Abstract
Nador lagoon sediments (East Morocco) are contaminated by industrial iron mine tailings, urban dumps and untreated wastewaters from surrounding cities. The lagoon is an ecosystem of biological, scientific and socio-economic interests but its balance is threatened by pollution already marked by biodiversity changes and a modification of foraminifera and ostracods shell structures. The aim of the study is to assess the heavy metal contamination level and mobility by identifying the trapping phases. The study includes analyses by ICP-AES and ICP-MS, of, respectively, major (Si, Al, Mg, Ca, Fe, Mn, Ti, Na, K, P) and trace elements (Sr, Ba, V, Ni, Co, Cr, Zn, Cu, As, Pb, Cd) in sediments and suspended matter, heavy metals enrichment factors calculations and sequential extractions. Results show that sediments contain Zn, Cu, Pb, V, Cr, Co, As, Ni with minimum and maximum concentrations, respectively, of 4–1190μg/g, 4–466μg/g, 11–297μg/g, 11–194μg/g, 9–139μg/g, 1–120μg/g, 4–76μg/g, 2–62μg/g. High concentrations in Zn are also present in suspended matter. The enrichment factors show contamination in Zn, Pb and As firstly induced by the mining industry and secondly by unauthorized dumps and untreated wastewaters. Cr and Ni are bound to clays, whereas V, Co, Cu and Zn are related to oxides. Thus, the risk in metal mobility is for the latter elements and lies in the oxidation–reduction-changing conditions of sediments.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
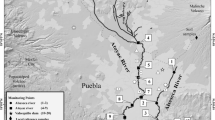
Nador lagoon is the only lagoon on the Moroccan Mediterranean coast (Fig.1). It has a 115km2 surface area and its water depth is at maximum of 8m. The ecosystem has a great social and economical interest (aquaculture, fishing, tourism), but has been subjected to environmental stresses since the last decades. Several towns have settled on its borders and rejected in the lagoon their untreated or partially treated wastewaters as, for example, Beni Ensar on the North-East (31,000 habitants), Arekman South East (18,000 habitants) or Nador South West (132,000 habitants). Moreover, some villages being all located around the lagoon, even in the northern island barrier, reject their wastewaters in the lagoon and leave all sorts of wastes in wild discharges. Furthermore, there are several potentially polluting industries such as ore treatment industry, which pollutes the Nador Lagoon by their activities.
The rejections of untreated urban wastewater induce an organic pollution resulting in high total carbon contents in the sediments and low dissolved oxygen in the water (Guelorget and Perthuisot 1983; Guelorget et al. 1993; Inani 1995; El Alami et al. 1998). Moreover, some lagoon areas show a starting contamination by heavy metals (Tesson and Gensous 1981; Inani 1995; Bloundi 2002; Bellucci et al. 2003; Ruiz et al. 2004, 2005) and the anthropogenic activities have an already proven negative impact on ostracods and foraminifera, which show tests deformations and nanism (Irzi 1987, 2002; Ruiz et al. 2004, 2005).
The environmental protection of the ecosystem requires a good knowledge of the present geochemical state and evolution of the medium. Some restricted studies were already been devoted to heavy metals distribution in Nador lagoon sediments (Tesson and Gensous 1981; Inani 1995; Bloundi 2002; Bellucci 2003; Aguila 2004; Gonzalez et al. 2006). This paper aims to present a more complete study on heavy metals monitoring the distribution in sediments and suspended matter in relation to anthropogenic activities, assessing the contamination level and identifying the phases, which possibly trap these elements.
The more precise objectives are to map the contaminated areas on the basis of the present data and those from the literature (Aguila 2004; Aguila et al. 2004; Gonzalez et al. 2006) and to specify the trapping way of the heavy metals in sediments and suspended matter.
The analyses to achieve these objectives are: (1) the mineralogy of surface sediments and suspended matter, (2) the heavy metal concentrations in sediments and suspended matter, and (3) study the heavy metals and minerals relations by leaching and statistical methods.
