Abstract
This study examined particulate phosphorus (PP) characteristics of terrestrial, stream bed and lake bed sediments along a common hydrological pathway. The objective was to quantify spatial variability of PP in the catchment of a eutrophic lake, Rotorua (New Zealand). Median total phosphorus (TP; dry weight) concentration in sediments (‘terrestrial’) deposited from overland flow on farmland in the upper stream catchment was high (2,157 mg kg−1), greatly exceeding that of stream bed sediments (212 mg kg−1; Waiteti Stream) which exhibited downstream decline in TP concentrations. Lake bed sediments were enriched in TP (median = 2,661 mg kg−1) with relatively low Fe:P and Al:P mass ratios. Sequential extraction of three PP fractions showed that the mean proportion of HCl-extracted P (Ca-bound, denoting the refractory fraction) in extracted P was higher in terrestrial sediments (17 %) than stream bed sediments (4 %). Accordingly, TP concentrations of terrestrial sediments were highly (p < 0.01) correlated with Ca (R = 0.89), whereas TP was most closely correlated with Al (R = 0.83) in the stream bed sediments. Nonetheless, the majority of sequentially extracted P in both terrestrial and stream bed sediments comprised fractions (bicarbonate dithionate- and NaOH-extractable) that are potentially bioavailable in Lake Rotorua. Furthermore, phosphate buffering experiments indicated that terrestrial sediments desorbed P to be a source of PO4–P to receiving waters, whereas stream bed sediments buffered PO4–P concentrations in stream water. Such quantification of landscape-scale variability in PP characteristics can support strategic management of TP loading to the lake.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Controlling phosphorus (P) loads to freshwaters is critical to abate water quality decline associated with eutrophication (Vollenweider 1968; Moss 2007). Phosphorus loads comprise a dissolved and solid phase, with the former generally considered to be bioavailable (Reynolds and Davies 2001) and hence a priority for control (Lewis et al. 2011). By contrast, the chemical composition of the particulate phosphorus (PP) pool can be complex and, consequently, the bioavailability and concomitant potential of PP to contribute to eutrophication can vary significantly (Reynolds and Davies 2001). Particulate P frequently comprises a major proportion of the P load to freshwaters and, therefore, catchment-specific knowledge of PP characteristics is necessary for resource managers to adequately assess eutrophication risk and develop efficient eutrophication control strategies (Ellison and Brett 2006; Kerr et al. 2011).
The composition of PP can be quantified using chemical extraction methods that isolate individual fractions (e.g. see Hupfer et al. 1995). Such methods use a range of extraction solutions which typically contain anions that displace phosphate from adsorption sites associated with increasingly refractory compounds. Determination of phosphate in the extractants subsequently allows different chemical forms of PP to be quantified which, although operationally defined, can be related to biogeochemical origins (Lukkari et al. 2007a) and bioavailability to phytoplankton (Boström et al. 1988; Okubo et al. 2012). Particulate P is subject to dynamic sorption processes between sediments and the aqueous phase (Mayer and Gloss 1980) and such sorption behaviour is also an important consideration for understanding the potential for PP to contribute to eutrophication (Machesky et al. 2010). The P sorption behaviour of sediments is related to ambient conditions, including PO4–P and total suspended sediment (TSS) concentrations, pH, oxidation–reduction potential, and ionic concentration (Mayer and Gloss 1980; McDowell et al. 2001; Uusitalo et al. 2003). Hence, as with assessing the bioavailability of sequentially extracted PP fractions, relating the P sorption characteristics of sediments to the potential for downstream impacts requires understanding of the biogeochemical characteristics of receiving waters.
The load of PP transported downstream through catchments is variable in space and time, reflecting the heterogeneous distribution of critical source areas and the importance of episodic rain events for driving PP delivery (Edwards and Withers 2008; Abell et al. 2013). A host of biological, chemical and physical processes can interact to influence the chemical composition of the PP pool, for example: mineralisation of organic PP (McDowell et al. 2001); dynamic sorption reactions (Mayer and Gloss 1980) and; selective erosion and associated winnowing of different sized sediments (Kerr et al. 2011). Hence, although the value of adopting a catchment-scale perspective to diffuse pollution control is well recognised (e.g. Moss 2007), the potential complexity surrounding variability of PP composition in catchments can constrain efficient design of control measures. When investigating PP cycling in catchments, it is important to consider stream bed sediments, in addition to potential source areas on land. Stream bed sediments can be a major source of PP for downstream transport (House 2003) and their overall contribution to the suspended sediment load is particularly high for streams with sand-dominated beds, low gradients and rainfall-driven supply processes (Salant et al. 2008). Stream bed sediments can also have an important role in regulating P in the overlying water column, dependent on the physicochemical properties of both the sediments (e.g. particle size) and the overlying water (e.g. ambient PO4–P concentration) (McDowell et al. 2001; Evans et al. 2004).
This study examined PP characteristics in a medium-sized agricultural catchment that drains into large and nationally iconic Lake Rotorua in New Zealand. Analysis is presented relating to sediment samples collected along a hydrological gradient that encompassed sites in terrestrial source areas on farmland in the upper catchment, ephemeral stream channels, permanent stream channels and the receiving lake. The aims of this study were to examine how PP characteristics vary downstream along a hydrological gradient and whether such information about PP characteristics can inform management actions to control P transport to the receiving lake.
Methods
Study site characteristics
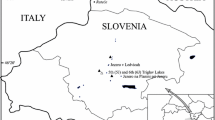
The Waiteti Stream drains a major surface sub-catchment (71 km2) of Lake Rotorua which is a large (81 km2) polymictic lake in the Bay of Plenty region in the central North Island, New Zealand (Fig. 1). Lake Rotorua is eutrophic (e.g. median TP concentration = 0.031 mg L−1; Fig. 2) and a national priority for remediation (PCE 2006). Agricultural land is the largest source of external P load to the lake and long-term changes to land management have been identified as necessary to achieve external P load reduction targets (ibid).
