Together, these results are equivalent to a total violation of 41 %, related to the SQG, for the DRB sediments.
According to the PCA and HCA performed on the sediment samples, there was a correlation between Pb, Zn, Hg, Fe, Mn, and OM%, which is related to the tendency of the Pb, Zn, and Hg to interact with Fe–Mn oxides and organic matter, and they may be associated with domestic and industrial discharges in the waters of the DRB. The PCA analysis also indicated that the Cr is probably from industrial activities occurring in the basin because of the anomalous behavior observed at site RD034. The Pearson correlation results were consistent with those found by PCA and HCA.
The classification using geochemical standards of quality of the collection points of the DRB as to the origin of the metals analyzed revealed that sites RD001, RD009, RD025, RD029, RD031, RD034, and RD035 were considered contaminated because of their I geo and EF values. When compared to the results obtained by analyzing the SQG and as indicated by PCA/HCA, it can be concluded that these sites are the most impacted in the DRB, probably because of anthropogenic activity; therefore, new monitoring and studies of speciation and mobility of these metals are very relevant and should be performed.
References
Abrahim GMS, Parker RJ (2008) Assessment of heavy metal enrichment factors and the degree of contamination in marine sediments from Tamaki Estuary, Auckland, New Zealand. Environ Monit Assess 136:227–238
Buccolieri A, Buccolieri G, Cardellicchio N, Dell’Átti A, di Leo A, Maci A (2006) Heavy metals in marine sediments of Taranto Gulf (Ionian Sea, Southern Italy). Mar Chem 99:227–235
CCME (1999) Canadian Council of Ministers of tne Environment. Canadian Sediment Quality Guidelines for the Protection of Aquatic Life. Canadian Environmental Quality Guideline. Canadian Council of Ministers of the Environment, Winnipeg
Chen CTA, Selvaraj K (2008) Evaluation of elemental enrichments in surface sediments off southwestern Taiwan. Environ Geol 54:1333–1346
Costa AT, Nalini Júnior HA, Lena JC, Friese K, Mages M (2003) Surface water quality and sediment geochemistry in the Gaulaxo do Norte basin, eastern Quadrilátero Ferrífero, Minas Gerais, Brazil. Environ Geol 45:226–235
Cotta JAO, Rezende MOO, Piovani MR (2006) Avaliação do teor de metais em sedimento do rio Betari no Parque Estadual Turístico do Alto Ribeira—Petar, São Paulo, Brasil. Quim Nova 29:40–45
Devesa-Rey R, Diaz-Fierros F, Barral MT (2011) Assessment of enrichment factors and grain size influence on the metal distribution in riverbed sediments (Anllons River, NW Spain). Environ Monit Assess 179:371–388
DOQ-CGCRE-008—Orientações sobre validação de métodos analíticos (2010) Instituto Nacional de Metrologia, Normalização e Qualidade Industrial—INMETRO
Duplay J, Semhi K, Bloundi MK, Khedhiri S, Ben Ahmed R, Darragi F (2012) Geochemical background and contamination level assessment in Tunisian and Moroccan Mediterranean coastal lagoon sediments. Environ Earth Sci 67:1201–1214
EMBRAPA (1997) Manual de métodos de análise de solo, 2a edn. EMBRAPA/Centro Nacional de Pesquisas de Solo, Rio de Janeiro
Esteves FA (2011) Fundamentos de Limnologia, 3ª edn. Interciência, Rio de Janeiro
Feng H, Han X, Zhang W, Yu L (2004) A preliminary study of heavy metals contamination in Yangtze river intertidal zone due to urbanization. Marine Pollut 49:910–915
Filgueiras AV, Lavilla I, Bendicho C (2004) Evaluation of disribution, mobility and binding behavior of heavy metals in surficial sediment of Louro River (Galicia, Spain) using chemometric analysis: a case study. Sci Total Environ 330:115–129
Franz C, Makeschin F, Weiss H, Lorz C (2013) Geochemical signature and properties of sediment sources and alluvial sediments within the Lago Paranoa catchment, Brasilia DF: a study on anthropogenic introduced chemical elements in an urban river basin. Sci Total Environ 452:411–420
Hortellani MA, Sarkis JES, Abessa DMS, Sousa ECPM (2008) Avaliação da contaminação por elementos metálicos dos sedimentos do estuário Santos—São Vicente. Quim Nova 31:10–19
Hu B, Li J, Zhao J, Yang J, Bai F, Dou Y (2013) Heavy metal in surface sediments of the Liaodong Bay, Bohai Sea: distribution, contamination, and sources. Environ Monit Assess 185:5071–5083
Hübner R, Astin KB, Hebert RJ (2009) Comparison of sediment quality guidelines (SQGs) for the assessment of metal contamination in marine and estuarine environments. J Environ Monit 11:713–722
IGAM, Instituto Mineiro de Águas (2010a) Monitoramento da qualidade das águas superficiais da bacia do Rio Doce—Relatório Anual 2009. IGAM, p 444
IGAM, Instituto Mineiro de Águas (2010b) Relatório Executivo—Qualidade das águas superficiais em Minas Gerais em 2010. IGAM, p 19
Lima EAM (2008) Avaliação da Qualidade dos Sedimentos e Prognóstico Geoquímico Ambiental da Zona Estuarina do Rio Botafogo. PhD Universidade Federal de Pernabuco, Pernambuco
Meurer EJ (2008) Fundamentos de Química do Solo, 3a ed, Porto Alegre
Mil-Homens M, Costa AM, Fonseca S, Trancoso MA, Lopes C, Serrano R, Sousa R (2013a) Characterization of heavy-metal contamination in surface sediments of the Minho River Estuary by way of factor analysis. Arch Environ Contam Toxicol 64:617–631