Study area
The lagoon drainage basin is characterized by three main structural domains; and it is bordered by (1) the Gourougou volcanic body on the North-West, (2) the Beni Bou Ifrour body W-SW, (3) the Kebdana body SE composed of marls, limestones, schist and sandstone formations (Paquet 1970; Mahjoubi et al. 2003).
From the physiographic point of view, the lagoon complex is divided in four main domains as follows: (1) the continental border with salt marshes and rivers such as Selwane and Bouaroug with irregular torrential runoff, most of the time dry, (2) Nador lagoon itself, the most extended lagoon in Morocco, (3) the island barrier broken off by a narrow pass (Bokhana) (Fig.1).
The climate is of Mediterranean type with contrasted seasons and with very specific local microclimates influenced by the height and the Saharan desert. The hydrological balance is controlled by (1) waters of the Mediterranean sea; (2) the rivers; (3) the anthropological discharges (agriculture, industry and wastewater); (4) waters of the water-treatment plant, and (5) waters of the groundwater.
Materials and methods
Sampling
The location of the sites of sampling was selected by using GPS (E - Map GARMIN). Sediment cores were collected by a diver equipped with a tube (30cm long, 4.5cm large). Surface sediments (0.5cm) were kept in polyethylene plastic bags and maintained at 4°C.
The surface sediments were selected to be on one hand representative of various zones of the lagoon and on the other hand located near the potentially contaminated sites.
The suspended matter (SM) was collected in January 2002 by filtration of 250ml of water in five sites close to the pollution sources. Filters were weighed before and after filtration with the aim of quantifying the contents in SM.
Mineralogy
The mineralogical analysis of the total sediment, the clays, and the suspended matter were obtained by X-ray diffraction using a PW1710 Philips diffractometer (CuKα) with a high-tension generator of 40kV and a current of 20mA. A software ‘Diffracplus EVA’ was used for diagram decomposition.
Geochemistry and enrichment factors
Chemical analyses were made by ICP-AES and MS (Jobin Yvon JY 124) on 61 sediments collected in winter and summer seasons (July 2000, January 2002, July 2002 and February 2003) (Fig.1). The analyzed major and trace elements are Si, Al, Mg, Ca, Fe, Mn, Ti, Na, K, P and Sr, Ba, V, Ni, Co, Cr, Zn, Cu, As, Pb, Cd, respectively. Calibration was performed using one blank and six international reference standard solutions. Table1 gives the inter-comparison of concentrations of major (% of oxide) and trace elements (μg/g) in these reference materials by ICP AES and ICP MS. The relative standard deviation (RSD) is obtained after three cycles of analyses. For ICP-AES, estimated RSD are to be of 2% for 1% element content and 10% for 10μg/g content. For ICP MS, estimated RSD are to be 10 and 15% for contents, respectively, of 1 and 0.01μg/g. Strongly toxic elements such as Pb, Cd and As were analyzed in these same samples by the Faculty of Chemistry in Sevilla within the framework of a common European project (Colasu ICA3-CT-2002-10012) (Aguila 2004; Aguila et al. 2004; Gonzalez et al. 2006).
The enrichment factor (FE) (Szefer et al. 1998) allows assessing the heavy metal contamination level of sediments; and it was calculated according to the following formula:
with:
- C sample :
-
trace element concentration in the sample;
- C crust :
-
trace element concentration in the continental crust (Taylor et al. 1995);
- Alsample :
-
Al content in the sample;
- Alcrust :
-
Al content in the continental crust (Taylor et al. 1995).
Al has been chosen as normalization element because of its origin being exclusively lithospheric.
Sequential extraction
Six sediment samples were chosen according to their location with regard to the pollution sources (Fig.1) and treated by sequential extraction. The choice of the extraction method was made according to the mineralogy of sediments, which essentially consist of silicates and carbonates. It is a simplified procedure inspired by the literature (Tessier et al. 1979; Leleyter 1998; Gomez et al. 2000). The extraction sequence includes four reagents from the weakest to the strongest: H2O, HCl, HNO3 and HF which dissolve gradually more resistant mineral phases.