Summary of total phosphorus (TP) and PO4–P concentrations measured in Lake Rotorua (2001–2012, n = 235) and the Waiteti Stream (2002–2010, n = 103) during state of the environment monitoring. Lake Rotorua was sampled at two central lake sites and the Waiteti Stream was sampled at site S2 (see Fig. 1). Symbols denote median values, boxes denote 25th and 75th percentiles and whiskers denote 10th and 90th percentiles. Data courtesy of Paul Scholes, Bay of Plenty Regional Council
The Waiteti Stream has mean discharge of approximately 1.4 m3 s−1 and is the sixth largest of nine surface stream inflows which collectively contribute about two-thirds of the hydraulic load to the lake (Hoare 1980). The stream catchment is hilly, predominantly comprises pastoral agriculture, and is located in one of the wettest areas of the lake catchment (mean annual rainfall ≈ 2,000 mm; ibid). The climatic, topographic and land use characteristics mean that the Waiteti Stream catchment contains numerous critical source areas for phosphorus loss and it has been identified as a primary candidate for the focus of research necessary to improve understanding of surface phosphorus fluxes to Lake Rotorua (Clarke et al. 2013). Soils comprise deep, porous sands and loams that are high in allophane (Newsome et al. 2000). Grazing is excluded from the stream banks which were mostly planted with native vegetation [predominantly flax (Phormium tenax) and native shrubs (e.g. Leptospermum scoparium)] in the 1980s (Williamson et al. 1996). Sections of the upper and middle reaches flow through steep gorges and there is an extensive network of ephemeral stream channels on farmland in the upper catchment. As with other local streams (see Abell et al. 2013), concentrations of PO4–P (median = 0.040 mg L−1; Fig. 2) are relatively consistent, whereas TP concentrations are more variable and correlate positively with discharge. Mean pH measured during monthly water quality monitoring during 2000–2010 was 7.0 (standard deviation = 0.2) and mean specific conductance was 80 μS cm−1 (standard deviation = 9.0 μS cm−1).
Sampling methods: terrestrial sediments
Synthetic grass mats (sensu Owens et al. 2007) were used to sample suspended sediments transported in overland flow (termed ‘terrestrial’ hereafter) across agricultural land in the upper catchment. The mats were placed upstream of earth detainment bunds that were constructed across ephemeral stream channels as part of catchment management works undertaken to attenuate the loss of PP from pastoral farms. Bunds were specifically designed to pond and then slowly release overland flow, thus promoting settling of suspended sediments onto pasture (Clarke et al. 2013). Such sediments represent a vector for PP which, in the absence of a detainment bund, would potentially contribute to the external PP load to the Waiteti Stream and Lake Rotorua. Mats were placed upstream of detainment bunds at two sites: a ‘pastoral site’ located on grazed paddocks on a dairy farm and an ‘arable site’ which was located on grazed paddocks sited c. 250 m from an arable field sown with a winter forage crop (Fig. 1). The arable field was bisected by the ephemeral stream channel upstream of the detainment bund and was identified as an important critical source area of sediment owing to the direct ephemeral hydrological connection between bare soil and downstream waters. The arable site was considered representative of similar arable land upstream of the Waiteti Stream, although it was situated c. 1 km to the north of the Waiteti Stream surface catchment (Fig. 1). The detainment bunds were c. 2 m high and 55 m (arable site) to 109 m (pastoral site) long.
Prior to forecasted rainfall, clean mats (45 cm × 50 cm) were secured to the ground with wire pins in the ponding area upstream of the detainment bunds. Twenty-two mats were deployed at the pastoral site and 14 mats were deployed at the arable site. Mats were placed 2–60 m perpendicularly from the bund and at elevations of 0–0.7 m above the bottom of the ponding area. Once ponded water had receded after the rainfall event (c. 1–3 d), mats were placed in clean plastic bags and transported to the laboratory where the mats and deposited sediments were dried (60 °C, 24 h). Mats were then shaken to remove deposited sediments which were then stored in clean, airtight plastic bags prior to analyses. Sediment samples were collected following three storms (March, May and July 2012) at the pastoral site and following one storm (July 2012) at the arable site.
Sampling methods: stream bed sediments
Samples of stream bed sediments were collected at twelve sites along the Waiteti Stream and tributaries during 4 days in December 2012 (Table 1). Sites were chosen that encompassed a range of lotic environments that included dry ephemeral tributary stream channels in the upper catchment as well as the lower reaches of the main Waiteti Stream channel, including one site 20 m upstream of Lake Rotorua. Either a sediment corer or a Ponar dredge (see Table 1) was used to collect three samples of surface (0–10 cm) sediments at each site. Samples were collected from different locations within the stream channel at each site to permit consideration of intra-site variability.
Sampling methods: lake bed sediments
Sediment cores were collected during August 2012 at 15 sites along a north–south transect through the lake. The transect bisected the deepest part of the lake and most sites had depth (z) >15 m. The sites were chosen to represent a range of depositional zones in the lake.
The cores were collected using a Swedish gravity corer (Pylonex HTH 70 mm) with a 60 × 600 mm Perspex (Plexiglas) core barrel to capture undisturbed sediments along with c. 20 cm of the overlying water. Once the core was retrieved, a custom-made, gas-tight sampling chamber designed to minimise exposure of potentially anoxic sediment to the air, was fitted to the core barrel and the core was extruded by a piston from the base of the core. Excess supernatant water overflowed from the top of the core upon extrusion until the sediment–water interface was reached at the lip of the barrel. The upper 2 cm of sediment was extruded and transferred into a 50-mL polypropylene centrifuge tube.
