Abstract
This work reports a geochemical study of sediments from the upper Paracatu River Basin. The objective is to define the influences of Au, Zn, and Pb mineral deposits and mining activities on the sediment metal sources, distribution, and accretion. The samples were analyzed using ICP/OES, AAS, and XRD techniques and were treated with principal components analysis and the geo-accumulation index. The main geochemical processes that control the sediment composition are pyrite oxidation, muscovite weathering, carbonate dissolution, and the erosion of oxisols enriched with Zn and Pb. The upper Rico Stream has high Al, Fe, Cu, Cr, Co, and Mn concentrations due erosion of oxisols and pyrite oxidation and muscovite alteration present in the parental rock. The artisanal alluvial gold mining increased the primary rock-minerals’ weathering and Hg sediment concentration. The lower Escuro River and Santa Catarina Stream are enriched with Zn and Pb due the erosion of metal-rich soils formed over galena, sphalerite, calamine, and willemite mineral deposits located upstream. Elements such as Ca, Mg, and Ba have low concentrations throughout the sampled area due the high solubility of these metals-bearing minerals. The dispersion of metals is limited by the basin geomorphology and their affinity to silt-clayey minerals and Fe and Mn oxides and hydroxides in circumneutral pH waters.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Environmental geochemistry includes studies of sources and distribution of chemical compounds in nature, interactions between rock, soil, and water, and their influence on the food chain and human health (Thornton 1993, 1996). Mining activities have led to disturbances of the Earth’s surface geochemical cycles and the contamination caused by such events is a worldwide issue (Plant et al. 2001). Therefore, understanding the processes that drive the metal composition of water and sediment and their influence on natural systems has been a highlighted subject in geochemistry.
Riverbed sediment carries information from natural and anthropogenic processes and its analysis is an important tool to measure the quality of aquatic systems (Power and Chapman 1992). Sediment is the unconsolidated geological material, distributed throughout the drainage system, characterized by the continuous and constant interaction of weathering and erosion within the catchment area (Licht 1998). Depending on the physicochemical features of the water, dissolved elements may precipitate as oxyhydroxides, carbonates, and other minerals, bind on fine solids surface, or form complexes with organic matter (Drever 1988). Through these processes the dissolved elements can incorporate the riverbed sediments together with suspended solids in low-energy hydraulic conditions.
Metals in sediments become bioavailable and toxic according to element speciation, pH, redox potential, and ligand availability. Generally, dissolved metals have higher bioavailability. However, the particulate phase can also have adverse effects, mainly on benthic communities (Chapman and Wang 2000; Luoma 1983). Elements that have biomagnification capacity, such as mercury, can enter the food chain to become harmful to the aquatic communities. According to Malm (1998), fish from higher trophic levels (e.g., piscivorous and carnivorous) present in rivers that drain gold mining areas have greater mercury concentration, followed by omnivorous, detritivorous, and herbivorous species that belong to lower trophic levels.
Mineralized areas are known to influence the chemical composition of aquatic systems. Studies carried out in rivers located in the Iberian Pyrite Belt region showed the influence of mineral deposits on the release of large amounts of metal ions into aquatic environments. Olias et al. (2004) demonstrated that sulfide oxidation influences the geochemical composition of Odiel River water. The authors reported pH < 2.50, and great concentrations of SO4 2− and dissolved Zn, Pb, Cu, Mn, and Fe due their great mobility in acidic waters. The presence of pyrite, chalcopyrite, arsenopyrite, and sphalerite in the alluvium near the mining areas inside the Tinto River Basin caused extremely high Pb and Zn enrichment in the sediment (Hudson-Edwards et al. 1999).
The upper Paracatu River Basin hosts Zn, Pb, and Au deposits with different types of ores distributed throughout the catchment area. In spite of the mineralized area’s wide distribution and related mining activities, riverbed sediment is liable to accumulate metals. To evaluate the influence of mineral deposits and mining activities on the chemical composition of sediments, this work aims to establish the concentrations of metals (Al, Fe, Mn, K, Mg, Ca, Ba, Cu, Cr, Co, Zn, Hg and Pb), define their sources and the geochemical processes that control the metal distribution and accumulation throughout the upper Paracatu River Basin, Minas Gerais, Brazil.