The reagent is renewed after every stage of extraction and added to the solid residue of the previous stage. The soluble extract is separated by centrifugation and analyzed by ICP AES and MS (Table2). The residual sample is dried on a heating plate and again dissolved in 15ml of 1M HNO3 for analyses by ICP AES and MS.
Suspended matter analysis
The mineralogical study was realized by scanning electron microscopy observations and X-ray energy dispersive chemical analyses (JEOL JSM 840).
For major and trace elements analyses in the suspended matter, the filters are heated at 650°C during 3h and the residue is mixed in Teflon containers with a volume of 1ml of HNO3 and 4ml of HF. Then the unit is heated at 70°C for 24h. The residue is then evaporated at 70°C and dissolved in 1ml of 1M HNO3 and 1ml of HClO4 with 140°C for 24h. The mixture is evaporated again at 140°C and the final residue is dissolved in 1M HNO3 and analyzed by ICP AES and MS.
Results
Mineralogy
Two main lagoon sectors (North and South) can be distinguished with regard to sediments mineralogy (Fig.2).
-
The Northern sector which gathers the NW and SW zones is characterized by a majority of feldspars and clays coming from the alteration of the Gourougou volcanic body. Additional minerals such as iron oxides (hematite and goethite) are also present; they can be of anthropogenic origin (old treatment plant of ores), and detrital (altered olivine of the Gourougou body).
The dominant argillaceous minerals are the smectites followed by kaolinite and illite; there is no chlorite. These smectites can have two origins: (1) heritage, coming from the Gourougou volcanic rocks (the argillaceous fraction of the Gourougou andesites contains approximately 90% of smectites) and, (2) neo-formation: this process is possible in alternate seasons countries such as Morocco; in summer, evaporation is intense and water concentrates. The lagoon waters exhibit high pH, and strong silica and basic cations concentrations favorable to the neoformation of smectite (Tardy et al. 1970; Ramirez et al. 2005).
The suspended matter is primarily composed of clays and feldspars.
-
The Southern sector gathers the SW and SE zones as well as the littoral border. Quartz and calcite are dominant and result from the hydrolysis of the limestones and sandy limestones of Kebdana and Beni Bou Ifrour bodies (Fig.1).
Dominant clays are illite and kaolinite, followed by chlorite; the smectite content is minor.
The suspended matter is composed of quartz, calcite, dolomite, illite and chlorite.
Heavy metal concentrations in lagoon sediments
The high standard deviation values show that the heavy metals distribution is inhomogeneous in the lagoon (Table3). The Zn, Pb, Cu, V, Ni, Cr, and Co highest contents are recorded North West in the vicinity of the old ore treatment plant (Attalyoun), around Nador town, the wastewater treatment plant and the mouth of Oued Bouaroug which drains urban wastewater of Zeghanghane municipality and partly of Nador city. One also finds high concentrations in Oued Selwane sector, which drains the industrial park (food-processing industry, etc.) and the quarry (Pb, Zn) (Fig.3), in the confined sector close to the cities of Beni Ensar and Arekman not endowed with water-treatment plant. In the centre of the lagoon higher Co and Pb contents were also recorded. It is along the littoral border that the lowest contents are found for all these metals. Cd and As are distributed differently. For the Cd, the lowest contents are recorded in the mouth of the Oued Selwane and near the pass; on the contrary the highest concentrations in As are observed near the pass and the lowest contents near the city of Beni Ensar.
Location of anthropogenic activities and iron ores around Nador lagoon (after Douieb 1971)
Figure4 illustrates the comparison of Nador lagoon with different hydro-systems where the anthropogenic impact is low [Oualidia, Morocco (Maanan et al. 2004); Ariana, Tunisia (Bloundi 2001)] or turned out [Venice, Italy (Bertolin et al. 1995); Thau and Berre (France) (Péna and Picot 1991; Giorgetti 1981)]. To be noted that the Zn and Cu contents are very high in Nador lagoon (Fig.4). The Cr concentrations are equivalent to those of Oualidia lagoon located on the Moroccan Atlantic coast and the Ni contents are of the same order of magnitude as in the other ecosystems.
