Abstract
Triton X-100 (TX100) and Brij 35 (B35) were used to investigate the elevated critical micelle concentration (CMC) induced by surfactant sorption and its influence on PAH removal in soil washing systems. The surface tension technique was applied to determine the CMC and the apparent CMC (CMCsoil) in soil–water systems. Surfactant sorption experiments were conducted by the batch equilibration technique. Surfactants sorbed on the soil at concentrations below the CMCsoil were quantified with data from the surface tension experiments for both an aqueous system and a soil–water system. Due to sorption, the CMCsoil values of the two surfactants are 2.75 and 6.31 times their corresponding CMC values in aqueous solutions, respectively. At concentrations below CMCsoil, the loss of B35 (92–99.7 %) was greater than that of TX100 (63–92 %). The PAH removal efficiencies are greatly dependent on the CMCsoil value. At surfactant concentrations below CMCsoil, the PAH removal is very low and remains almost invariable. Whereas, at concentrations above CMCsoil, the PAH removal increases greatly. B35 inhibited PAH desorption at concentrations below its CMCsoil. For TX100, some degree of PAH desorption enhancement was observed at concentrations below its CMCsoil. CMCsoil is a key parameter while selecting a surfactant for a specific soil washing system, only surfactant concentrations above their CMCsoil should be evaluated.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Polycyclic aromatic hydrocarbons (PAHs) are ubiquitous pollutants in the environment, and are mainly derived from anthropogenic activities, such as biomass burning, incomplete fossil fuel combustion, oil spills and some industrial processes (Bernardez 2008; Nganje et al. 2012; Wang et al. 2012; Tay and Biney 2013). Among which coal processing is one of the most important sources of PAHs. Soils from many sites such as areas of coal storage, coke oven plants, manufactured gas plants, and areas of coal tar spillage are highly contaminated by PAHs (Paria and Yuet 2006; Viglianti et al. 2006). These contaminated sites pose risks to the human and the environment, especially those located in urban areas that have been abandoned by pollution intensive industries (Sousa 2001). Due to their known or potential genotoxicity and carcinogenicity, remediation of these PAH-contaminated sites has been always a major environmental concern (Woo et al. 2001; Ahn et al. 2008, Guo et al. 2013).
Many technologies have been used to remediate PAH-contaminated soils such as bioremediation (Hughes et al. 1997), phytoremediation (Huesemann et al. 2009), chemical oxidation (Alderman et al. 2007), photocatalytic degradation (Zhang et al. 2008), and electrokinetic remediation (Reddy et al. 2006). Compared with the forenamed techniques, soil washing provides an effective and relatively low cost alternative for the remediation of PAH-contaminated soil. Due to their hydrophobic nature, PAHs are strongly sorbed to soil. As a consequence, the remediation of PAHs in soil–water systems depends strongly on their desorption rates from the soil surface and the subsequent incorporation of the pollutant into the bulk aqueous phase (Jin et al. 2007; Din et al. 2009). Surfactants have frequently been used to enhance desorption of PAHs from soil and the subsequent transfer into water through solute solubilization into aqueous micelles (Paterson et al. 1999; Yuan and Marshall 2007; Alcantara et al. 2009; Petitgirard et al. 2009). Surfactant-enhanced remediation has been suggested as a promising technology for the remediation of contaminated soils and groundwater (Harwell et al. 1999; Mulligan et al. 2001).
Nonionic surfactants can sorb onto soil to some extent, and anionic surfactant will precipitate with divalent cations in soils (e.g., Ca2+, Mg2+) (Wang and Keller 2009). Understanding the sorption characteristics of the surfactant can greatly assist in designing and optimizing the surfactant-enhanced remediation technologies (Grasso et al. 2001; Zhu and Zhou 2008). In a soil–water system, the surfactant dose required for micelle formation is greater owing to surfactant sorption, which results in a higher measured CMC for soil–water systems compared to the CMC in aqueous solutions. This elevated CMC is referred to the apparent CMC and is denoted by CMCsoil in this paper. Former researches have demonstrated that surfactants can significantly enhance PAH desorption only at concentrations above their CMCsoil (Grasso et al. 2001; Zhu and Zhou 2008). Sorbed surfactant may account for the majority of added surfactant in surfactant amended remediation applications, and this may result in increased hydrophobic organic compound (HOC) partitioning onto soil until HOC solubilization by micellar phase surfactant successfully competes with increased HOC sorption on surfactant-modified soil (Zhou and Zhu 2008; Laha et al. 2009).