Study area
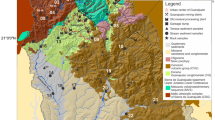
The study area is located on the upper course of the Paracatu River (one of the most important tributaries of the São Francisco Basin), in the northwest of Minas Gerais State (Brazil), between coordinates 46°20′ and 47°20′W and 17°10′ and 18°15′S (Fig. 1). It covers the cities of Paracatu, Guarda-Mor, Vazante and Lagamar; areas of agriculture; mining industries of Au (Paracatu), Zn–Pb (Morro Agudo), Zn (Vazante), limestone (Lagamar); and artisanal alluvial gold mining in the upper Rico Stream.
The upper Paracatu Basin is inserted in the Brasília Fold Belt within the geological units of the Vazante and Canasta Groups (Dardenne 2000). The Vazante Group metasediments are composed basically of a clayey and dolomitic-clayey stromatolite sequence. It is Neoproterozoic passive sedimentation along the western side of the Craton. The formations of this Group in the study area are (1) Morro do Calcário: stromatolitic dolomites that host galena (PbS) and sphalerite (ZnS) mineralization in Morro Agudo; (2) Serra do Poço Verde: algal dolomites with willemite (Zn2SiO4) and calamine (Zn4Si3O(OH)2) mineralization in Vazante; (3) Lagamar: psamo-pelitic carbonate with alternation of conglomerates, quartzites, slates, and metasiltstones; (4) Serra do Garrote: interbedded carbonate or pyrite slates and quartzite; and (5) Serra da Lapa: carbonaceous phyllites, carbonate metasiltstones, dolomite, and quartzite lenses (Valeriano et al. 2004).
The Canasta Group consists of siliciclastic metasedimentary rocks, composed of carbonaceous phyllite layers (Paracatu Formation) covered by quartzites and sericitic and chloritic phyllites (Campos Neto 1984; Freitas-Silva and Dardenne 1992). The depositional anoxic conditions of the paleoenvironment favored the precipitation of sulfide-metal minerals, e.g., pyrite and chalcopyrite (Fuck et al. 1993). Gold mineralization occurs free in quartz boudins or in iron sulfides with residual gold, resulting from pyrite decomposition (DNPM 1988).
Materials and methods
Sediment samples were collected in 2008 from 33 sites (Fig. 2). Samples were dried and sieved in fraction <63 μm. Sediment decomposition used lithium metaborate fusion for major elements (Al, Fe, Mg, Ca and K), acid attack (HNO3, HCl, HF and HClO4) for trace elements (Cu, Zn, Ba, Cr, Pb, Co, and Mn), and acid attack with HCl and HNO3 in closed vessels for Hg. The metal determination used: (1) optical emission spectrometer with plasma (ICP/OES) of spectro analytical instruments GmbH, model spectroflame FVM03 for Al, Fe, Mg, Ca, Cu, Zn, Ba, Cr, Co, and Mn measurement; (2) ICP/OES of varian, model liberty for Pb measurement; (3) atomic absorption spectrometer (AAS) double-beam Perkin Elmer, model 603 for K measurement; and (4) atomic absorption spectrometer (AAS), model MHS-400-ENDIF (Flow Injection Mercury System—Mercury Hydrate System) Perkin Elmer, with hydride generation (NaBH4—0.05%) for Hg measurement. The analyses were validated with the Standard Reference Material San Joaquin Soil, 2709, NIST (National Institute of Standards and Technology). The mineralogical analyses were performed by X-Ray Diffraction (XRD) on Rigaku D/MAX. The diffraction pattern interpretation and mineral identification used JADE 3.0 software.
To assist the sediment data interpretation, water samples were collected at the same sites described above. Aliquot parts were filtered using cellulose ester membrane filters, Millipore 0.45 μm. PO4 3−, SO4 2−, NO3 −, and NH3 were measured with a spectrophotometer (Hach model DR2500) and Cl− and HCO3 − by volumetric techniques. Electrical conductivity (EC) and pH were determined in the field using a multi-parameter Hach—Sension 156.