Mil-Homens M, Costa AM, Fonseca S, Trancoso MA, Lopes C, Serrano R, Sousa R (2013b) Natural heavy metal and metalloid concentrations in sediments of the Minho River estuary (Portugal): baseline values for environmental studies. Environ Monit Assess 185:5937–5950
MMA Ministério do Meio Ambiente (2005) http://www.riodoce.cbh.gov.br/bacia_caracterizacao.asp. Accessed 22 May 2013
Mulholland DS, Boaventura GR, Araujo DF (2012) Geological and anthropogenic influences on sediment metal composition in the upper Paracatu River Basin, Brazil. Environ Earth Sci 67:1307–1317
Müller G (1979) Schwermetalle in den Sedimenten des Rheins-Veränderungem seit 1971. Umschau 79:778–783
Nasrabadi T, Bidhendi GN, Karbassi AR, Mehrdadi N (2010) Evaluating the efficiency of sediment metal pollution indices in interpreting the pollution of Haraz River sediments, southern Caspian Sea basin. Environ Monit Assess 171:395–410
Palmieri HEL, Nalini Júnior HA, Leonel LV, Windmöller CC, Santos RC, Brito W (2006) Quantification and speciation of mercury in soils from the Tripuí Ecological Station, Minas Gerais, Brazil. Sci Total Environ 368:69–78
Papastergios G, Fernandez-Turiel JL, Georgakopoulos A, Gimeno D (2009) Natural and anthropogenic effects on the sediment geochemistry of Nestos river, Northern Greece. Environ Geol 58:1361–1370
Pereira JC, Guimarães-Silva AK, Nalini Júnior HA, Pacheco-Silva E, Lena JC (2007) Distribuição, fracionamento e mobilidade de elementos traço em sedimentos. Quim Nova 30:1249–1255
Poleto C (2008) Ambiente e Sedimentos. ABRH, Porto Alegre
Qi S, Leipe T, Rueckert P, Di Z, Harff J (2010) Geochemical sources, deposition and enrichment of heavy metals in short sediment cores from the Pearl River Estuary, Southern China. J Mar Syst 82:S28–S42
Ramiro Pastorinho M, Telfer TC, Nogueira AJA, Soares AMVM, Ranville JF (2012) An evaluation of trace metal distribution, enrichment factors and risk in sediments of a coastal lagoon (Ria de Aveiro, Portugal). Environ Earth Sci 67:2043–2052
Rodriguez-Barroso MR, Garcia-Morales JL, Coello Oviedo MD, Quiroga Alonso JM (2010) An assessment of heavy metal contamination in surface sediment using statistical analysis. Environ Monit Assess 163:489–501
Santos JS, Santos MLP, Oliveira E (2008) Estudo da mobilização de metais e elementos traços em ambientes aquáticos do semiárido brasileiro aplicando análises de componentes principais. Quim Nova 31:1107–1111
Shelton LR, Capel PD (1994) Guidelines for collecting and processing samples of stream bed sediments for analysis of trace elements and organic contaminants for the National Water-Quality Assessment Program. USGS, Open-file Report
Silva PSC, Damatto SR, Maldonado C, Favaro DIT, Mazzilli BP (2011) Metal distribution in sediment cores from Sao Paulo State Coast, Brazil. Mar Pollut Bull 62:1130–1139
Silva AF, Lima GRS, Alves JC, Santos SH, Garcia CAB, Alves JPH, Araujo RGO, Passos EA (2012) Evaluation of trace metal levels in surface sediments of the Sergipe River Hydrographic Basin, Northeast Brazil. J Braz Chem Soc 23:1669–1679
Sundaray SK, Nayak BB, Lin S, Bhatta D (2011) Geochemical speciation and risk assessment of heavy metals in the river estuarine sediment—a case study: Mahanadi basin, India. J Hazard Mater 186:1837–1846
Turekian KK, Wedepohl KH (1961) Distribution of elements in some major units of the Earth’s crust. Geol Soc Am Bull 72:175–192
Vieira EC, Kamogawa MY, Lemos SG, Nobrega JD, Nogueira ARD (2005) Microwave-assisted decomposition of soil samples: Strategies to avoid formation of insoluble fluoride salts. Revista Brasileira De Ciencia Do Solo 29:547–553
Windmöller CC, Santos RC, Athayde M, Palmieri HEL (2007) Distribuição E Especiação De Mercúrio Em Sedimentos De Áreas De Garimpo De Ouro Do Quadrilátero Ferrífero (Mg). Quim Nova 30:1088–1094
Zhang J, Liu CL (2002) Riverine composition and estuarine geochemistry of particulate metals in China—weathering features, anthropogenic impact and chemical fluxes. Estuar Coast Shelf Sci 54:1051–1070
Zhao S, Feng C, Yang Y, Niu J, Shen Z (2012) Risk assessment of sedimentary metals in the Yangtze Estuary: new evidence of the relationships between two typical index methods. J Hazard Mater 241:164–172
Zheng W, Liang L, Gu B (2012) Mercury reduction and oxidation by reduced natural organic matterin anoxic environments. Environ Sci Technol 46:292–299
Acknowledgments
This study was financially supported by The National Council for Scientific and Technological Development of Brazil (CNPq), Foundation for Supporting Research in the State of Minas Gerais, FAPEMIG, in project APQ 03861-09, and laboratorial support was provided by National Institutes of Science and Technology on Mineral Resources, Water and Biodiversity, INCT-Acqua.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Santolin, C.V.A., Ciminelli, V.S.T., Nascentes, C.C. et al. Distribution and environmental impact evaluation of metals in sediments from the Doce River Basin, Brazil. Environ Earth Sci 74, 1235–1248 (2015). https://doi.org/10.1007/s12665-015-4115-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12665-015-4115-2