In this study, Triton X-100 (TX100) and Brij 35 (B35) were used to facilitate desorption of PAHs from aged-contaminated soils. The objectives of this research were (1) to investigate the surfactant loss due to sorption, and its influence on PAH removal efficiency; and (2) to evaluate the elevated CMC induced by surfactant sorption and its implication on surfactant selecting for soil washing systems.
Materials and methods
Study site and sample collection
The soil used in this study was collected from a former coke oven plant in Beijing, China, which mainly produced coke, coal gas, coke tar and some other coal chemical products. The contamination of this site is about 50 years. Before the start of the 2008 Olympic Games, Beijing coke oven plant was moved out to other city in order to improve the environmental quality. The contaminated land needs to be remediated according to laws before its reuse. 15 sample sites in horizontal direction and three sample sites in vertical direction (from 0 to 2.5 m below the ground surface) were set to get representative soil samples. Oversize materials were removed. Individual samples were mixed for purpose of treatability study for soil washing. The mixed samples were air-dried. Particles that passed through a 2-mm sieve were used for subsequent experiment. Selected physical and chemical properties of the soil samples are listed in Table 1. The initial PAH concentrations are shown in Table 2.
Chemical reagents
TX100 and B35 were purchased from Amresco. They were selected due to their wide use in soil washing systems. All the surfactants were used as obtained without further purification. 16 US EPA priority PAHs standard mixture consisted of naphthalene (Nap), acenaphthylene (Acy), acenaphthene (Ace), fluorene (Flu), phenanthrene (Phen), anthracene (Ant), fluoranthene (FlA), pyrene (Pyr), benzo[a]anthracene (BaA), chrysene (Chr), benzo[b]fluoranthene(BbF), benzo[k]fluoranthene (BkF), benzo[a]pyrene (BaP), indeno[1,2,3-cd]pyrene (InP), dibenzo[a,h]anthracene (DBA), benzo[g,h,i]perylene (BgP) and deuterated surrogate naphthalene-d8, phenanthrene-d10, chrysene-d12 and Perylene-d12 were obtained from AccuStandard, and internal standard of hexamethylbenzene was obtained from Sigma. Alumina (100–200 mesh), silica gel (80–100 mesh), anhydrous sodium sulfate, diatomite, and copper powder were analytical grade obtained from Beijing Chemical Reagents Company (China). All solvents used for sample processing and analysis (dichloromethane, acetone, hexane and ethanol) were HPLC grade purchased from J.T. Baker. Deionized water was produced by a Milli-Q system (Millipore).
Surface tension measurements
The surface tension technique was applied to determine the CMC and the CMCsoil. The surface tension measurements were carried out with a tensiometer (JWY-200A, Beijing Jinshengxin Testing Machine) following the method of Grasso et al. (2001). Triplicate measurements were made for each sample, which was comprised of surfactant solutions and filtered supernatant from a centrifuged soil-aqueous system. The samples were tested in increasing concentrations and the ring was rinsed with deionized water between samples.
Sorption of surfactants on the soil
Surfactant sorption experiments were conducted in duplicate by the batch equilibration technique. Surfactant solutions were prepared in 0.02 % sodium azide using deionized water. 30-mL surfactant solution was added to 3.0 g of soil sample in 50-mL Teflon centrifuge tubes. The tubes were equilibrated on a reciprocating shaker at 150 rpm and 30 °C for 48 h. Then the soil suspensions were centrifuged at 10,000 rpm for 15 min. A 20 mL aliquot of supernatant was removed to measure the surface tension. Surfactants sorbed on the soil at a concentration lower than the CMCsoil were quantified from the surface tension experiments described above for both an aqueous system and a soil–water system.