Correlations between major and trace elements
The high percentages of some of the metal elements can be of origin, as well anthropogenic as one awaits it around the mine dumps of the old ore treatment plants in Western North, than natural; in this last case the trace elements come from the hinterland formations which are rich in V, Zn, Cr and Ni (El Bakkali 1995; El Azzouzi et al. 1999; Bloundi 2005). In order to specify affinities between the trace elements and the different mineral phases, the study of correlations between trace and major elements was undertaken.
Ni and Cr are strongly correlated (the correlation coefficient is 0.91) (Fig.5). It is the same for Co and Cu (R²=0.59), as well as Zn and Pb (R²=0.77).
The Zn, Pb, Ni, Cr, Co and Cu are strongly correlated to Fe (Fig.5). Cr and Ni are besides also correlated to Al (R²=0.59). This would indicate that all these trace elements are related to iron rich phases, which can be either iron oxides or clays of the smectite or chlorite type (Fig.5).
The trace elements are generally negatively correlated with Ca and Mg. This might indicate that they are not related to the carbonated phases in the sediment.
Contamination level
The lagoon zones, where the contamination in trace elements is observed, are identified by an enrichment factor of value higher than 2 (Szefer et al. 1998; Rubio et al. 2000).
The cartography of the factor values of all campaigns (Fig.6) shows that the contamination is present in several zones of the lagoon such as the central part and the seacoast of the lagoon being the least affected.
To be mentioned that all the positively correlated elements appear enriched in the same sectors of the lagoon. Thus, Zn and Pb have factors of enrichment higher than 2 in every studied sector even in confined zones of the North West and South East, in the South border around Nador city or in the mouth of Oueds. In addition Pb and Cd are enriched in the centre.
It is clear that the Zn and Pb enrichment locally around the old treatment plant of ores is related to the richness in these metals of the spoil heaps. The soils located around the old factory are besides also enriched in Zn and Pb (Gonzalez et al. 2006). However the contamination in the whole lagoon indicates that enrichment is not only of anthropogenic but also of natural origin, related to the release of these elements by alteration of the surrounding volcanic rocks.
The other studied elements are slightly enriched in the whole lagoon except some restricted and distinct zones: thus Cr and Ni are enriched on the level of the Oued Bouaroug (I), Arekman town (II) and around the old positions of the pass (III). Zones I and II are clearly distinguished by the industrial anthropogenic (iron ore) and urban activities, in this case water and domestic wastes rejections, and not controlled wild dumps charged out of plastics. Cu and Co as for them are enriched close to the spoil heaps (V) and the purification station (IV).
As although not correlated with the other trace or major elements, is enriched like the preceding elements in sectors I with V, but also in the confined zone Northern West and near the irrigation canal which drains the agricultural plain waters rich in fertilizers. Cd is enriched in the sector of the mine dumps (V), in the centre of the lagoon as for Pb, and on the seacoast.
Heavy metals trapping phases
The treated samples are representative of the two sectors of the lagoon: stations I, II and III of the Northern sector (feldspars and smectite dominant sediments) and stations IV, V and VI of the Southern sector (quartz, calcite, illite and chlorite dominant sediments) (Fig.1).
The results of the sequential extractions of major and trace elements were synthesized by component analysis (Fig.7). For the statistical treatment, the element concentrations obtained after the three stages of extraction: L2, extraction by HCl, L3, extraction by HNO3 and L4, extraction by HF were taken into consideration. The stage of extraction by water was not considered, given the weak concentrations in heavy metals put in solution.
This statistical study allows having a better analyse of the nearness and the distances between the various variables, and in this manner to isolate the “atypical” individuals and to group together the “similar” individuals.
Three groups can be distinguished (Fig.7):
-
Group 1: represents the group of the carbonates which are dissolved by the hydrochloric acid; it consists of Ca as dominant element, followed by Mg, Mn and Sr which show negative correlations with the rest of elements.
