Abstract
The transport and leaching potential hazards of various pesticides were studied in a shallow unconfined aquifer located in Northwest Bangladesh. Pesticide leaching potential was quantified using a one-dimensional advective–dispersive transport equation for a non-conservative chemical that follows first-order decay and linear adsorption in soils. Leaching potential index (LPI) was calculated for 69 sites in the study area to evaluate the relative vulnerability to pesticide leaching and to prioritize sites for model study and soil sampling. The numerical ranks of computed LPI were grouped by quantiles into very high, high, moderate, low and very low categories; and based on these rankings, the most vulnerable site was selected. The fate and transport of pesticides in this most vulnerable site was modeled using MT3D. The model results indicate that pesticides with high sorptivity and moderate to high persistence have low potential impact on groundwater. Top soils are found to be particularly vulnerable to the accumulation of organochlorine pesticides. Results also revealed that decreasing the soil organic matter and increasing the half-life of the pesticides at deeper depths did not make any significant change. Finally, six soil samples were collected from the same site at depths of 0.0, 1.5, 3.0, 4.5, 6.0, and 7.5 m for the analysis of pesticide residues. The soil–water was extracted from the samples following standard extraction technique and tested using gas chromatography (GC) and high-performance liquid chromatography (HPLC) for pesticide residues. Results showed no trace of pesticide residues in the soil–water; however, a few unknown peaks were detected indicating the use of some unknown brand of chemicals in the study area.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Due to the increased use of agro-chemicals in agricultural fields, contamination of soil and groundwater by pesticide leaching has become one of the major threats to the subsurface environment. Prediction of leaching potential and transport processes of agro-chemicals in the subsurface is an essential prerequisite for their efficient and safe management as well as to establish realistic regulatory controls. Groundwater pollution from pesticide leaching has been detected in many countries. Several investigators have studied the factors contributing to pesticide leaching (Gish et al. 1991; Truman and Leonard 1991; Sichani et al. 1991; Agertved et al. 1992; Yunus 2004). Their investigations have shown that chemical solubility in water, sorptive properties, pesticide formulation and soil persistence determine the pesticide leaching. Similarly, the important environmental and agronomic factors include soil properties, climatic conditions, crop type, water management methods and cropping practices. Many of the environmental factors are highly time-variable and soil properties are spatially variable. In short, the hydrological cycle interacts with the chemical properties and characteristics to transform and transport pesticides within and out of the soil profile. Vertical movement out of and below the root zone can result in groundwater contamination. An important part of managing the occurrence of pesticides in groundwater is the identification of areas susceptible to contamination by leaching.
A number of techniques have been developed for assessing the vulnerability of groundwater to leaching contamination by organic contaminants. Some of these methods are based on subjective scoring. A model known as DRASTIC (Aller et al. 1985) involves assigning weightage to various factors influencing leaching. Other qualitative assessment schemes include those of Teso et al. (1988), Le Seur et al. (1987), and Breeuwsma and van Duijvenbooden (1987). Methods that offer a more quantitative assessment of groundwater vulnerability include those of Dean et al. (1984), Rao et al. (1985), and Meeks and Dean (1990).
Groundwater quality and quantity have been of concern in Bangladesh as a result of increasing reliance on groundwater for irrigation. The impact of agro-chemicals on groundwater quality is of particular concern because groundwater is the predominant source of domestic water in rural areas of Bangladesh (Anwar and Yunus 2010). Most of the private wells are shallow and generally not tested for quality; thus, unsafe levels of contamination may go undetected. Soil, water and climatic resources of the country make it ideally suited for intensive agriculture. Although annual average rainfall is about 150 cm, non-uniform distribution often causes water stress in crops. Such drought conditions and availability of groundwater supply have led to multiple cropping with several fold increases in irrigated area. Since the early 1960s, agricultural land use changed markedly in Bangladesh with the introduction of high yielding variety (HYV) crops, expansion of irrigation and the use of chemical fertilizers and pesticides. Groundwater irrigation expanded more rapidly because of its inherent technical and cost advantages and covers about 68 % of the irrigated land. The overall use of fertilizers and pesticides has been significantly increased over the last three decades because of the production of HYV rice (Anwar and Yunus 2010). The focus of attention has now moved to the study of pesticide fate and its leaching potential in Bangladesh groundwater.