PAH desorption with surfactants
Polycyclic aromatic hydrocarbon desorption experiments were carried out in duplicate by the batch equilibration technique. Surfactant solutions were prepared in 0.02 % sodium azide using deionized water. 30-mL surfactant solution was added to 3.0 g of contaminated soil in 50-mL Teflon centrifuge tubes. The tubes were equilibrated on a reciprocating shaker at 150 rpm and 30 °C for 48 h. Then the soil suspensions were centrifuged at 10,000 rpm for 15 min. A 20-mL aliquot of supernatant was removed to extract PAHs for analysis.
PAH extraction and analysis
Polycyclic aromatic hydrocarbons in the original soil samples were extracted with acetone and dichloromethane mixture (1:1, v:v) using an Accelerated Solvent Extractor (ASE 300, Dionex Corp.). 1.0 g of dried, homogenized soil sample was mixed with diatomite and activated copper powder, then filled into the stainless steel extraction cells. Prior to extraction, the soil was spiked with 1-mL (4 mg L−1) deuterated recovery surrogate standards of naphthalene-d8, phenanthrene-d10, Chrysene-d12, Perylene-d12. The extracts were concentrated to nearly dryness by rotary evaporation and changed solvent to hexane for further chromatographic separation.
A 20-mL aliquot of surfactant solution containing dissolved PAH compounds was extracted with 3 × 20 mL hexane. Ethanol was added to break the hexane-surfactant emulsion. Deuterated PAH surrogate standards naphthalene-d8, phenanthrene-d10, chrysene-d12 and Perylene-d12 were added to monitor the procedures of cleanup and analysis.
Polycyclic aromatic hydrocarbons in concentrated hexane extract were separated using a 2:1 silica gel/Alumina glass chromatography column (1.0 × 30 cm) with 1-g anhydrous sodium sulfate overlaying the Alumina to remove small quantities of water (Guo et al. 2013). First, 15 mL of hexane was used to remove the aliphatic hydrocarbons, the eluate was discarded. Then PAHs were eluted with 70-mL hexane/dichloromethane (7:3, v:v), the eluate was collected in a 100-mL pear-shaped flask. The sample volume was reduced via rotary evaporation, exchanged into hexane, and concentrated to 1 mL with a gentle purified N2 stream. Prior to transfer to GC–MS vials, known quantities of internal standard (hexamethylbenzene) were added.
Sample extracts were analyzed for 16 US EPA priority PAHs using a ThermoQuest Trace 2000 GC/MS (Finigan). The PAHs were separated using a DB-5MS 30 m × 0.25 mm fused silica column (J&W) in selected ion mode. GC/MS operating conditions were as follows: the injection port, interface line, and ion source temperature were maintained at 280 °C. Column temperature was programmed at 80 °C (hold for 3 min), increased to 250 °C at the rate of 10 °C min−1 (hold for 3 min), then increased at 5 °C min−1 to 290 °C (hold for 7 min). Helium was the carrier gas at a flow of 1.0 mL min−1 and a linear velocity of 24.6 cm s−1. 1 μL volume of each sample was injected manually in the splitless mode. The ionization was carried out in the electron impact mode at 70 eV. Identifications of 16 US EPA priority PAHs were based on the retention time and ion m/z ratio of an authentic PAH mixed standard. Concentrations of individual compound were estimated from their areas under the chromatographic peaks using the internal standard peaks as instrument references.
Quality control
All experiments were carried out in duplicate. Two blank samples were included in every batch of samples. No PAH was detected in blank samples. Recoveries of the four deuterated surrogates added to the soil samples were 55 ± 8 % for naphthalene-d8, 74 ± 6 % for phenanthrene-d10, 69 ± 7 % for Chrysene-d12, and 90 ± 12 % for Perylene-d12. Recoveries of the four deuterated surrogates added to the soil washing effluents were 75–92 %. All the values reported in this paper were not corrected to achieve 100 % recovery.