-
Group 2: gathers the iron (oxides and clays) correlated to P, Co, Cu, Zn and V.
-
Group 3: represents the group of alumino-silicates, the dominating element being Al. It is strongly correlated to K, Cr, Ni and V. Fe is also present in this group and bound to Ni.
These circles of correlations show that the iron-bearing minerals (oxides, hydroxides and clays) are preferential trapping sites of heavy metals. Co and Cu have a great affinity for iron oxides followed by Pb and to a lesser extent by Zn. Cr and Ni have a strong affinity for clays, followed of V and Co, then Cu and Zn, which are more slightly related to these minerals. The carbonates on the contrary, do not take part in the process of trapping. The organic matter on the other hand, traps only a small percentage of elements.
These results indicate that the most available metals are those which are trapped by the oxidized phase. Indeed, this fraction is very fragile during any change of oxidation–reduction conditions and there is a strong potential risk of toxicity. This is all the more true for Zn, which is strongly accumulated in the sediments.
Heavy metals in suspended matter
The suspended matter is a compartment, which takes an active part in the processes of trapping or transfer of the trace elements either towards the sediments, or towards water. No previous study on the lagoon of Nador considered this compartment before; that is why these complementary data were acquired in this work.
The analyses of suspended matter show that the average concentrations of heavy metals follow the decreasing order: Zn (101μg/g)>Cr (15μg/g)>V (13μg/g)>Cu (12μg/g)>Ni (6μg/g)>Pb (4μg/g)>Co (2.4μg/g)>As (1.7μg/g)>Cd (0.4μg/g). As in the sediments, Zn is the dominating trace element in suspended matter.
Figure8 shows the distribution in the lagoon of heavy metals from the suspended matter. It is the station situated at the release with the water-treatment plant of Nador that is most concentrated in heavy metals. The contents of the latter are above the averages calculated except for Cd and Zn. In the vicinity of the city of Nador, the suspended matter is enriched in Cu, As, Cd, Cr and Ni. The station of the old treatment plant of ores presents as for it, the strongest contents in Zn and important contents in Pb in comparison with the other stations. As regards the stations of the South of the lagoon (Selwane, Mrader and Arekman; Fig.1), the heavy metal contents are lower in comparison with the stations North of the lagoon. Near the sector of Selwane, there is enrichment in Cr and Ni and farther southward (station of Mrader) only Pb is enriched. At the Southern border of the lagoon (Arekman), the suspended matter is impoverished in metals and no content exceeds the averages.
The strong contents in heavy metals in suspended matter of the Northern sector coincide with the massive presence of smectites in suspended matter (Fig.9) and also with rather important particulate organic matter contents. This is also the case in the vicinity of Nador and of the wastewater treatment plant. Smectites and organic matter are phases with strong adsorption capacities on which the elements can be combined and thus be transported from the continent to the lagoon.
The high values in Zn are recorded on the level of the old iron ore treatment plant. This element results very probably from mine dumps left on the border of the lagoon. More in the South of the lagoon the contents of heavy metals in suspended matter decrease, and only the contents of Ni and Cr exceed the averages. This is related to the presence of chlorites, which trap these two elements (Fig.9).
Conclusion
This study enabled us to identify the sites in Nador lagoon where a beginning of heavy metals enrichment in sediments is observed, and thus to make in evidence the impact of anthropogenic activities; these stations are: (1) the old treatment plant of ores; (2) the vicinity of Nador town; (3) the mouth of Oued Bouaroug; (4) the discharge of the wastewater treatment plant; (5) the South East end of the lagoon (in the vicinity of Arekman town).
The most contaminant anthropogenic activities are the mining industry, which is responsible for the input of Zn, Pb and As, the unauthorized discharges and the untreated wastewaters releases.
The heavy metal contents in suspended matter depend on the mineralogical composition. Thus in the sectors with oxides in majority (old treatment plant of ores), the suspended matter is rich in Zn and Pb. There where smectites dominate (Nador town basin and discharge of wastewater treatment plant), the contents of Cu, As, Pb, Cd, Cr, Ni, Co are higher. And south of the lagoon where chlorites dominate, Cr and Ni contents are high.