For this research, nine of the most commonly used pesticides were chosen to study their fate and transport in a shallow unconfined aquifer located in Northwest Bangladesh. First, the Leaching Potential Index (LPI) was calculated for sixty-nine sites in the study area to evaluate the relative vulnerability to pesticide leaching and to prioritize sites for model study and soil sampling. The fate and transport of pesticides in the most vulnerable site were studied using the MT3D model. Next, soil samples were collected from the same site at different depths and analyzed for pesticide residues in soil or groundwater.
Methodology
Leaching potential index
Many studies have been carried out for the assessment of groundwater vulnerability using different methods. The most common approaches are statistical/empirical methods and methods based on solute transport theory. The most widely used methods based on chemical transport equations were given by Rao et al. (1985), Jury et al. (1987), Meeks and Dean (1990) and Kookana and Aylmore (1994). Kookana and Aylmore (1994) modified the Jury et al. (1987) model for decreasing the organic matter content in soil with respect to depth. More recently, Schlosser et al. (2002) presented a modified method of Meeks and Dean (1990) for the assessment of aquifer vulnerability to pesticide leaching and introduced a term vulnerability index (VI). These methods are based on the one-dimensional advective–dispersive transport equation for a non-conservative chemical that follows first-order decay and linear adsorption in soils. Using simplified assumptions and steady state conditions, the following equation can be derived relating the contaminant concentration C 0 at the surface and the concentration C at any depth z.
where, R = retardation factor; C = chemical concentration (g m−3); V = soil–water velocity (m year−1); λ = first-order decay rate of the chemical in the soil (year−1); and z = vertical depth (m). The term C/C 0 is the attenuation factor. The factor λz(V/R)−1 specifies the relative concentration, C at any depth, z. This factor is a dimensionless number whose magnitude indicates the susceptibility of groundwater to be contaminated by leaching of chemicals through the overlying soil if the depth of interest, z is set equal to the depth of the water table. Meeks and Dean (1990) took the inverse of this quantity, V/Rλz as the indicator of leaching potential. To convert the index to a practical range, the value is multiplied by 1,000 which is designated as the LPI:
Higher values of LPI indicate a greater susceptibility of groundwater to contamination. This susceptibility index depends on the soil–water velocity, retardation factor, chemical decay rate, and groundwater depth. The method is physically-based and uses chemical and environmental properties. This offers planners and agencies a simple, but quantitative tool for managing pesticide use and identifying areas susceptible to groundwater contamination by pesticide leaching.
LPI computation
Information required for computing the leaching potential index are given in those from Meeks and Dean (1990) and Anwar and Yunus (2010). The following data are required for LPI computation: physical properties of soil (bulk density, field capacity, organic matter content, soil water velocity) and chemical properties of pesticide (decay rate or half-life, organic carbon partitioning coefficient or water solubility). The soil water velocity (V) is the average rate at which recharge water moves vertically through the unsaturated zone to the groundwater. This value can be calculated if the field capacity of the soil and the recharge rate from precipitation, irrigation and any other source is known. Recharge rate from precipitation can be estimated as the excess of precipitation over evaporation and runoff. The recharge rate from irrigation can be estimated as the difference between the rate at which water is applied to crops and the crop evapotranspiration rate. The total recharge rate is limited by the hydraulic conductivity of the soil. The soil water velocity is the total recharge rate divided by the soil moisture content (θ). As soil moisture content varies with time, it can be taken as the field capacity (θc), which represents the practical upper limit to the water content. Field capacity can be estimated for a particular soil type.
The retardation factor (R) measures how much slower the dissolved chemical travels relative to the soil water velocity which can be calculated as:
where, k d = adsorption partition coefficient (cm−3g) and is equal to f oc × k oc, f oc = fraction of organic carbon content, k oc = organic carbon partition coefficient (cm−3g); ρb = soil bulk density; and θ = soil–water content. The adsorption partition coefficient (k d) reflects the ratio of adsorbed to dissolved concentrations of the chemical at equilibrium in the presence of a solvent (water) and adsorbent media (soil). Value of k oc can be estimated using an equation of the form: log k oc = a log (S w or k ow) + b, where S w = aqueous solubility of a compound, k ow = octanol–water partition coefficient (ratio of concentration of solute in octanol phase to concentration of solute in aqueous phase), a = slope on log–log plot, b = value of log k oc when log (S w or k ow) equals zero. Mercer and Waddell (1992) describe a number of regression equations of the above form, from which ones useful for pesticides can be chosen. Soil bulk density (ρb) can be estimated for a particular soil type in the laboratory. The decay rate (λ) is a measure of how persistent a compound is in a specific environment, which can be represented by the rate law: “The rate of decomposition is proportional to the amount of substance present”. In equation form, it can be written as: N = N oe−λt, where N is the number of atoms remaining after time t and N 0 is the original number present at time t = 0. Decay rate (λ) is related to half-life (t 1/2), which is the time at which the number of atoms remaining is one-half the original number. Thus, the above equation may be written as:
where t 1/2 = chemical half-life (years). Decay rate (λ) is determined as λ = 0.693/t 1/2. Chemical properties such as like S w, log k ow, and t 1/2 of different pesticides can be found in Worthing and Hance (1991) and Vogue et al. (1994). Groundwater depth (z) can be obtained from national or local water agency. As groundwater depth fluctuates seasonally and in response to pumping, weighted average depth or minimum depth should be taken for conservative calculation of LPI.