Results and discussion
The apparent CMC of surfactants in soil–water systems
Understanding the surfactants behavior in soil–water systems is important for the design of soil washing projects. Surfactant loss due to sorption may significantly increase the surfactant doses required for ex-situ soil washing (Vreysen and Maes 2005). Furthermore, surfactant adsorption will increase the organic carbon content of the soil, favoring the adsorption of HOCs (Lee et al. 2004; Rodriguez-Cruz et al. 2005). Since the surfactants can only effectively desorb HOCs at concentrations above their CMCsoil in soil–water systems, the measurement of CMCsoil can provide scientific basis for a proper surfactant dose.
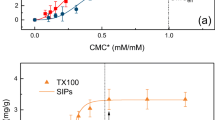
The measured CMC and CMCsoil are shown in Fig. 1. The results are the average of triplicate measurements. In general, the surface tension curve has two linear segments for each dose of surfactant. The breakpoint between the two segments indicates the value of CMC in a soil–water system (Chu et al. 2005). The sorption of surfactants is a function of physicochemical and mineralogical properties of soils and the characteristics of surfactants themselves (Rodriguez-Cruz et al. 2005). In this research, the CMCsoil values of TX100 and B35 are 2.75 and 6.31 times their corresponding CMC values in aqueous solutions, respectively. This is comparable to the results of some other researchers. For example, Chu and Chan (2003) found that the apparent CMC of B35 in a soil–water system is 6.25 times its CMC value in aqueous solution at a soil/water ratio of 1:6. In our research, the CMCsoil of B35 is 6.31 times their CMC value in aqueous solution at a soil/water ratio of 1:10. Grasso et al. (2001) found that the apparent CMC values of Alfonic 1412-7 in soil–water systems are one to two orders of magnitude of their CMC in aqueous solution at a soil/water ratio of 1:1–1:10.
Loss of surfactants resulted from sorption
The sorption of surfactant to the soil is mainly caused by surfactant monomers. Since the monomer concentration in solution is a constant at surfactant doses above CMCsoil, the surfactant sorption should remain unchangeable (Chu et al. 2006; Muherei et al. 2009). Therefore, the surfactant sorption achieves its maximum value at a concentration equals to CMCsoil. At concentrations below the CMC, there exists a linear relationship between the surfactant concentration and the surface tension; therefore, the surfactant sorption can be obtained through surface tension data (Zheng and Obbard 2002; Chu and Chan 2003; Chu et al. 2005; Muherei et al. 2009). The surfactant loss due to sorption can be estimated by the following relationship
where Q e is the surfactant loss due to sorption to the soil (mg kg−1); C soil is the bulk surfactant dose in the soil/aqueous system that produces a surface tension value of σ in the supernatant (mg L−1); C a is the corresponding surfactant concentration required to produce the same surface tension value of σ in the absence of soil (mg L−1); V a is the volume of aqueous solution (L); and W soil is the mass of soil (kg).
Freundlich isotherms were used to fit the sorption data of nonionic surfactants at concentrations below CMCsoil:
where C S is the amount of adsorbed surfactant (mg kg−1), K F is a measure of sorption capacity, C e is the equilibrium concentration of surfactant in solution (mg L−1), and n is the constant which indicates the curvature of the isotherm.
The Freundlich isotherm fitting results and the surfactant loss data are shown in Table 3. The sorption of TX100 and B35 onto soils is nonlinear, and can fit well with Freundlich isotherm. At concentrations below CMCsoil, most of the surfactants were lost due to sorption. The loss of B35 was greater than that of TX100 with more than 92 % of the surfactant sorbed onto soil at concentrations below its CMCsoil.
Implications of CMCsoil on PAH removal efficiencies and surfactant selecting
Surfactant-enhanced desorption of PAHs in soils from a former coke oven plant were conducted using TX100 and B35. The removal efficiency was defined as the fraction of PAHs removed from the soils. Figure 2 presents PAH removal efficiency as a function of surfactant concentrations.
As shown in Fig. 2, depending on the surfactant concentrations below or above their CMCsoil, the PAH removal by surfactants presented different patterns. At concentrations below their CMCsoil, due to severe surfactant loss (Table 3), only a small quantity of surfactant molecules were left to exist in the form of monomers. Earlier studies have demonstrated that surfactant monomers have a weak or no HOC desorption enhancement abilities (Chu and Chan 2003). Therefore, the PAH removal is very low and remains almost invariable. Whereas, at concentrations above their CMCsoil, surfactant micelles begin to form in the solution and the PAH removal began to increase greatly.