Laboratory analysis: ICP-MS
Dried terrestrial, stream bed and lake bed sediments were analysed using inductively coupled plasma mass spectrometry (ICP-MS) to determine dry weight concentrations of total phosphorus (TP) and the following key elements involved in P cycling: Fe, Al, Mn and Ca (Reynolds and Davies 2001). Sample preparation was based on a standard procedure (Martin et al. 1994). Dried sediments were first ground with a pestle and mortar and then digested overnight with Aqua Regia (3:1 v:v of 1:5 conc. HCl and 1:2 conc. HNO3) in polypropylene tubes. Tubes were then placed in a water bath at 80 °C for 1 h before a 12 mL sub-sample was filtered (0.45 µm, Whatman GF/F) and analysed (ICP-MS model ELAN DRC II; Perkin–Elmer SCIEX). Concentrations of Al exceeded the detection limit (3,000 mg kg−1) in nine of the 14 terrestrial sediment samples. Absolute concentrations of this element are not reported although concentrations of Al were set to the detection limit for these nine samples for calculation of Al:P. Such values of Al:P will, therefore, be underestimated but it was considered desirable to retain them for data completeness and the associated error introduced to statistical tests (Principal Components Analysis and non-parametric Spearman rank order correlation) was deemed acceptable.
Laboratory analysis: sequential phosphorus extraction
A sequential phosphorus extraction scheme based on the method described by Psenner et al. (1984) and modified by Hupfer et al. (1995) was used to quantify three operationally defined phosphorus fractions in samples of terrestrial and stream bed sediments. Sediments from the following stream sites were chosen for sequential P extraction: S1, S2, S4, S6, S8, S9, S10, and S11 (see Fig. 1). For each of these stream sites, sediments in one of the three sub-samples were analysed. Sediments collected from the arable site during one storm in July 2012 and the pastoral site during storms in May and July 2012 were analysed separately. The pastoral sediments were separated into ‘lower basin’ (sediments collected using mats positioned 0–200 mm vertically from the base of the sedimentation basin) and ‘upper basin’ (sediments collected using mats positioned 200–700 mm vertically from the base) samples to account for the potential for variability in sediment characteristics between different elevations in this large sedimentation basin. Thus, five separate samples of terrestrial sediments were used for sequential P extraction analysis.
The sequential extraction was as follows:
-
1.
BD–P: bicarbonate dithionate (0. 11 M Na2S2O2 and 0.11 M NaHCO3 for 1 h) to remove redox-sensitive P. This predominantly comprises P bound to Fe hydroxides and Mn compounds (Lukkari et al. 2007a).
-
2.
NaOH–P: sodium hydroxide (0.1 M NaOH for 16 h) to remove organic forms of P and P bound to oxides of Al and Fe that are non-redox sensitive.
-
3.
HCl–P: hydrochloric acid (0.5 M HCl for 16 h) to remove Ca-bound P and apatite P.
For each sample, 0.5 g of sediment was analysed in triplicate. At each step, 40 mL of extractant was added to the sediment in 50-mL capacity polypropylene tubes which were placed horizontally on a shaker plate (150 rpm). After the requisite extraction time, tubes were centrifuged (3,000 rpm, 10 min) and the supernatant decanted and filtered (Whatman GF/F, 0.45 µm) prior to PO4–P determination. For extraction steps 2 and 3, PO4–P was determined by colorimetric analysis using a standard molybdenum-blue method (Murphy and Riley 1962; APHA 1998) with a Lachat QuickChem flow injection analyser (Zellweger Analytics Inc.). The bicarbonate dithionate extractant interfered with the colorimetric method hence PO4–P in extraction step 1 was determined using ICP-MS (see above) to detect TP, following acidification with HNO3. This method may have overestimated PO4–P slightly due to detection of colloidal-bound P. Although drying has been shown to potentially alter the PP composition of sediments, e.g. due to oxidation (Lukkari et al. 2007b), the use of dried sediments was considered appropriate in this study because periodic wetting and drying occur naturally for both sets of sediments, and analysis of dried sediments provides greater precision than the use of wet sediments.
Laboratory analysis: phosphate buffer experiments
Phosphate buffer experiments were undertaken to investigate the phosphate buffering characteristics of sampled sediments. Sediments from the following sites were selected for this analysis: S1 (at the stream mouth), S9 (mid-reaches) and the pastoral site (a combined sample of sediments collected during storms in May and July 2012). The experiments were based on the method developed by Nair et al. (1984) and involved adding sediments to a range of PO4–P standards, allowing an assumed equilibrium to be reached between sediments and the solutions, and then measuring PO4–P in the treatments to determine change in PO4–P concentration (ΔPO4–P).
Experiments were conducted in 60-mL capacity centrifuge tubes using 40 mL of standard solution. For each site, 4 g of sediment was added in duplicate to the following eight PO4–P (as KH2PO4) standards in 0.01 M CaCl2 solution: 0, 0.0005, 0.0025, 0.005, 0.025, 0.05, 0.25 and 1 mg P L−1. Samples were then placed on an orbital shaker plate for 14 h (150 rpm) before they were filtered (Whatman GF, 0.45 µm) and analysed for PO4–P using flow injection analysis (see above). Samples from the pastoral site (higher fine sediment component) were centrifuged (4 min, 3,000 rpm) prior to filtration of the supernatant.
Equilibrium P concentration (EPC0) was determined for each site in accordance with Bridgham et al. (2001). Accordingly, ΔPO4–P was regressed on initial solution PO4–P concentration and the value for which ΔPO4–P = 0 determined as the x-axis intercept (±regression standard error). Consequently, the EPC0 represents the concentration at which sediments display maximum buffering capacity and there is no net adsorption or desorption of PO4–P from the sediments.