Modelling with MT3D
MT3D is a comprehensive three-dimensional numerical model for simulating solute transport in complex hydrogeologic settings (Zheng 1992). In this study, the most vulnerable site, based on LPI, was chosen for MT3D application. MT3D has a modular design that permits simulation of transport processes such as, three-dimensional advection, dispersion, equilibrium-controlled linear or non-linear sorption, and first-order irreversible decay or biodegradation. In the MT3D code, dispersion, sink/source and reactions are solved by explicit finite-difference method without using an iterative solver. The advection term can be solved by either mixed Eulerian–Lagrangian methods including the forward-tracking method of characteristics (MOC), the backward tracking modified method of characteristics (MMOC), and a hybrid of these two methods (HMOC) or explicit finite-difference method. MT3D model was chosen to investigate the percentage of remaining pesticides at different soil depths after 2 months of pesticide application.
Soil sampling and analysis
The same site (most vulnerable site used in MT3D) was also chosen for the collection of soil samples. Soil sampling procedure and analysis details are given in those of Anwar and Yunus (2010). Six soil samples (>500 g) were collected from this site at the soil surface and at depths of 1.5, 3.0, 4.5, 6.0, and 7.5 m below the ground surface. The soil samples were kept in a cooler box and analyzed at the Institute of Food and Radiation Biology (IFRB) of the Bangladesh Atomic Energy Commission. Each soil sample was mixed thoroughly with a glass rod and oven-dried at 50 °C. Fifty grams of oven-dried sample was mixed with 50 ml double-distilled hexane and 50 ml double-distilled acetone in a conical flask. After shaking for 4 h with an electric shaker, the mixture was allowed to settle for 10 min. The supernatant layer was extracted and transferred into another conical flask. This was re-extracted 3 times by adding 10 ml double-distilled hexane and manually shaken for 5 min each time. Then, 5 g of anhydrous Na2SO4 was added and transferred into a flat-bottomed flask and the solution was placed in a rotary vacuum evaporator at 40 °C until 2–3 ml of volume was left. The concentrated extract was transferred into a vial and cleaned up with Florisil by eluting with 100 ml double-distilled hexane. Again, evaporation leaving 2–3 ml volume by rotary vacuum evaporator was performed. The cleaned concentrated extract was then transferred into a vial and dried fully with an N 2 gas blower. After all these processes, the samples were analyzed by GC and HPLC. Chromatograms were produced for different pesticides and were checked for pesticide residues by comparing with standard chromatograms of the nine selected pesticides.
Study area
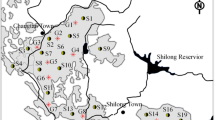
Rangpur Sadar Thana in Northwest Bangladesh was selected, as the study area (Fig. 1) is situated between longitudes 89°05′E and 89°20′E and between latitudes 25°39′N and 25°50′N. It has a total area of 319.1 km2 (SRDI 1990). Abundant groundwater supplies occur throughout the region in a shallow unconfined aquifer. The maximum depth to groundwater lies between 3 and 5 m below the ground. The reason for choosing this site is that the intensity of irrigation and the amount of groundwater use are higher than in other areas. Moreover, the area is located in the Teesta River flood plain, where alluvial soils tend to be more coarse grained and hence, more susceptible to leaching contamination. A review of available soil information (Soil Resources and Development Institute-SRDI 1990; Joshua and Rahman 1983) revealed that the most complete soil data were available for this region. The record for Rangpur Sadar Thana was found to be more complete in terms of necessary soil parameters, and as such this Thana was chosen for investigating the pesticide leaching threats to groundwater.