Principal component analysis (PCA) and Geo-accumulation index (Igeo) were used for data interpretation. PCA was applied to the geochemical data to assess the relationships among metals. It was interpreted together with the XRD results to define how mineral alteration dynamics influenced sediment composition. PCA was performed according to Farnham et al. (2003). The factor loads and the sample factor scores were calculated in a correlation matrix with the varimax rotation. The components that had eigenvalues higher than one were selected (Voudouris et al. 1997). The Igeo was used to assess the sediment metal accumulation intensity (Table 1) compared with background values using the Eq. 1
where, Cn is the measured concentration of the element in the sediment, and Bn is the geochemical background value. The constant 1.5 allows for the natural fluctuations of a given substance in the environment (Förstner 1983).
Results and discussion
Geological and anthropogenic sources
The metal composition of the upper Paracatu River sediment is shown in Table 2.
PCA summarizes all data in three principal components (PC), representing 75.3% of the data total variance. PC1 had high factor loads (>0.7) on K, Al, Fe, Co, Mn, Cu, Cr, and Hg, representing 44% of the variance; PC2 on Ca, Mg, and Ba, representing 16%; and PC3 on Pb and Zn, representing 15% (Fig. 3).
PC1 displays two distinct groups of minerals that occur together in the upper Rico Stream. The first is related to hematite (Fe2O3) and goethite (FeO(OH)), originating from pyrite (FeS2) oxidation and erosion of oxisols. The second is represented by illite ((K,H3O)Al2Si3AlO10(OH)2), kaolinite (Al2Si2O5(OH)4), and gibbsite (Al(OH)3), originating from muscovite (KAl2(AlSi3O10)(OH)2) alteration present in the carbonaceous phyllites. It is associated mainly with the BCR1, BCR2, BCR3, and BCR4 sites. Co, Cu, Cr, and Mn have primarily geological origin since they are trace elements typical of pyrite and chloritic/sericitic phyllites (Andrew-Jones 1968). According to Starostin and Yapaskurt (2007), the black shale sedimentary rocks that form the carbonaceous phyllites are also known to be concentrators of metals. Hg is geologically associated with sulfide minerals that host Au mineralization (Lentz 2005), and is also introduced in its metallic form by artisanal gold mining activities. Hg is used to separate fine gold particles from the sediment minerals through amalgamation. During this process, large amounts of Hg are lost to the sediment riverbed and alluvium due unsuitable gold extraction techniques. Hg-rich tailings are usually left in most of the gold mining areas (Lacerda and Salomons 1998).
Major contents of gibbsite were found in the upper Rico Stream and are related to intense mineral leaching caused by morphological changes in the riverbed due alluvial gold mining activities. Acid drainage caused by upstream sulfide oxidation and the removal of the riparian vegetation also contributes to mineral leaching. Equations 2–4 show gibbsite formation from primary rock-forming mineral degradation. Equation 2 shows the aluminosilicates (muscovite and illite) alteration into a clay-mineral (kaolinite), caused by interaction with water and atmospheric CO2, where M is a metal, usually K. Equation 3 demonstrates kaolinite dissolution in an acidic medium, producing 2Al3+ and 2H4SiO4. Equation 4 shows the precipitation of aluminum hydroxide, forming gibbsite (Banks et al. 1997).
Pyrite oxidation is controlled by chemical constituents such as pH, temperature, dissolved oxygen, Fe3+ chemical reactivity, and the mineral surface area exposed to weathering (Salomons 1995). Equations 5–8 explain higher SO4 2− and H+ concentrations in the water of the upper Rico Stream (Table 3). In Eq. 5 iron sulfide, exposed to air and water, undergoes oxidation, producing Fe2+, SO4 2−, and two protons. In Eq. 8, Fe3+ is reduced by pyrite oxidation, producing Fe2+, SO4 2− and 16 protons. The circumneutral pH in the Rico downstream demonstrates a rapid neutralization of acid drainage by carbonate dissolution. Neutralization of pH occurs when calcite, dolomite or other carbonate minerals are present. The combined reaction of pyrite oxidation and acid neutralization by carbonates was described by Williams (1982), where two moles of calcium carbonate are used to neutralize the acids produced by one mole of pyrite, as exemplified in Eq. 9.
Equations 7, 9 explain the presence of hematite and goethite in the sediments of the upper Rico Stream. The weathering processes form Fe3+ amorphous hydroxides (Fe(OH3)) that precipitate in a circumneutral pH environment. According to Mackay (1960) goethite is formed by the continuous dissolution and reprecipitation of Fe3+ hydroxide (reconstructive transformation) and, according to Fischer and Schwertman (1974), hematite is formed by the internal dehydration of amorphous Fe3+ hydroxide.