Laboratory analysis: particle size analysis
Particle size distribution of terrestrial and stream bed sediments was analysed using laser diffraction (Mastersizer 2000, Malvern Instruments) to quantify the volume of sediment corresponding to 48 size classes in the range 0.05–2,000 μm. These data were used to calculate values of two particle size distribution metrics: (1) the median particle size (D 50); and (2) the percentage of particles with a diameter <63 μm, corresponding to the clay and silt fractions (Wentworth 1922). Stream bed sediments comprised larger particles and were first sieved to remove sediments >2 mm.
Data analysis
Spearman rank order correlation analysis was undertaken to examine relationships between physicochemical properties of sediments and concentrations of both TP and sequentially extracted P fractions. Principal Component Analysis (PCA) was used to examine inter-site differences in the composition of the sampled sediments. Specifically, variation was examined in the TP concentration and the following elemental ratios (by mass): Al:P, Ca:P, Fe:P and Mn:P. PCA is a multivariate exploratory technique that seeks to describe relationships in datasets comprising numerous inter-correlated independent variables using a reduced number of orthogonal (uncorrelated) principal components. Prior to this analysis, data were standardised by subtracting the mean and dividing by the standard deviation to reduce data to a common scale. Statistical analyses were undertaken using Statistica (version 11; Statsoft, Tulsa) and a significance threshold of p < 0.05 assumed.
Results
Between-site variability in total phosphorus concentrations and relationships with sediment characteristics
Total P concentrations differed markedly between terrestrial, stream and lake bed sites (Table 2). Concentrations were highest in the lake bed sediments (median = 2,661 mg kg−1), followed by the terrestrial sediments (median = 2,157 mg kg−1) and lowest in the stream bed sediments (median = 212 mg kg−1). Among the stream sites, there was a trend of increasing TP concentration with distance upstream from the lake [Spearman rank order correlation coefficient (R) = 0.55; Fig. 3] with the highest concentrations measured at ephemeral sites. For stream bed sediments, Al:P was positively correlated (R = 0.42) with distance upstream from the lake, while Fe:P was negatively correlated (R = −0.44) with this variable (Fig. 3). Note that these trends are within the context of marked within-site variability at some sites (Fig. 3), reflecting the range of stream geomorphic zones that were sampled. For TP, the mean coefficient of variation for the three sites was 23 % (range 3–68 %).
For terrestrial sediments, there were statistically significant positive correlations between concentrations of TP and both Ca (R = 0.89) and Mn (R = 0.58) (Table 3). In contrast, TP concentrations of stream bed sediments were most strongly positively correlated with Al concentrations (R = 0.83). For stream bed sediments, there was also moderate positive correlation between TP concentrations and concentrations of both Ca (R = 0.50) and Mn (R = 0.39), although these results at least partly reflect inter-correlation between Al, Ca and Mn (note higher correlation coefficients between these elements and Al than for TP; Table 3). Total P concentrations of stream bed sediments were positively correlated with organic matter (R = 0.73; analyte not determined for other sites) although again this at least partly reflects inter-correlation with Al. The TP concentrations of lake bed sediments were not significantly correlated with concentrations of any of the elements that were considered.
Stream bed sediments were generally coarser than the terrestrial sediments, e.g. median D 50 for the stream bed sediments was 525 μm (range = 60–975 μm) compared with 93 μm (range = 53–475 μm) for the terrestrial sediments (Table 2; D 50 not determined for lake bed sediments). There was no significant correlation between TP concentrations and D 50 for either terrestrial or stream bed sediments, although the concentration of TP in the stream bed sediments was weakly positively correlated with the percentage of fine (<63 μm) sediments (R = 0.46). This size fraction was also positively correlated with concentrations of Ca (R = 0.40) and Al (R = 0.56).
Results of PCA further elucidated between-site variability in the PP characteristics by quantifying the influence of independent variables in explaining variability (Table 4; Fig. 4). Only principal components (PCs) 1 and 2 had Eigenvalues (E) ≥1 and hence only those PCs were considered (Legendre and Legendre 1998). Principal component 1 (E = 3.1) accounted for 62 % of variability in the data and PC 2 (E = 1.0) accounted for 20 % of the variability. Factor loadings indicated that PC 1 was most strongly correlated (negatively) with TP concentration and Fe:P, while PC 2 was most strongly correlated (positively) with Ca:P and Al:P (Table 4; Fig. 4a). Consequently, terrestrial sediments are represented in the upper left (i.e. negative PC 1 and positive PC 2 values) quadrant, reflecting high TP concentration and higher Ca:P (median = 5.4). Stream bed sediments predominantly had positive values for PC 1, reflecting low TP concentrations (Figs. 3, 4). Among stream bed sediments, distance upstream and PC 1 values were negatively correlated (R = −0.64, p < 0.05), reflecting the general trend of increasing TP concentration and Al:P with distance upstream. Lake bed sediments are represented in the lower left (i.e. negative values for both PCs) quadrant of the factor plane, reflecting high TP concentration and relatively low Fe:P (median = 7.5) and Al:P (median = 5.4). Absolute values for both PCs 1 and 2 were significantly different between the three groups of sediments (one-way ANOVA, p < 0.05).
Sequentially extracted phosphorus fractions
For both stream bed and terrestrial sediments, NaOH–P was the dominant of the three sequentially extracted fractions, comprising 48–77 % (mean = 63 %) of sequentially extracted P for the stream bed sediments and 59–67 % (mean = 62 %) for the terrestrial sediments. BD–P was typically the second dominant fraction, comprising 20–46 % (mean = 33 %) of sequentially extracted P for the stream bed sediments and 10–25 % (mean = 21 %) for the terrestrial sediments. HCl–P comprised the smallest proportion of sequentially extracted P although this proportion was higher for terrestrial sediments (range = 15–22 %, mean = 17 %) than stream bed sediments (range = 2–6 %, mean = 4 %). For stream bed sediments, the above-mentioned positive correlation between Al:P and distance from the stream mouth was consistent with higher concentrations of NaOH–P measured at upstream sites (S9, S10 and S11) relative to stream sites further downstream (Fig. 5). The composition of sequentially extracted P was relatively consistent between the samples of terrestrial sediments, despite the fact that the samples were collected at two different sites and, in the case of the pastoral site, following two different storm events and at different elevations in the sedimentation basins.