Land use in Bangladesh is predominantly agricultural and rice is the principal crop. Three types of rice (Aus, Aman and Boro) are grown in Bangladesh. Among them, Boro rice covers the highest amount of irrigated areas. In the study area, wheat, pulses, oil seeds, potatoes and other vegetables, sugarcane, cotton, and some other crops are also grown. The increase of irrigated area for different crops in the study area is shown in Fig. 2 for the period 1996–2000. The rice type, Boro, covers more than 60 % of the irrigated area in 1996 which has increased to 70 % of the area in the year 2000. Although the overall use of pesticides in Bangladesh was relatively low as compared to other countries, pesticide use grew rapidly in this country in the early 1970s following the introduction of high-yielding rice varieties. Significant amounts of pesticides are being used in Boro rice fields. The pattern of pesticide use in Bangladesh is shown in Fig. 3 for the period 1987–2000 (Islam 2000). Increasing trend of pesticide use in crops indicates that there might be an occurrence of groundwater contamination because of pesticide leaching in the area.
Irrigated area for different crops in the study area (BBS 2000)
Pesticide use in Bangladesh (Islam 2000)
Data collection
Data needed for LPI estimation
Information on the organic matter content and the soil type at 69 locations in the Thana was obtained from the Land and Soil Resources Use Guidebook (Soil Resources and Development Institute-SRDI 1990). Grid lines of the area were constructed on the soil association map at 2-km intervals running from north to south and from west to east. This allows for easy reference of the locations selected for LPI computation. Information on the field capacity and bulk density for different soil series was obtained from Joshua and Rahman (1983) and is shown in Table 1.
As soil–water velocity is obtained by dividing the recharge rate by the field capacity of the soil, the recharge rate was taken as the annual fluctuation of the depth of the groundwater table (Karim 1984) during the calculations. The estimated recharge rate was 0.396 m/year. This parameter was considered constant for all soil types in the Thana as there was no basis for differentiating recharge rates among sampled sites. In general, recharge rates and soil–water velocity are higher at irrigated sites than at non-irrigated sites; therefore, the corresponding leaching potential is also higher. Depth to groundwater was obtained from observation well data collected by the Bangladesh Water Development Board, a national agency responsible for countrywide groundwater data collection. The maximum depth of the groundwater table varies between 3 and 5 m and the average depth ranges between 0.69 and 4.45 m over a year. The minimum depth of 0.69 m was taken for a conservative estimation of LPI. The quantity of organic matter for each of the sampled sites was taken from the Land and Soil Resources Use Guidebook (Soil Resources and Development Institute-SRDI 1990). Percent organic carbon content was calculated by multiplying the percent of organic matter by 0.5263 (Mercer and Waddell 1992). A lower fraction of organic carbon content will result in higher LPI values.
LPI values were computed for the nine selected pesticides at 69 locations in the study area. A preliminary survey was conducted to select the most widely used pesticides in the area. Based on the survey data, the following pesticides were selected from three pesticide groups: (i) organophosphorous—diazinon, malathion, chlorpyrifos, cypermethrin and fenitrothion; (ii) carbamate—carbofuran and carbaryl and (iii) organochlorine—heptachlor and endosulfan. Heptachlor is permitted only for use in sugar cane; however, several farmers in the area were found to be using it in potato fields and other crops. Therefore, it was also included in this investigation. However, organophosphorous and carbamate groups are moderately persistent while organochlorine is highly persistent in soil and groundwater. The properties of the selected pesticides are given in Table 2 (Vogue et al. 1994).
Data for MT3D application
The solute transport program MT3D (Zheng 1992) was used to investigate the remaining percentage of different pesticides at different depths of soil after a 2 month period. The chosen site is divided into five layers and physical properties of the soil, chemical properties and recommended dose of different pesticides are invoked in the MT3D program. Table 3 shows the physical properties of the soil at different depths of the chosen site (Joshua and Rahman 1983) which was used in MT3D modelling. Recommended dose of various pesticides are taken from Department of Agricultural Extension (DAE) of the Rangpur Sadar Thana and are presented in Table 2 together with the chemical properties of different pesticides.