PC2 shows the association of metals typical of calcite (CaCO3) and dolomite (Ca.Mg(CO3)2). The study area has relatively constant and low Ca, Mg, and Ba concentrations due the high dissolution of these metal-bearing minerals (Eqs. 10, 11) (Morse and Arvidson 2002). Carbonate weathering produces great HCO3 − concentrations in waters of the Santa Catarina Basin (Table 3). XRD results showed the presence of dolomite only in two sites of the studied area (BCR5 and BRE10). These sites had the highest Ca, Mg, and Ba concentrations throughout the region. The presence of dolomite in BCR5 is explained by the proximity to a rainwater drainage effluent located in the city of Paracatu. Lee et al. (2005) showed that minor amounts of carbonate minerals (calcite and dolomite) are present in roadside sediments. They are minerals of construction material residues transported by runoff water to the riverbed. The presence of dolomite in BRE11 is explained by runoff water that removes and transports tailings materials from Morro Agudo mine into the Traíras Stream riverbed.
PC3 is related to galena, calamine, willemite, and sphalerite that occur in the Vazante Group. The sampling sites with highest Zn and Pb values are BRSC6, BRSC7, and BRE10. They are affected by Zn and Pb sulfide minerals that oxidize similarly to pyrite, releasing metals and sulfate without any acid drainage production as show in Eq. 12 (Banks et al. 1997). The increased SO4 2− contents in the Santa Catarina River (Table 3) indicate that Zn and Pb mineral sulfides are oxidized by runoff water and subsequently incorporated in the aquatic system. The lower Santa Catarina River is also influenced by the dissolution of Zn-silicate minerals located in the Serra do Poço Verde Formation. The low concentrations of SO4 2− in the Escuro River water (Table 3) indicates that the river’s geochemical composition is mainly related to erosion of Zn/Pb-rich oxisols, instead of the direct weathering of reduced sulfide-metal minerals present in the parental rock.
Background values
The different geological features and anthropogenic activities in the upper Paracatu River basin hamper the establishment of a control area that can provide background values for calculating Igeo. Moreover, the mean for each parameter also does not offer an ideal reference value compared with other studies. The presence of mineral deposits produces a high average, especially for Zn. Thus, the background values are defined as the mean of four sites (BRSC11, BRE5, BRE6, and BRE13). They are located in the main geological units (Vazante Group and Paracatu Formation), in catchments that do not contain mineral deposits and intense anthropogenic activities. The reference values are compared with others used in the US, Germany, and Canada, and the earth’s crust average in Table 4. The background (BG) values defined for this work agree with those used in other studies in different regions of the world.
Metal distribution and accumulation
The sampled sites are assessed according to the Igeo obtained for each element (Table 5). A distinct geochemical composition of the Rico Stream samples in relation to other rivers is observed. Al, Fe, Mn, Cu, Cr, and Co accumulation is assessed as absent to moderate due to the intense weathering of pyrite, muscovite and oxisols. Hg accumulation is assessed as moderate to heavy, making it the metal with the highest class among Rico Stream samples. This demonstrates the influence of human activities related to artisanal auriferous mining dating back to 1730. Presently, the artisanal mining activities are negligible due the exhaustion of the alluvial deposit. However, Hg concentration is almost seven times higher than the background values, with maximum concentrations of 0.77 mg/kg in BCR3. This site is located downstream from the mining area with more silt-clayey sediment that is suitable for metal scavenging. Comparing Rico Stream sediment data, Hg, Zn, and Pb have a positive correlation with K (correlations coefficients of 0.89, 071, and 0.86, respectively). The K+ (Al-silicates) (e.g., illite) are known to bind metals on the mineral surface due their cation exchange capacity. Rodrigues Filho and Maddock (1997) also found a relationship between Hg and silt-clayey minerals in the sediments located downstream from an alluvial gold mining in the Amazon Region. The authors suggest that elemental Hg is associated with coarse fractions in the mining area, whereas Hg2+ is associated with fine fraction sediment that is easily transported downstream.