Concentrations of sequentially extracted phosphorus fractions in stream bed and terrestrial sediments. Locations of stream sites and both pastoral (P) and arable (A) terrestrial sites are shown in Fig. 1. For the pastoral site, separate samples were analysed of sediments sampled following two storms (May 2012, ‘M’; June 2012, ‘J’) and at different elevations within the sedimentation basin (lower, ‘1’; upper, ‘2’) (see “Methods”)
Note that the sum of the sequentially extracted P fractions was typically lower than the TP concentrations determined using ICP-MS. Concentrations of residual P (i.e. TP minus sequentially extracted P) are not reported and this fraction is not necessarily comparable to the other three fractions as TP determination was undertaken using separate sub-samples to those used for sequential extraction. It was noted, however, that such residual P was slightly less than the sum of sequentially extracted P for four of the 13 samples (one stream bed sample and three terrestrial samples; residual P for these samples = −114 to −15 mg P kg−1). This may reflect the above-mentioned difference in the material analysed or, potentially, some minor ‘carryover’ of P between extracts, as has been noted in other studies (Condron and Newman 2011).
The results of Spearman correlation analysis of relationships between the relative proportion of the three sequentially extracted P fractions and sediment size metrics of sediment samples showed a significant relationship only for the HCl–P fraction. Specifically, there was indication that finer sediments comprised a higher relative proportion of HCl–P as this variable was positively correlated with the percentage of fine (<63 μm) sediments (R = 0.61) and negatively correlated with D 50 (R = −0.65). This result reflected the higher relative proportion of HCl–P in the finer textured terrestrial sediments; the relationship was not present when only the stream bed sediments were analysed.
Phosphate buffer experiments
Relationships between the initial PO4–P concentration and measured ΔPO4–P were linear for the range of initial PO4–P concentrations that was considered, indicating that the buffering capacity of the sediments was high. Standard deviation of ΔPO4–P measured for replicate samples was on average 29 % of the mean final PO4–P concentration measured after the incubation period. The EPC0 determined for the stream bed sediments was comparable (0.025 compared to 0.022 mg P L−1; Fig. 6) indicating that these sediments had similar sorption characteristics, despite one site (S9) being c. 6.7 km upstream of the other (S1). By contrast, the EPC0 determined for the terrestrial sediments was much higher (0.140 mg P L−1; Fig. 6) and in excess of the range of PO4–P concentrations measured in the Waiteti Stream (Fig. 2).
Results of PO4–P buffer experiments conducted using stream bed sediments from two stream sites and terrestrial sediments from the pastoral site (see Fig. 1). Equilibrium phosphorus concentration (EPC0) was defined as x-axis intercept ± regression standard error. Thus, EPC0 represents the initial PO4–P concentration at which no net adsorption or desorption of PO4–P occurs from the sediments. Experiments were conducted using six initial PO4–P concentrations (see “Methods”) and vertical bars denote standard deviation of duplicates
Discussion
Spatial variability in PP characteristics along the hydrological gradient
The TP concentrations of terrestrial sediments were high, e.g. median TP concentration exceeded the TP concentration of 23 of a diverse range of 24 New Zealand pasture soils (mean = 937 mg kg−1, range = 116–2,746 mg kg−1) studied by McDowell et al. (2005). Higher concentrations of TP in terrestrial as compared to stream bed sediments reflect relative P enrichment of the terrestrial sediments (e.g. from agricultural sources and geological sources), as well as potential preferential downstream transport of the most mobile stream sediments that are high in TP (Evans et al. 2004). Lakes are typically long-term sinks for P (Vollenweider 1968) and the high TP concentrations in lake bed sediments relative to the contributing terrestrial soils that were sampled are indicative of selective retention of PP arising from the efflux of C and N in sediments during mineralisation and diagenesis in the lake (Hecky et al. 1993). The TP concentrations of lake bed sediments were comparable to local eutrophic lakes (Trolle et al. 2008), although they were relatively high compared to eutrophic lakes elsewhere. For example, median TP concentration (2,661 mg kg−1; Table 2) exceeds the sediment TP concentration in three of the six shallow eutrophic Danish lakes (range = 740–4,100 mg kg−1) studied by Søndergaard et al. (2013) and is nearly five times greater than the mean TP concentration (mean = 560 mg kg−1, range = 295–1,322 mg kg−1) of surface sediments reported for Lake Taihu (China) which is renowned for its eutrophication problem (Liu et al. 2013). It is important to note, however, that while measurements of TP concentrations in lake bed sediments provide context for the interpretation of data for terrestrial and stream bed sediments, the lake bed sediments integrate chemical characteristics of a wide range of other contributing sources aside from the Waiteti Stream catchment, including the influence of historic inputs of treated waste water which occurred for some decades until 1991 [PCE (Parliamentary Commissioner for the Environment) 2006].