Results and discussion
Frequency distribution of LPI
To fit frequency (empirical) distribution, computed LPI values for all nine pesticides were first grouped into class intervals and then absolute frequency, relative frequency, and summary statistics were calculated. Empirical distribution in the form of frequency tables and histograms can provide an estimate of probabilities of environmental exposures. The computed relative frequency provides an estimate of the probability of an LPI value falling in the indicated range or class intervals. Relative frequencies are plotted as histograms in Fig. 4.
An inspection of the histograms shows that LPI values follow an exponential distribution as expected for any decay process. The great diversity of environments within the study area is reflected by the large variation of LPI values. The strong skew of histograms and the relatively small mean illustrate that the vast majority of sections were ranked rather low and only a few sections had large LPIs. This distribution suggests that an effective sampling effort need not be uniform but rather should focus on a small number of high LPI areas.
Selection of most vulnerable site
To select the most vulnerable site, the spatial distribution of LPI in the study area was checked for the selected pesticides. The results for carbofuran are presented as a bar graph in Fig. 5. Higher LPI values are found in sites 13–20 and then again in sites 25–29. Higher LPI values indicate higher susceptibility to pesticide leaching in groundwater. This result revealed that an effective groundwater sampling effort need not be uniform, but should focus on a small number of high LPI areas. All other pesticides showed a similar pattern of spatial variation of LPI. In fact, similar spatial variation of LPI will be exhibited by any other pesticides if the organic carbon content at a given site remains the same (see Eqs. 1–4).
The numerical ranks of computed LPI for different pesticides were grouped by quantiles into very high, high, moderate, low and very low categories. This categorization was designed to facilitate the identification of areas susceptible to groundwater contamination in the study area. The assigned leaching category and quantiles and the number of sections in the study area in each category are presented in Table 4. Of the 69 sites, 10 % of the areas were ranked very high, 13 % were high, 30 % were moderate, 30 % were low and 16 % were very low. Three (moderate, low and very low ranks) of the five categories contain larger geographic area, except for those ranked high and very high. These categories were made smaller because of the exponential nature of the scale, which at higher values causes each increment of the LPI to be more significant.
Based on the LPI ranking and distribution, site 16 was found to be the most vulnerable location for pesticide leaching and hence, selected for model study and soil sampling for pesticide residues.
MT3D simulation results
MT3D was first used to model the percentage of pesticides remaining at varying depths in the soil 2 months after their application. Figure 6a, b presents the results for fenitrothion and diazinon, respectively, where the groundwater level is 1.35 m below the surface. Figure 6a reveals that the fenitrothion remained at negligible amounts (<2 × 10−4 %) at the surface and at different soil depths. Fenitrothion is a low persistent pesticide with a half-life of 8 days (Table 2) which breaks down completely within 2 months and hence the remaining percentage is very small. Similar results are also found for malathion of the organophosphorous group indicating that low persistent pesticides like malathion and fenitrothion (half-life <10 days) have no contamination possibility for Rangpur sadar thana. For diazinon, as shown in Fig. 6b, about 24 and 7 % remains (persists) in the surface and groundwater level, respectively. As diazinon has a recommended dose rate similar to fenitrothionin (Table 2), the results imply that diazinon has more contamination potential than fenitrothion. Simulation was also performed for other pesticides and the results revealed that significant amounts of pesticides remain in surface (≥24 %) and groundwater level (≥5 %) for those with high persistency (t 1/2 >30 days). Thus, these pesticides have more leaching potential for groundwater contamination. However, this would also depend on the sorptivity of the pesticides and the groundwater depth. In general, high sorptivity pesticides tend to be confined within the surface level and hence extensive use of low sorptivity and high persistence pesticides would be expected to have more contamination potential for groundwater than that of high sorptivity pesticides with a similar persistence. Sorption usually reduces the concentration of the solute plume to some fraction because it reduces the solute migration velocity relative to the average pore water velocity. Again, the mobility of pesticides is inversely related to their sorption. Sorption may be classified broadly into three classes based on their \( K_{\text{oc}} \) values (Hornsby 1995) as shown in Table 5.
Simulation results further revealed that the organochlorine pesticides (heptachlor, endosulfan) accumulate in the topsoil. Accumulation of pesticides in a soil compartment depends on less hydrophobicity, persistency, and low volatility of pesticides and the binding properties of the soil. The organochlorine pesticides (heptachlor, endosulfan) are more persistent in soil due to their lower biodegradability than organophosphorous (diazinon, chlorpyrifos, malathion, fenitrothion). Therefore, all top soils are particularly vulnerable to organochlorine accumulation.