The data obtained for the Rico Stream is consistent with other studies conducted in Brazil. Windmöller et al. (2007) found Hg concentrations between 0.04 and 1.1 mg/kg in sediments of an alluvial gold mining, also located in Minas Gerais State (Brazil). In a gold mining located in Mato Grosso State the sediments Hg concentrations ranged from 0.30 to 1.85 mg/kg (Rodrigues Filho and Maddock 1997). Other studies determined Hg sediment concentrations higher than the values observed in this work, e.g., 7.4 mg/kg in the North Carolina mining district (Callaham et al. 1994), 25 mg/kg in areas of the Amazon Region (Lacerda et al. 1991), and 157 mg/kg in the Madeira River (Malm et al. 1990).
The lower Escuro River and Santa Catarina Stream have heavy Zn accumulation, and moderate to heavy Pb accumulation. These high values are found in the BRSC6, BRSC7, BRSC13, BRE8, and BRE10 sites. The first two are located downstream from the Vazante mine (Serra do Poço Verde Formation—Vazante Group), where willemite and calamine mineral deposits occur. The BRE8, and BRE10 sites are located downstream from the Morro Agudo mine (Morro do Calcário Formation—Vazante Group), where sphalerite and galena mineral deposits occur. The BRSC13 site is not close to any mine, but has moderate to heavy Zn e Pb accumulation that indicates the presence of these element-bearing minerals in the catchment area.
Monteiro (2002) analyzed the mineralized dolomites of the Vazante Group and found Pb/Zn ratios between 0.005 and 0.023 while Burak et al. (2010) studied the metal content of the soils from the same region and found Pb/Zn ratios between 0.35 and 1.5. The authors suggest that the enrichment of Pb in the soils is caused by the greater binding affinity of Pb with Mn and Fe oxides. In this study, the sediment Pb/Zn ratios of the sampling sites located near the Zn and Pb mineral deposits (BRE10, BRSC6 and BRSC7) were 0.13, 0.07, and 0.08, respectively. The sediment Pb/Zn ratios below the values found in the soils indicates that Zn is easily transported from the soils to the sediments when compared with Pb. The comparison of Pb and Zn concentrations between soils and sediments enforce this hypothesis. The maximum Pb concentration found by Burak et al. (2010) in the soils was 465.32 mg/kg, which is almost four times greater than the values found in the sediment. On the other hand, the maximum Zn concentration found in the soils was 556.26 mg/kg, which is almost four times less than the values found in the sediments. The greater affinity of Pb than Zn on Mn and Fe oxides and hydroxides of tropical soils is also described by Fontes and Gomes (2003). The Zn sediment concentrations are consistent with the values presented by Hudson-Edwards et al. (1999) for the sediments of the Iberian Pyrite Belt Rivers, but Pb concentrations are lower since the weathered oxisols work as a reservoir of this metal. Comparing the Escuro River and Santa Catarina Stream sediment data, a high positive correlation of Zn and Pb with Mn and Fe was not observed. Therefore, these metals are not mainly associated with Mn and Fe oxides and hydroxides in the sediment as they are in the soils. In a sediment sequential extraction of a river that drains Zn and Pb mineral deposits, Svete et al. (2001) showed that 25–60% of Zn in the sediment is incorporated in the crystalline silicate lattice, while <10% is bound to amorphous Fe and Mn oxides and hydroxides. The authors also show that Pb is distributed mainly between organic matter and sulfides, or it is adsorbed in the silicate lattice.
Fe, Mn, Cu, Cr, and Co accretion in the Santa Catarina Stream coincides with the sites that have goethite and hematite. It is caused by the intense dissolution of soluble minerals (e.g., calcite and dolomite), which increases the concentrations of weathering resistant minerals and the elements associated with them (Fe, Mn, Cu, Co and Cr). These elements are also present as traces in the same type of Zn–Pb deposits in the dolostones of the Mississippi Valley (Leach et al. 1995). The influence of pyritic slate in the Serra do Garrote Formation and conglomerates cemented by iron oxides and hydroxides, located in Lagamar Formation, also explain high concentrations of these elements. According to Rose et al. (1979) Cu, Co, and Hg are associated with Zn–Pb deposits, explaining the accumulation on the BRSC6 and BRSC7 sites. These metals’ behavior is also related to Fe and Mn dynamics in aquatic systems. Comparing the samples of the Santa Catarina and Escuro Basins there is a positive correlation of Cr, and Cu with Fe (correlation coefficients of 0.71, and 0.67, respectively) and Co with Mn (correlation coefficient of 0.62). Haese (2006) describes how Fe and Mn oxides and hydroxides control the behavior of metals. The large surface area of oxides and the abundance of binding sites influence metals’ mobility and distribution. The oxides and hydroxides precipitate in circumneutral pH water, adding other metals by adsorption or occlusion of ions in the particles (Axe and Trivedi 2002, Kabata-Pendias and Pendias 2005).