The main difference in P fractionation between the terrestrial and stream bed sediments was the higher contribution of HCl–P to the sequentially extracted P pool in terrestrial sediments. The HCl extraction removes Ca-bound P that is mainly associated with apatite minerals (Lukkari et al. 2007a), as evidenced by the higher positive correlation between Ca and TP concentrations for the terrestrial sediments. This result may indicate preferential downstream transport of non-apatite forms of PP, as found by Kerr et al. (2011) who undertook a longitudinal survey in an Australian sub-tropical catchment and found evidence for the selective erosion and downstream export of fine (<63 µm) sediments that were enriched in more labile forms of P. The relative proportion (range = 2–22 %, mean = 9 %) of HCl–P in all sediments in this study was still, however, relatively low. For example, a study of sediments in a New York (USA) river found HCl–P to comprise 27–96 % (mean = 57 %, n = 30) of TP (Wang and Pant 2011), whereas a study of sediments from farmland in the catchment of Chesapeake Bay (USA) reported a range of 48–85 % (mean = 62 %, n = 4; McDowell and Sharpley 2003). The majority of the sequentially extracted P was, therefore, present in either the BD–P or NaOH–P fractions which have been shown to be conditionally bioavailable to phytoplankton (e.g. Boström et al. 1988; Okubo et al. 2012). The BD–P represents redox-sensitive P predominantly bound to Fe and Mn (Lukkari et al. 2007a) and can, therefore, be released from lake bed sediments (at least temporarily) during periods of hypolimnetic anoxia which can occur in polymictic Lake Rotorua during extended (e.g. 1 month) stratified periods in summer (Burger et al. 2007). The potential bioavailability of NaOH–P in the lake is more uncertain as this fraction primarily represents P bound to Al oxides, non-reducible Fe oxides and organic P (Lukkari et al. 2007a). Thus, variable composition of this fraction has meant that laboratory bioassays have measured a range in the extent of NaOH–P utilisation by P-limited phytoplankton (reviewed by Boström et al. 1988). Given the high Al concentrations (terrestrial sediments) and strong correlation between Al and P (stream bed sediments), it is plausible that much of this fraction represents Al-bound P in the sediments that were sampled. Aluminium-bound P is presumed not to be readily bioavailable to phytoplankton (Reynolds and Davies 2001), except perhaps under high pH conditions that may be associated with phytoplankton blooms (Gao et al. 2012). There is some uncertainty, however, with this inference as both Al and P concentrations were also correlated with the proportion of fine sediments in stream bed samples (Table 3) and it is possible that a significant proportion of the NaOH–P fraction may reflect P associated with fine organic particles.
Comparison of the range of PO4–P concentrations measured in receiving waters (Fig. 2) with EPC0 determined for the terrestrial and stream bed sediments (Fig. 6) provides insight into the potential for P sorption processes to regulate dissolved P flux to Lake Rotorua. The high EPC0 of terrestrial sediments relative to the ambient PO4–P concentrations in the stream indicates that PO4–P will readily desorb from such sediments. Such desorption is a two-step process that comprises an initial fast step (minutes–hours, 50–90 % of reaction) and a second step with slow kinetics (days–months; Froelich 1988). By contrast, the EPC0 of the stream bed sediments (0.022–0.025 mg L−1) was much lower and less than the median PO4–P concentration of the Waiteti Stream (0.039 mg L−1; Fig. 2), thus suggesting that stream bed sediments typically have a buffering effect on water column PO4–P concentrations in the stream. This conclusion is consistent with other studies in the lake catchment which have noted apparent adsorption of bioavailable P to stream sediments during bioassay experiments (Abell and Hamilton 2013), as well as relative consistency of PO4–P concentrations in tributary streams during high discharge, interpreted as evidence of buffering by stream sediments to maintain a semi-steady state PO4–P concentration (Abell et al. 2013). Elsewhere, the EPC0 of stream bed sediments and PO4–P concentration of stream water have been shown to correspond closely, thereby indicating that stream bed sediments can have a role in modulating ambient stream water PO4–P concentrations (Machesky et al. 2010).
Differences in EPC0 are consistent with the much lower Fe:P and Mn:P in terrestrial relative to stream bed sediments (Fig. 4). In particular, Fe:P has been shown to reflect the capability of aerobic sediments to regulate P release as it provides a measure of the availability of orthophosphate adsorption sites on iron oxide and oxyhydroxide surfaces (Jensen et al. 1992). Measured Fe:P in the terrestrial sediments (mean = 5.2:1) was considerably lower than both the stream bed sediments (mean = 25.6:1) and the range of 10–15:1 that has been proposed as sufficient to promote effective binding of P by iron species (Søndergaard et al. 2003). This supports the conclusion that the terrestrial sediments represent a source of bioavailable P to downstream waters. Likewise, relatively low Fe:P in lake sediments (mean = 8.1:1) emphasises that the availability of Fe compounds in lake sediments is insufficient to effectively bind PO4–P during oxic conditions in the lake.
Implications for environmental management
This study highlights the potential for sediments transported in overland flow from farmland in the upper catchment of Lake Rotorua to contribute to downstream eutrophication. Given the often large disparity between the costs of controlling agricultural nutrient pollution at source and downstream in the lake, it is both environmentally and economically prudent to actively control export of this sediment downstream. There are numerous measures that, when implemented as part of a strategic landscape-scale approach, could attenuate sediment export; examples include installation of vegetative buffers and silt traps in ephemeral stream channels (McDowell and Nash 2012). While a programme of riparian planting has been undertaken in the catchment, such linear buffer features have been shown to be susceptible to low efficacy during sustained periods of overland flow due to the creation of ‘breakpoints’ where buffers are breached (Owens et al. 2007). Given the high rainfall and steep topography of parts of the upper stream catchment, wider implementation of detainment bunds, which both trap sediment and reduce overland flow downstream, therefore holds promise for effectively reducing PP export without substantially reducing pastoral productivity (Clarke et al. 2013).
Distinct differences in sediment P characteristics between broad categories of source area have been observed elsewhere (Wang and Li 2010) and suggest that further research in the catchment to characterise PP in sediments from different origins (e.g. stream banks, forested gullies, unsealed roads) could yield integrated understanding of the relative risk posed by different PP sources to eutrophication downstream. Such research could contribute towards development of spatially resolved (i.e. GIS-based) index models that combine new and existing knowledge about source (e.g. PP characteristics of catchment soils) and transport (e.g. proximity to a hydrological flow path) factors to guide management actions in a strategic and efficient manner (Drewry et al. 2011).