For B35, at concentrations below its CMCsoil, the PAH removal efficiencies were even less than that in the absence of surfactant. This is similar to the results of some other researchers who have demonstrated that the addition of nonionic surfactants at concentrations below CMC may enhance the retardation of HOCs (Sun et al. 1995; Park and Bielefeldt 2003; Zhou and Zhu 2005). For TX100 which has lesser sorption loss, removal efficiencies less than that in the absence of surfactant were observed only at low surfactant concentrations of 100 and 200 mg L−1 for some PAHs (FlA, Pyr and BaA).
A desorption efficiency coefficient, E, can be defined according to that of Wang and Keller (2008):
where D s and D w are the fractions of PAHs desorbed from the soils in the presence (D s) and absence (D w) of surfactant. An E > 1 indicates enhanced PAH desorption, while E < 1 represents an inhibited PAH desorption.
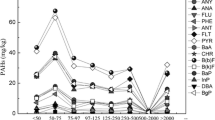
As the heavy molecular PAHs (B(b)F, B(K)F, BaP, DBA and InP) were not detected in the experiment with no surfactant added, the E value was not obtained for all the PAHs.
Figure 3 shows the E values. Due to severe surfactant sorption (Table 3), B35 inhibited PAH desorption at concentrations below its CMCsoil. For TX100 which has lesser sorption loss, some degree of PAH desorption enhancement was observed at concentrations below its CMCsoil. It seemed that surface tension reduction mechanism took effect in the PAH removal. Only when PAHs desorbed into water by surface tension reduction exceed those sorption on surfactant-modified soil, can desorption enhancement be observed.
In the design and application of surfactant-enhanced remediation processes for contaminated sites, the selection of surfactant is critical (Grasso et al. 2001). Deshpande et al. (1999) have recommended that both anionic and nonionic surfactants at concentrations below and above their CMC should be evaluated in selecting a surfactant for a given soil-contaminant system. However, according to our research, nonionic surfactant TX100 and BJ35 can greatly enhance PAH desorption only at concentrations well above their CMCsoil. We think CMCsoil is a key parameter while selecting a surfactant for a specific soil washing system. In order to reduce time and money cost, only surfactant concentrations above their CMCsoil should be evaluated in surfactant selecting.
Conclusions
TX100 and B35 were used to facilitate the desorption of PAHs from soils. The surfactant loss due to sorption and the PAH removal efficiencies by each surfactant is evaluated. Results showed that the CMCsoil values of the two surfactants are 2.75 and 6.31 times their corresponding CMC values in aqueous solutions, respectively. At concentrations below CMCsoil, the loss of B35 (92–99.7 %) was greater than that of TX100 (63–92 %). The PAH removal efficiencies are greatly dependent on the CMCsoil value. At surfactant concentrations below CMCsoil, PAH removal is very low and remains almost invariable. Whereas, at concentrations above their CMCsoil, the PAH removal increases greatly. B35 inhibited PAH desorption at concentrations below its CMCsoil. For TX100, some degree of PAH desorption enhancement was observed at concentrations below its CMCsoil. CMCsoil is a key parameter while selecting a surfactant for a specific soil washing system, only surfactant concentrations above their CMCsoil should be evaluated.