The Mn and Fe oxides and hydroxides, the silt-clayey minerals, and the water circumneutral pH work as a geochemical barrier (Perel´man 1986) that has a significant impact on the metals’ mobility and transport throughout the studied basin. The basin geomorphology also influences metal dispersion. The alluviums that involve the Paracatu River and its tributaries’ lower courses work as a sediment deposition reservoir where the floodplains, terraces, and fluvial plain occur. The enrichment of metals is close to their sources and does not affect the composition of the main river of the basin. No significant metal accretion was observed in the upper Paracatu River main course where Zn, Pb, and Hg concentrations were close to the background values. The sediment mixture and dilution with other catchment systems located at the east side of the upper Paracatu River Basin also contributes to the low metal concentrations in the basin’s main course. These eastside rivers drain the Paraopeba Group and the Mata da Corda Formation geological units that did not present mineral deposits related to the elements studied here.
Conclusion
The geochemical and mineralogical composition of sediments in the upper Paracatu River Basin is described. Principal Component Analysis, the mineralogical data, and the geo-accumulation index permit distinguishing the influences of mineral deposits and human activities in the metal distribution and accumulation of the sediment. The main geochemical processes that control the metal behavior are pyrite oxidation, muscovite weathering, carbonate dissolution, and the erosion of oxisols enriched with Zn and Pb. The metal sources are basically from the geological mineral features, except Hg which also has an anthropogenic input and Al that is accumulated due increased mineral leaching, both caused by artisanal alluvial gold mining.
The primary minerals that form the basin riverbed sediment are quartz, illite, and kaolinite. Their presence in all sampling sites reflects the high weathering environment typical of the Brazilian Central Plateau. Quartz is a leach-resistant mineral and illite and kaolinite are fillossilicates originating from primary rock-mineral alteration. Gibbsite, goethite, and hematite are residual minerals of intensely weathered soils.
The tributaries have different sediment chemical compositions due the different geologic units that compose the catchment areas. The upper Rico Stream has Fe, Al, Mn, Cu, Cr, Co, and Hg accumulation due pyrite oxidation, erosion of oxisols, and anthropogenic activities related to the alluvial gold mining. The Escuro River and Santa Catarina Stream low courses have accumulation of Zn and Pb in sediments due the erosion of metal-rich soils formed over Zn–Pb deposits. The silt-clayey minerals, the Mn and Fe oxides and hydroxides, and the water circumneutral pH perform a key role in the distribution and transport of metals, working as a geochemical barrier that limits the spread of metals downstream. The basin geomorphology also contributes to metal accumulation in the tributaries’ lower courses. The accretion of these elements is located near their mineral deposits and anthropogenic sources, restricted to the Paracatu River’s tributaries. The river basin’s main course shows no critical metal enrichment, especially for Hg, Zn, and Pb.