Conclusion
Physicochemical characteristics of terrestrial, stream bed and lake bed sediments were examined to investigate how particulate P varies along a common hydrological pathway. Particulate P concentration and composition differed markedly between the three groups of sediments. Approximately, 20 % of sequentially extracted P in sediments sampled in overland flow on farmland comprised the most refractory fraction, although the high total P and equilibrium P concentrations of these sediments indicated that such sediments have considerable potential to impair water quality downstream. Total P concentrations were lowest in the stream bed sediments which had lower values of EPC0 that were comparable with typical ambient stream water PO4–P concentrations, suggesting that such sediments have a buffering effect on stream water. Total P concentrations were high in the lake sediments, reflecting a legacy of high external loading and a major potential source of P to overlying waters. Characterising how particulate P composition varies between sediments sampled from different source areas can support risk-based approaches for controlling diffuse pollution.
References
Abell JM, Hamilton DP (2013) Bioavailability of phosphorus transported during storm flow to a eutrophic polymictic lake. N Z J Mar Freshw 47:481–489
Abell JM, Hamilton DP, Rutherford JC (2013) Quantifying temporal and spatial variations in sediment, nitrogen and phosphorus transport in stream inflows to a large eutrophic lake. Environ Sci: Process Impacts 15:1137–1152
APHA (1998) Standard methods for the examination of water and wastewater, 20th edn. American Public Health Association, Washington, DC
Boström B, Gunnar P, Broberg B (1988) Bioavailability of different phosphorus forms in freshwater systems. Hydrobiol 170:133–155
Bridgham SD, Johnston CA, Schubauer-Berigan JP, Weishampel P (2001) Phosphorus sorption dynamics in soils and coupling with surface and pore water in riverine wetlands. Soil Sci Soc Am J 65:577–588
Burger DF, Hamilton DP, Pilditch CA, Gibbs MM (2007) Benthic nutrient fluxes in a eutrophic, polymictic lake. Hydrobiol 584:13–25
Clarke D, Paterson J, Hamilton D, Abell J, Moore R, Scarsbrook M, Thompson K, Bruere A (2013) Overview of using detainment bunds for mitigating diffuse source phosphorus and soil losses from pastoral farmland. In: Currie LD, Christensen CL (eds) Accurate and efficient use of nutrients on farms. Occasional report no. 26. Fertilizer and Lime Research Centre, Massey University, Palmerston North
Condron LM, Newman S (2011) Revisiting the fundamentals of phosphorus fractionation of sediments and soils. J Soils Sediments 11:830–840
Drewry JJ, Newham LTH, Greene RSB (2011) Index models to evaluate the risk of phosphorus and nitrogen loss at catchment scales. J Environ Manage 92:639–649
Edwards AC, Withers PJA (2008) Transport and delivery of suspended solids, nitrogen and phosphorus from various sources to freshwaters in the UK. J Hydrol 350:144–153
Ellison ME, Brett MT (2006) Particulate phosphorus bioavailability as a function of stream flow and land cover. Water Res 40:1258–1268
Evans DJ, Johnes PJ, Lawrence DS (2004) Physico–chemical controls on phosphorus cycling in two lowland streams. Part 2–the sediment phase. Sci Total Environ 329:165–182
Froelich PN (1988) Kinetic control of dissolved phosphate in natural rivers and estuaries: a primer on the phosphate buffer mechanism. Limnol Oceanogr 33:649–668
Gao Y, Cornwell JC, Stoecker DK, Owens MS (2012) Effects of cyanobacterial–driven pH increases on sediment nutrient fluxes and coupled nitrification–denitrification in a shallow fresh water estuary. Biogeosciences Discuss 9:1161–1198
Hecky RE, Campbell P, Hendzel LL (1993) The stoichiometry of carbon, nitrogen, and phosphorus in particulate matter of lakes and oceans. Limnol Oceanogr 38:709–724
Hoare RA (1980) Inflows to Lake Rotorua. J Hydrol (NZ) 19:49–59
House WA (2003) Geochemical cycling of phosphorus in rivers. Appl Geochem 18:739–748
Hupfer M, Gächter R, Giovanoli R (1995) Transformation of phosphorus species in settling seston and during early sediment diagenesis. Aquat Sci 57:305–324
Jensen HS, Kristensen P, Jeppesen E, Skytthe A (1992) Iron: phosphorus ratio in surface sediment as an indicator of phosphate release from aerobic sediments in shallow lakes. Hydrobiol 235:731–743
Kerr JG, Burford MA, Olley JM, Bunn SE, Udy J (2011) Examining the link between terrestrial and aquatic phosphorus speciation in a subtropical catchment: the role of selective erosion and transport of fine sediments during storm events. Water Res 45:3331–3340
Legendre P, Legendre L (1998) Numerical ecology, 2nd edn. Elsevier Science, Amsterdam
Lewis WM, Wurtsbaugh WA, Paerl HW (2011) Rationale for control of anthropogenic nitrogen and phosphorus to reduce eutrophication of inland waters. Environ Sci Technol 45:10300–10305
Liu E, Shen J, Yuan H, Zhang E, Du C (2013) The spatio–temporal variations of sedimentary phosphorus in Taihu Lake and the implications for internal loading change and recent eutrophication. Hydrobiol 711:87–98
Lukkari K, Leivuori M, Hartikainen H (2007a) Fractionation of sediment phosphorus revisited. I: Fractionation steps and their biogeochemical basis. Limnol Oceanogr Methods 5:433–444
Lukkari K, Leivuori M, Hartikainen H (2007b) Fractionation of sediment phosphorus revisited. II: Changes in phosphorus fractions during sampling and storing in the presence or absence of oxygen. Limnol Oceanogr Methods 5:445–456
Machesky M, Holm T, Slowikowski J (2010) Phosphorus speciation in streambed sediments from an agricultural watershed: solid–phase associations and sorption behavior. Aquat Geochem 16:639–662