With regard to the mode of trapping of the pollutants, the results of the sequential extraction and statistical studies show that Cr and Ni are related to clays whereas V, Co, Cu and Zn have stronger affinities for oxides. Thus the risk in metal mobility is for V, Co, Cu and Zn and lies in the oxidation–reduction-changing conditions.
References
Aguila E (2004) Distribucion espatial y fuentes de los elementos traza en la laguna de Nador (Maruecos). Memoir, University of Sevilla, Spain, p 74 (in Spanish)
Aguila E, Gonzalez I, Galàn E, Hamoumi N, Labraimi M, Bloundi M-K (2004) Distribucion de elementos traza en los sedimentos de la laguna de Nador (Marruecos). Estudio preliminar. Geotemas 6(1):195–198
Bellucci L-G, El Moumni B, Collavini F, Frignani M, Albertazzi S (2003) Heavy metals in Morocco lagoon and river sediments. J Phys 107:139–142
Bertolin A, Frizzo P, Rampazzo G (1995) Sulphide speciation in surface sediments of the Lagoon of Venice: a geochemical and mineralogical study. Mar Geol 123(1-2):73–86
Bloundi M-K (2001) Mode de piégeage des éléments polluants dans les sédiments de la Sebkha d’Ariana (Tunisie); Application de la méthode d’extraction séquentielle. Memoir, University of Louis Pasteur, Strasbourg, France, p 35 (in French)
Bloundi M-K (2002) Cartographie et supports des éléments métalliques dans les sédiments de la lagune de Nador: Application de la technique d’extraction séquentielle. Memoir, University of Mohamed V, Rabat, Morocco, p 29 (in French)
Bloundi MK (2005) Etude géochimique de la lagune de Nador: impact des facteurs anthropiques. Ph.D., 480 University of Strasbourg, France, p 238 (in French)
Douieb M (1971) Mines et Géologie, 34, Rabat, Maroc, p 52
El Alami M, Mahjoubi R, Damnati B, Kamel S, Icole M, Taieb M (1998) Sédimentologie et géochimie organique des sédiments superficiels de la lagune de Nador (Maroc nord - oriental). J Afr Earth Sci 26(2):249–259
El Azzouzi M, Griffiths JB, Bellon H, Maury R-C, Piqué A, Fourcade S, Cotten J, Hernandez J (1999) Evolution des sources du volcanisme marocain au cours du Néogène. CR Acad Sci Paris 395:95–102
El Bakkali S (1995) Volcanologie et Magmatologie du système du Gourougou (Rif Oriental, Maroc), Ph.D., University of Clermont-Ferrand, France, p 256 (in French)
Giorgetti C (1981) Etude de l’état de pollution des sédiments de l’étang de Berre. 2 eme partie: la pollution inorganique. Ph.D., University of Marseille, France, p 132 (in French)
Gomez J-L, Giralgez I, Sanchez-Rodas D, Morales E (2000) Metal sequential extraction procedure optimized for heavily polluted and iron oxide rich sediment. Anal Chim Acta 414(1-2):151–164
Gonzalez I, Aguila E, Galàn E (2006) Partitioning, bioavailability and origin of heavy metals from the Nador lagoon sediments (Morocco) as a basis for their management. Environ Geol 52:1581–1593
Guelorget O, Perthuisot J-P (1983) Le domaine paralique: Expressions géologiques, Biologiques et Economiques du Confinement. Memoir, Ecole Normale Supérieure, Paris, France, p 136 (in French)
Guelorget O, Lefebvre A, Orbi A, Shafee M-S (1993) Etude de la lagune de Nador (Maroc): Résultats de la mission de juin 1993. Memoir, Institut Scientifique des pêches Maritimes, Casablanca. Morocco, p 53 (in French)
Inani I (1995) Dynamique sédimentaire et état de pollution dans la lagune de Nador. Ph.D., University Mohamed V, Rabat. Morocco; p 186 (in French)