References
Andrew-Jones DA (1968) The application of geochemical techniques to mineral exploration. Miner Ind Bull 2(6):1–31
Axe L, Trivedi P (2002) Intraparticle surface diffusion of metal contaminants and their attenuation in microporous amorphous Al, Fe and Mn oxides. J Colloid and Interface Sci 247:259–265
Banks D, Younger PL, Arnesen RT, Iversen ER, Banks SB (1997) Mine-water chemistry: the good, the bad and the ugly. Environ Geol 32(3):157–174
Burak DL, Fontes MPF, Santos NT, Monteiro LVS, Martins ES, Becquer T (2010) Geochemistry and spatial distribution of heavy metals in oxisols in a mineralized region of the Brazilian Central Plateau. Geoderma 160:131–142
Callaham JE, Miller JW, Craig JR (1994) Mercury pollution as a result of gold extraction in North Carolina, USA. Appl Geochem 9(2):235–241
Campos Neto MC (1984) Litoestratigrafia, relações estratigráficas e evolução paleogeográfica dos grupos Canastra e Paranoá (região de Vazante-Lagamar, MG). Rev Bras Geocienc 14(2):81–91
Chapman PM, Wang F (2000) Issues in ecological risk assessment of inorganic metals and metalloids. Hum Ecol Risk Assess 6:965–988
Dardenne MA (2000) The Brasília fold belt. In: Campos DA, Cordani UG, Thomaz Filho A (eds) Tectonic evolution of South America. International Geological Congress, Rio de Janeiro, pp 231–263
DNPM (1988) Principais depósitos minerais do Brasil. CPRM, Brasília
Drever JI (1988) The Geochemistry of natural waters, 2nd edn. Prentice Hall, New Jersey
Farnham IM, Johannesson KH, Singh AK, Hodge VF, Stetzenbach KJ (2003) Factor analytical approaches for evaluating groundwater trace element chemistry data. Anal Chim Acta 490:123–138
Fischer WR, Schwertman US (1974) The formation of hematite from amorphous iron(III) hydroxide. Clays Clay Miner 23:33–37
Fontes MPF, Gomes PC (2003) Simultaneous competitive adsorption of heavy metals by the mineral matrix of tropical soils. Appl Geochem 18:1143–1149
Förstner U (1983) Assessment of metal pollution in rivers and estuaries. In: Thornton I (ed) Applied environmental geochemistry. Academic Press, Londres, pp 395–423
Freitas-Silva FH, Dardenne MA (1992) Controles litoestruturais do depósito de ouro do Morro do Ouro, Paracatu, MG. Rev Esc Minas 45(3):216
Fuck RA, Jardim De Sá EF, Pimentel MM, Dardenne MA, Soares ACP (1993) As faixas de dobramento marginais do Craton do São Francisco. In: Dominguez JML, Misi A (eds) Sociedade Brasileira de Geologia
Grosbois CA, Horowitz AJ, Smith JJ, Elrick KA (2001) The effect of mining and related activities on the sediment trace element geochemistry of lake coeur D’Alene, Idaho, USA. Part III: the Spokane River Basin. Hydrol Process 15:855–875
Gruiz K, Muranyi A, Molnar M, Horvath B (1998) Risk assessment of heavy metal contamination in Danube sediments from Hungary. Water Sci Tech 37:273–281
Haese RR (2006) The biogeochemistry of iron. In: Schulz HD, Zabel M (eds) Maritme Geochemistry, 2nd edn, pp 241–270
Hudson-Edwards KA, Schell C, Macklin MG (1999) Mineralogy and geochemistry of alluvium contaminated by metal mining in the Rio Tinto area, southwest Spain Karen. Appl Geochem 14:1015–1030
Kabata-Pendias A, Pendias H (2005) Trace elements in soils and plants, 3rd edn. CRC Press, Boca Raton
Lacerda LD, Salomons W (1998) Mercury from gold and silver mining: a chemical time bomb?. Springer, Berlin
Lacerda LD, Pfeiffer WC, Marins RV, Rodrigues Filho S, Souza CMM, Bastos WR (1991) Mercury dispersal in water, sediments and aquatic biota of a gold mining tailing deposit drainage in Poconé Brazil. Water Air Soil Pollut 55:283–294
Leach DL, Viets JB, Foley-Ayuso N, Klein DP (1995) Mississippi Valley-Type Pb–Zn deposits (Models 32a,b; Briskey, 1986 a,b). In: Du Bray EA (ed) Preliminary compilation of descriptive geoenvironmental mineral deposit models: US Government Consulting Group, open file report 95–831, pp 234–243
Lee P, Yu H, Yun S, Mayer B (2005) Metal contamination and solid phase partitioning of metals in urban roadside sediments. Chemosphere 60:672–689
Lentz DR (2005) Mercury as a Lithogeochemical exploration vectoring technique: a review of methodologies and applications, with selected VMS case histories. Gangue 85:1–8