References
Ahn CK, Kim YM, Woo SH, Park JM (2008) Soil washing using various nonionic surfactants and their recovery by selective adsorption with activated carbon. J Hazard Mater 154:153–160
Alcantara MT, Gomez J, Pazos M, Sanroman MA (2009) PAHs soil decontamination in two steps: desorption and electrochemical treatment. J Hazard Mater 166:462–468
Alderman NS, N’Guessan AL, Nyman MC (2007) Effective treatment of PAH contaminated superfund site soil with the peroxy-acid process. J Hazard Mater 146:652–660
Bernardez LA (2008) Investigation on the locus of solubilization of polycyclic aromatic hydrocarbons in non-ionic surfactant micelles with 1H NMR spectroscopy. Colloid Surf A Physicochem Eng Asp 324:71–78
Chu W, Chan KH (2003) The mechanism of the surfactant-aided soil washing system for hydrophobic and partial hydrophobic organics. Sci Total Environ 307:83–92
Chu W, Choy WK, Hunt JR (2005) Effects of nonaqueous phase liquids on the washing of soil in the presence of nonionic surfactants. Water Res 39:340–348
Chu W, Chan KH, Choy WK (2006) The partitioning and modelling of pesticide parathion in a surfactant-assisted soil-washing system. Chemosphere 64:711–716
Deshpande S, Shiau BJ, Wade D, Sabatini DA, Harwell JH (1999) Surfactant selection for enhancing ex situ soil washing. Water Res 33:351–360
Din KU, Shafi M, Bhat PA, Dar AA (2009) Solubilization capabilities of mixtures of cationic Gemini surfactant with conventional cationic, nonionic and anionic surfactants towards polycyclic aromatic hydrocarbons. J Hazard Mater 167:575–581
Grasso D, Subramaniam K, Pignatello JJ, Yang Y, Ratte D (2001) Micellar desorption of polynuclear aromatic hydrocarbons from contaminated soil. Colloid Surf A 194:65–74
Guo W, He MC, Yang ZF, Lin CY, Quan XC, Wang HZ, Tian ZJ (2013) The distribution, sources and toxicity risks of polycyclic aromatic hydrocarbons and n-alkanes in riverine and estuarine core sediments from the Daliao river watershed. Environ Earth Sci 68:2015–2024
Harwell JH, Sabatini DA, Knox RC (1999) Surfactants for grounder water remediation. Colloid Surf A Phys Eng Asp 151:255–268
Huesemann MH, Hausmann TS, Fortman TJ, Thom RM, Cullinan V (2009) In situ phytoremediation of PAH- and PCB-contaminated marine sediments with eelgrass (Zostera marina). Ecol Eng 35(10): 1395–1404
Hughes JB, Beckles DM, Chandra SD, Ward CH (1997) Utilization of bioremediation processes for the treatment of PAH-contaminated sediments. J Ind Microbiol Biotechnol 18:152–160
Jin DJ, Jing X, Ou ZQ (2007) Effects of concentration, head group, and structure of surfactants on the degradation of phenanthrene. J Hazard Mater 144:215–221
Laha S, Tansel B, Ussawarujikulchai A (2009) Surfactant-soil interactions during surfactant-amended remediation of contaminated soils by hydrophobic organic compounds: a review. J Environ Manag 90:95–100
Lee JF, Hsu MH, Chao HP, Huang HC, Wang SP (2004) The effect of surfactants on the distribution of organic compounds in the soil solid/water system. J Hazard Mater 114:123–130
Muherei MA, Junin R, Merdhah ABB (2009) Adsorption of sodium dodecyl sulfate, Triton X100 and their mixtures to shale and sandstone: a comparative study. J Petrol Sci Eng 67:149–154
Mulligan CN, Yong RN, Gibbs BF (2001) Surfactant-enhanced remediation of contaminated soil: a review. Eng Geol 60:371–380
Nganje TN, Edet AE, Ibok UJ, Ukpabio EJ, Ibe KA, Neji P (2012) Polycyclic aromatic hydrocarbons in surface water and soil in the vicinity of fuel-oil spillage from a tank farm distribution facility, Esuk Utan, Calabar municipality, Nigeria. Environ Earth Sci 67:81–90
Paria S, Yuet PK (2006) Solubilization of naphthalene by pure and mixed surfactants. Ind Eng Chem Res 45:3552–3558
Park SK, Bielefeldt AR (2003) Aqueous chemistry and interactive effects on non-ionic surfactant and pentachlorophenol sorption to soil. Water Res 37:4663–4672