Finally, the effect of soil organic matter on pesticide movement was examined. Soil organic matter is the key factor that influences the plant nutrient cycle, soil moisture relationships, and soil structure including numerous chemical and/or biological properties. Model results revealed that decreasing soil organic matter and increasing half-life of pesticides in the deeper depth did not make any significant change.
Pesticide residues in soil water
Soil samples were collected from site 16 within 1 month after the harvesting of Boro rice from the field. Discussion with the local farmers of the site indicated that several pesticides such as, carbaryl, carbofuran, diazinon, chlorpyrifos, cypermethrin, fenitrothion and malathion are usually applied in Boro season over the site and on adjacent areas to the site. Samples were collected from the site at different depths using a soil auger. The groundwater table at the time of sampling was located at a depth of 1.35 m. After collection of samples, they were kept in pre-washed glass bottles which were sealed by leukoplast and were preserved in a cool box until chemical analysis for pesticide residues was performed. The chemical analysis for pesticide residues was carried out at the Institute of Food and Radiation Biology of Bangladesh Atomic Energy Commission. GC was used for determining the organochlorine pesticide residues and HPLC was used for detecting organophosphorous and carbamate pesticides. Chromatograms were produced for each of the samples (Fig. 7) and compared with a standard chromatogram for the detection of any pesticide residues in soil–water. Results showed that there was no trace of pesticide residues in any of the soil; however, some unknown peaks were detected which indicated the presence of some unknown chemicals. This might be due to the use of some unknown varieties of pesticides in the study area or some other organic chemicals coming from unknown sources. Pagiola (1995) reported that some highly toxic pesticides continue to be produced and sold in the neighboring country. Some unknown brand pesticides are readily available in border areas of Bangladesh, where they are known to farmers for their effectiveness, high toxicity and relatively low price. Although there was no trace of known pesticides found in the samples collected from the most vulnerable site during the study period, it does not mean that it would not happen in the future. Any pesticide used in the study area should provide similar LPI ranking as long as the organic matter content of the area remains the same.
Conclusions
It is important to identify the areas susceptible to groundwater contamination by pesticides for the management of pesticides in agricultural fields. This initial effort involved selecting the most vulnerable site based on its leaching potential index (LPI) under field conditions where abundant groundwater supplies occur in a shallow unconfined aquifer and crop fields are intensively irrigated. LPI values were ranked by quantiles into very high, high, moderate, low and very low categories and based on these rankings, the most vulnerable site was identified. Higher values of LPI indicate a greater susceptibility of groundwater contamination by pesticide leaching. Spatial distribution of LPI suggests that an effective sampling effort need not be uniform, but should focus on the small number of high LPI areas. Next, pesticide movement in this most vulnerable site was studied using the MT3D model. Model results revealed that low persistence pesticides are generally non-threatening to health, while pesticides with low sorptivity should be taken as a serious concern as they pose a significant impact on groundwater. Decreasing organic matter of the soil and increasing the half-life of pesticides at deeper depths did not show any significant change. Results revealed that top soils are particularly vulnerable to the accumulation of organochlorine pesticides. Finally, soil samples at different depths were collected from the same site and analyzed for the presence of pesticide residues. Chromatogram results revealed that there was no trace of pesticide residues; however, some unknown chemicals were detected. This might be because of the use of some unknown varieties of chemicals in the study area.