Licht OAB (1998) Prospecção geoquímica: princípios técnicas e métodos. Rio de Janeiro, CPRM
Luoma SN (1983) Bioavailability of trace metals to aquatic organisms: a review. Sci Total Env 28:1–22
Mackay AL (1960) Some aspects of the topochemistry of the iron oxides and hydroxides.In: 4th International Symposium React Solids, Amsterdam, pp 571–583
Malm O (1998) Gold mining as a source of mercury exposure in the Brazilian Amazon. Environm Res 77:73–78
Malm O, Pfeiffer WC, Souza CMM, Reuther A (1990) Mercury pollution due to gold mining in the Madeira River basin, Brazil. Ambio 1:11–15
Monteiro LVS (2002) Modelamento metalogenico dos depósitos de zinco de Vazante, Fagundes e Ambrósia, associados ao Grupo Vazante, Minas Gerais. PhD Thesis, Universidade de São Paulo
Morse JW, Arvidson RS (2002) The dissolution kinetics of major sedimentary carbonate minerals. Earth Sci Rev 58:51–84
Olias M, Nieto JM, Sarmiento AM, Cerón JC, Cánovas CR (2004) Seasonal water quality variations in a river affected by acid mine drainage: the Odiel River (South West Spain). Sci Total Env 333:267–281
Perel´man AI (1986) Geochemical barriers: theory and practical applications. Appl Geochem 1:669–680
Plant J, Smith D, Smith B, Williams L (2001) Environmental geochemistry at the global scale. Appl Geochem 16:1291–1308
Power EA, Chapman PM (1992) Assessing sediment quality In: Sediment Toxicity Assessment. Lewis Publishers, Boca Raton, pp 1–18
Rodrigues Filho S, Maddock JEL (1997) Mercury pollution in two gold mining areas of the Brazilian Amazon. J Geochem Explor 58:231–240
Rose AW, Hawkes HE, Webb JS (1979) Geochemistry in mineral exploration. Academic Press, New York
Salomons W (1995) Environmental impact of metals derived from mining activites: processes, predictions, prevention. J Geochem Explor 52:5–23
Starostin VI, Yapaskurt OV (2007) Aspects of genetic formational typification of metalliferous higly carbonaceous sedimentary complexes. Mosc Univ Geol Bull 62:131–142
Svete P, Milacic R, Pihlarb B (2001) Partitioning of Zn, Pb and Cd in river sediments from a lead and zinc mining area using the BCR three-step sequential extraction procedure. J Environ Monit 3:586–590
Thornton I (1993) Environmental geochemistry and health in the 1990s: a global perspective. Appl Geochem 2:203–210
Thornton I (1996) Impacts of mining on the environment; some local, regional and global issues. Appl Geochem 11:355–361
Turekian KK, Wedepohl KH (1961) Distribution of the elements in some major units of the earth’s crust. Bull Geol Soc Am 72:175–192
Valeriano CM, Dardenne MA, Fonseca MA, Simões LS, Seer HJ (2004) A evolução tectônica da Faixa Brasília. In: Bartorelli A, de Brito BB, Carneiro CDR, Neto VM (eds) Geologia do Continente Sul Americano: Evolução da Obra de Fernando Flavio Marques de Almeida. Beca, São Paulo, pp 355–368
Voudouris K, Lambrakis N, Papatheodorou G, Daskalaki P (1997) An application of factor analysis for the study of the hydrogeological conditions in plio-pleistocene aquifers of NW achaia (NW Peloponnesus, Greece). Math Geol 29(1):43–59
Williams EG (1982) Factors controlling the generation of acid mine drainage. Report to the United States Bureau of Mines
Windmöller CC, Santos RC, Athayde M, Palmieri HEL (2007) Distribuição e especiação de mercúrio em sedimentos de áreas de garimpo de ouro do Quadrilátero Ferrífero (MG). Quim Nova 30(5):1088–1094
Acknowledgments
This work was supported by CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico) that provided the project financial support and a Masters scholarship (CNPq-Process 555953-06-08). We thank Dr. Luis Fabrício Zara from Atomic Spectroscopy Laboratory of Catholic University of Brasilia and Dr. Olaf Malm from Radio Isotope Laboratory of Federal University of Rio de Janeiro for Pb and Hg measurements support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mulholland, D.S., Boaventura, G.R. & Araújo, D.F. Geological and anthropogenic influences on sediment metal composition in the upper Paracatu River Basin, Brazil. Environ Earth Sci 67, 1307–1317 (2012). https://doi.org/10.1007/s12665-012-1574-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12665-012-1574-6