Paterson IF, Chowdhry BZ, Leharne SA (1999) Polycyclic aromatic hydrocarbon extraction from a coal-contaminated soil using aqueous solutions of nonionic surfactants. Chemosphere 38:3095–3107
Petitgirard A, Djehiche M, Persello J, Fievet P, Fatin-Rouge N (2009) PAH contaminated soil remediation by reusing an aqueous solution of cyclodextrins. Chemosphere 75:714–718
Reddy KR, Ala PR, Sharma S, Kumar SN (2006) Enhanced electrokinetic remediation of contaminated manufactured gas plant soil. Eng Geol 85:132–146
Rodriguez-Cruz MS, Sanchez-Martin MJ, Sanchez-Camazano M (2005) A comparative study of adsorption of an anionic and a non-ionic surfactant by soils based on physicochemical and mineralogical properties of soils. Chemosphere 61:56–64
Sousa CD (2001) Contaminated sites: the canadian situation in an international context. J Environ Manag 62:31–154
Sun SB, Inskeep WP, Boyd SA (1995) Sorption of nonionic organic compounds in soil-water systems containing a micelle-forming surfactant. Environ Sci Technol 29:903–913
Tay CK, Biney CA (2013) Levels and sources of polycyclic aromatic hydrocarbons (PAHs) in selected irrigated urban agricultural soils in Accra, Ghana. Environ Earth Sci 68:1773–1782
Viglianti C, Hanna K, de Brauer C, Germain P (2006) Removal of polycyclic aromatic hydrocarbons from aged-contaminated soil using cyclodextrins: experimental study. Environ Pollut 140:427–435
Vreysen S, Maes A (2005) Remediation of a diesel contaminated, sandy-loam soil using low concentrated surfactant solutions. J Soil Sediment 5:240–244
Wang P, Keller AA (2008) Particle-size dependent sorption and desorption of pesticides within a water-soil-nonionic surfactant system. Environ Sci Technol 42:3381–3387
Wang P, Keller AA (2009) Partitioning of hydrophobic pesticides within a soil-water-anionic surfactant system. Water Res 43:706–714
Wang YH, Xue R, Zhu HX, Xu YY, Xue BM, SH Q, Yuan DX, Theodore OI (2012) Compositional fractionation of polyaromatic hydrocarbons in the karst soils, South China. Environ Earth Sci 66:2013–2019
Woo SH, Park JM, Rittmann BE (2001) Evaluation of the interaction between biodegradation and sorption of phenanthrene in soil-slurry systems. Biotechnol Bioeng 73:12–24
Yuan T, Marshall WD (2007) Optimizing a washing procedure to mobilize polycyclic aromatic hydrocarbons (PAHs) from a field-contaminated soil. Ind Eng Chem Res 46:4626–4632
Zhang LH, Li PJ, Gong ZQ, Li XM (2008) Photocatalytic degradation of polycyclic aromatic hydrocarbons on soil surfaces using TiO2 under UV light. J Hazard Mater 158(2–3):478–484
Zheng ZM, Obbard JP (2002) Evaluation of an elevated non-ionic surfactant critical micelle concentration in a soil/aqueous system. Water Res 36:2667–2672
Zhou WJ, Zhu LZ (2005) Distribution of polycyclic aromatic hydrocarbons in soil-water system containing a nonionic surfactant. Chemosphere 60:1237–1245
Zhou WJ, Zhu LZ (2008) Influence of surfactant sorption on the removal of phenanthrene from contaminated soils. Environ Pollut 152:99–105
Zhu LZ, Zhou WJ (2008) Partitioning of polycyclic aromatic hydrocarbons to solid-sorbed nonionic surfactants. Environ Pollut 152:130–137
Acknowledgments
Financial supports are from Beijing Municipal Science and Technology Commission (SF2008-02), the National Natural Science Foundation of China (21207049), Shandong Provincial Higher Educational Science and Technology Program (J12LC02), and the Research Starting Foundation for Doctors from University of Jinan (XBS1227).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, H., Chen, J. & Jiang, L. Elevated critical micelle concentration in soil–water system and its implication on PAH removal and surfactant selecting. Environ Earth Sci 71, 3991–3998 (2014). https://doi.org/10.1007/s12665-013-2783-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12665-013-2783-3