References
Agertved J, Rugge K, Barker JF (1992) Transformation of the herbicide MCPP and atrazine under natural aquifer conditions. Groundwater 30(4):500–506
Aller LT, Lehr JH, Petty RJ (1985) DRASTIC: a standardized system for evaluating groundwater pollution potential using hydrogeologic settings, US EPA (600/2-85/0180)
Anwar AHMF, Yunus A (2010) Pesticide leaching potential in a shallow unconfined aquifer. J Water Environ Technol 8(1):1–16
Bangladesh Bureau of Statistics (BBS) (2000) Yearbook of agricultural statistics of bangladesh. Bangladesh Bureau of Statistics, Bangladesh
Breeuwsma A, van Duijvenbooden W (1987) Mapping of groundwater vulnerability to pollutants in the Netherlands. In: van Duijvenbooden W, van Waegeningen HG (eds) Proceedings of international conference on vulnerability of soil and groundwater to pollutants. National Institute of Public Health and Environmental Hygiene, pp 309–320
Dean JD Jowise PP, Donigan AS, Jr (1984) Leaching evaluation of agricultural chemicals handbook (LEACH),”EPA-600/3-84-068, US Envir. Protection Agency Environmental Research Laboratory, Athens
Gish TJ, Isensce AR, Nash RG, Helling CS (1991) Impact of pesticides on shallow groundwater quality, soil and water division. Trans ASAE 34(4):1745–1753
Hornsby AG (1995) How contaminants reach groundwater. Soil Science Fact Sheet, SL-48, Institute of Food and Agriculture Sciences, University of Florida, Gainesville
Islam S (2000). Integrated pest management and green farming in rural poverty alleviation in Bangladesh. In: Proceedings of the regional workshop on integrated pest management and green farming in rural poverty alleviation, Suwon, Republic of Korea, 11–14 Oct 2000
Joshua WD, Rahman M (1983) Physical properties of soils on the Teesta river floodplain and barind tract of Bangladesh. Soil Research and Development Institute, Dhaka
Jury WA, Focht DD, Farmer WJ (1987) Evaluation of pesticide ground water pollution from standard indices of soil-chemical adsorption and biodegradation. J Environ Qual 16(4):422–428
Karim MA (1984) Upazilla-wise groundwater recharge conditions of Bangladesh. Groundwater Investigation Circle, Bangladesh Water development Board, Dhaka
Kookana RS, Aylmore LAG (1994) Estimating the pollution potential of pesticides to groundwater. Aust J Soil Res 32:1141–1155
Le Seur LP, Wehrmann HC, Schock SC, Shafer JM (1987) Prioritizing Areas for State-wide Groundwater Monitoring. J Water Resour Plan Manag 113(2):204–215
Meeks YJ, Dean JD (1990) Evaluating Groundwater Vulnerability to Pesticides. J Water Resour Plan Manag 116(5):693–707
Mercer JW, Waddell RK (1992) Contaminant Transport in Groundwater, Chapter 16. In: Maidment DR (ed) Handbook of Hydrology. McGraw Hill, New York
Pagiola S (1995) Environmental and natural resource degradation in intensive agriculture in Bangladesh. Environmental Economic Series, Paper No. 15, Environmentally Sustainable Development, The World Bank
Rao PSC, Hornsby AG, Jessup RE (1985) Indices for ranking the potential for pesticide contamination of groundwater. In: Proceedings of Soil and Crop Science Society of Florida, pp 1–8
Schlosser AA, McCray JE, Murray KE, Austin B (2002) A subregional-scale method to assess aquifer vulnerability to pesticides. Groundwater 40(4):361–367
Sichani SA, Engel BA, Monke EJ, Kladivko EJ (1991) Validating GLEAMS with pesticide field data on a Clermont silt loam soil. Trans ASAE 34(4):1732–1737
Soil Resources and Development Institute-SRDI (1990) Land and Soil Resources Use Guidebook-Gangachara Thana. Ministry of Agriculture, Government of Bangladesh, Rangpur district
Teso RR, Younglove T, Peterson MR, Sheeks DL, Gallavan RE (1988) Soil taxonomy and surveys: classification of areal sensitivity to pesticide contamination of groundwater. J Soil Water Conserv 43(4):348–352
Truman CC, Leonard RA (1991) Effect of pesticide, soil, and rainfall characteristics on potential pesticide loss by percolation: a GLEAMS simulation Soil Water Div Trans ASEE 34(6):2461–2468
Vogue PA, Kerle EA, Jenkins JJ (1994) OSU Extension pesticide properties database, National pesticide information centre, Oregon State University. http://npic.orst.edu/ppdmove.htm
Worthing CR, Hance RJ (1991) The Pesticide Manual, 9th edn. British Crop Protection Council, Surrey
Yunus A (2004) An investigation of pesticide transport in soil and groundwater. Department of Water Resources Engineering, Bangladesh University of Engineering and Technology, Dhaka, MSc Eng Thesis
Zheng C (1992) MT3D: a modular three-dimensional model for simulation of advection, dispersion, and chemical reactions of contaminants in ground water systems. Version 1.5, S.S. Papadopulos & Assoc., Bethesda
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Faisal Anwar, A.H.M., Yunus, A. Groundwater vulnerability to pesticides in Northwest Bangladesh. Environ Earth Sci 70, 1971–1981 (2013). https://doi.org/10.1007/s12665-013-2708-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12665-013-2708-1