Martin TD, Creed JT, Brockhoof CA (1994) Sample preparation procedure for spectrochemical determination of total recoverable elements. Method 200.2 (Revision 2.8). Environmental Monitoring Systems Laboratory Office of Research and Development U. S. Environmental Protection Agency. Cincinnati, Ohio
Mayer LM, Gloss SP (1980) Buffering of silica and phosphate in a turbid river. Limnol Oceanogr 25:12–25
McDowell RW, Nash D (2012) A review of the cost–effectiveness and suitability of mitigation strategies to prevent phosphorus loss from dairy farms in New Zealand and Australia. J Environ Qual 41:680–693
McDowell RW, Sharpley AN (2003) Uptake and release of phosphorus from overland flow in a stream environment. J Env Qual 32:937–948
McDowell RW, Sharpley AN, Condron LM, Haygarth PM, Brookes PC (2001) Processes controlling soil phosphorus release to runoff and implications for agricultural management. Nutr Cycl Agroecosys 59:269–284
McDowell RW, Condron LM, Cave SV (2005) Chemical nature and diversity of phosphorus in New Zealand pasture soils using 31P nuclear magnetic resonance spectroscopy and sequential fractionation. Nutr Cycl Agroecosys 72:241–254
Moss B (2007) The art and science of lake restoration. Hydrobiol 581:15–24
Murphy J, Riley JP (1962) A modified single solution method for the determination of phosphate in natural waters. Anal Chim Acta 27:31–36
Nair PS, Logan TJ, Sharpley AN, Sommers LE, Tabatabai MA, Yuan TL (1984) Interlaboratory comparison of a standardized phosphorus adsorption procedure. J Environ Qual 13:591–595
Newsome PFJ, Wilde RH, Willoughby EJ (2000) Land and Resource Information System Spatial Data Layers Volume 1: ‘Label Format’. Landcare Research, Palmerston North, New Zealand
Okubo Y, Inoue T, Yokota K (2012) Estimating bioavailability of soil particulate phosphorus to Microcystis aeruginosa. J Appl Phycol 24:1503–1507
Owens PN, Duzant JH, Deeks LF, Wood GA, Morgan RPC, Collins AJ (2007) Evaluation of contrasting buffer features within an agricultural landscape for reducing sediment and sediment–associated phosphorus delivery to surface waters. Soil Use Manage 23:165–175
PCE (Parliamentary Commissioner for the Environment) (2006) Restoring the Rotorua lakes: the ultimate endurance challenge. Parliamentary Commissioner for the Environment, Wellington, New Zealand
Psenner R, Puckso R, Sager M (1984) Die Fraktionierung orgariischer und an organischer phosphorverbindungen von sedimenten. Arch Hydrobiol/Suppl 70: 111–155
Reynolds CS, Davies PS (2001) Sources and bioavailability of phosphorus fractions in freshwaters: a British perspective. Biol Rev 76:27–64
Salant NL, Hassan MA, Alonso CV (2008) Suspended sediment dynamics at high and low storm flows in two small watersheds. Hydrol Process 22:1573–1587
Søndergaard M, Jensen JP, Jeppesen E (2003) Role of sediment and internal loading of phosphorus in shallow lakes. Hydrobiol 506:135–145
Søndergaard M, Bjerring R, Jeppesen E (2013) Persistent internal phosphorus loading during summer in shallow eutrophic lakes. Hydrobiol 710:95–107
Trolle D, Hamilton DP, Hendy C, Pilditch C (2008) Sediment and nutrient accumulation rates in sediments of twelve New Zealand lakes: influence of lake morphology, catchment characteristics and trophic state. Mar Freshw Res 59:1067–1078
Uusitalo R, Turtola E, Puustinen M, Paasonen-Kivekäs M, Uusi-Kämppä J (2003) Contribution of particulate phosphorus to runoff phosphorus bioavailability. J Environ Qual 32:2007–2016
Vollenweider RA (1968) Scientific fundamentals of the eutrophication of lakes and flowing waters, with particular reference to nitrogen and phosphorus as factors of eutrophication. Organisation for Economic Co-operation and Development, Paris
Wang Q, Li Y (2010) Phosphorus adsorption and desorption behavior on sediments of different origins. J Soils Sediments 10:1159–1173
Wang J, Pant HK (2011) Assessments of potential spatial–temporal variations in phosphorus distribution and fractionation in river bed sediments. Clean Soil Air Water 39:148–156
Wentworth CK (1922) A scale of grade and class terms for clastic sediments. J Geol 30:377–392
Williamson R, Smith C, Cooper A (1996) Watershed riparian management and its benefits to a eutrophic lake. J Water Resour Plan Manage 122:24–32
Acknowledgments
This study is indebted to the landowners who permitted fieldwork access and detainment bund construction. John Paterson (Bay of Plenty Regional Council) managed construction of the detainment bunds and provided considerable technical support. Michael Hupfer (IGB, Berlin) provided guidance regarding sequential extraction methods and Phil Owens (University of Northern British Columbia) provided advice about the use of synthetic grass mats. Paul Scholes provided monitoring data and Alastair MacCormick provided management support (both from Bay of Plenty Regional Council). Steve Cameron undertook ICP-MS analysis and Annette Rogers assisted with particle size analysis (both from University of Waikato). Funding was primarily provided by Bay of Plenty Regional Council with additional support from the Ministry of Business, Innovation and Employment (UOWX0505) and DairyNZ.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Peryer-Fursdon, J., Abell, J.M., Clarke, D. et al. Spatial variability in sediment phosphorus characteristics along a hydrological gradient upstream of Lake Rotorua, New Zealand. Environ Earth Sci 73, 1573–1585 (2015). https://doi.org/10.1007/s12665-014-3508-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12665-014-3508-y