Irzi Z (1987) Etude sédimentologique et micropaléontologique de la lagune de Nador (Maroc Oriental). Ph.D., University of Pierre and Marie Curie, Paris, France, p 172 (in French)
Irzi Z (2002) Les environnements du littoral méditerranéen oriental du Maroc compris entre l’oued Kiss et le cap des trois fourches dynamique sédimentaire et évolution Ecologie des Foraminifères benthiques de la lagune de Nador. Thesis, University of Mohamed I, Oujda, Morocco, p 279 (in French)
Leleyter L (1998) Spéciation chimique des éléments majeurs, traces et des terres rares dans les matières en suspension et dans les sédiments de cours d’eau: application aux fleuves de Patagonie (Argentine), à la Piracicaba (Brésil), à l’oued Sebou (Maroc) et à l’Ill (France). Ph.D., University of Louis Pasteur, Strasbourg, France, p 297 (in French)
Maanan M, Zourarah B, Carruesco C, Aajjane A, Naud J (2004) The distribution of heavy metals in the Sidi Moussa lagoon sediments (Atlantic Moroccan Coast). J Afr Earth Sci 39(3-5):473–483
Mahjoubi R, Kamel S, El Moumni B, Noack Y, Parron C (2003) Nature et répartition de la phase argileuse de la lagune de Nador (Maroc Nord Oriental). Geol Belg 6(1-2):31–42
Paquet H (1970) Evolution géochimique des sédiments argileux dans les altérations et les sols des climats méditerranéens et tropicaux à saisons contrastées. Bull Mem Serv Carte Geol Alsace Lorraine 30:31–42
Péna G, Picot B (1991) Métaux traces dans les sédiments d’une lagune méditerranéenne: l’étang de Thau. Oceanol Acta 15(5):459–472
Ramirez S, Righi D, Petit S (2005) Alteration of smectites induced by hydrolytic exchange. Clays Miner 40:15–24
Rubio B, Nombela M-A, Vilas F (2000) Geochemistry of major and trace elements in sediments of the Ria de Vigo (NW Spain): an assessment of metal pollution. Mar Pollut Bull 40(11):968–980
Ruiz F, Abad M, Hamoumi N, Labraimi M, Bouimetarhan I, Boumaggard E-H (2004) Los ostracodos como indicadores ambientales: la laguna de Nador (NE de Marruecos). Geotemas 6(2):307–310
Ruiz F, Abad M, Bodergat A-M, Carbonel P, Rodriguez-làzaro J, Yasuhara M (2005) Marine and brackish-water ostracods as sentinels of anthropogenic impacts. Earth Sci Rev 72:89–111
Szefer P, Kusak A, Szefer K (1998) Evaluation of the anthropogenic influx of metallic pollutants into Puck Bay, southern Baltic. Appl Geochem 13:293–304
Tardy Y, Paquet H, Millot G (1970) Trois modes de genèse des montmorillonites dans les altérations et les sols. Bull Groupe Français Argiles 22:69–77
Taylor S-R, McLennan S-M (1995) The geochemical evolution of the continental crust. Rev Geophys 33(2):241–265
Tessier A, Campbell P-G-C, Bisson M (1979) Sequential extraction procedure for speciation of particulate traces metals. Anal Chem 51(7):844–851
Tesson M, Gensous B (1981) Quelques caractères de la géochimie d’une lagune microtidale: la Sebkha Bou-Areg (Maroc). Paper presented at the 106ème Congress, Perpignan, France. Soc Savantes vol 3, pp 183–194
Acknowledgments
This work was financed within the framework of an European INCO project (Colasu ICA3-CT-2002-10012) and of a French-Moroccan Integrated Action (MA/01/09).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bloundi, M.K., Duplay, J. & Quaranta, G. Heavy metal contamination of coastal lagoon sediments by anthropogenic activities: the case of Nador (East Morocco). Environ Geol 56, 833–843 (2009). https://doi.org/10.1007/s00254-007-1184-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00254-007-1184-x