Abstract
Arid and semiarid areas face major challenges in the management of scarce groundwater. This valuable resource is under pressures of population, economic expansion, contamination and over-exploitation. This research investigates groundwater vulnerability to pesticide contamination in the Al-Kharj area of Saudi Arabia. It explores the spatial distribution of pesticide concentrations in groundwater and other relevant factors. Thin permeable soils, permeable aquifers and shallow water tables, which are prevalent in the area, are especially vulnerable to pesticides. Analyses of 40 groundwater samples were performed using a gas chromatograph mass spectrometer coupled with a quadrupole mass spectrometer with a GC column. The analysis was conducted to detect 32 pesticides from different chemical families, and a total of 22 pesticides were detected. All 40 water samples were positive for at least one of the pesticides studied. In total, 21 compounds were above the quantification limit and 10 of them exceeded the legal limit. Total pesticide levels ranged from 0.18 to 2.21 μg/L, and 68 % of the analyzed samples exceeded the maximum allowable pesticide concentrations established by the European Community. Comparison of the daily intake peak (DIP) and daily intake mean (DIM) relative to the acceptable daily intake (ADI) shows that groundwater contamination with pesticides is a serious problem. Prolonged exposure to pesticides can cause adverse effects to human health and the ecosystem. Spatial distribution maps of groundwater contamination were developed using GIS. These maps will help risk managers identify vulnerable sources and provide a relative assessment of pesticide hazards to human health and the environment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Groundwater is one of the most important resources in arid countries like Saudi Arabia, being the main source of water for agriculture, industry and the public drinking water supply. In Saudi Arabia, groundwater provides >83 % of the water used in agriculture, and >81 % of the public water supply (The World Bank 2012). The unwise widespread use of pesticide has been of growing concern in agricultural areas. It has been estimated that 2.5 million tons of pesticides are being applied worldwide each year and there is an increasing trend in usage (Pimentel 1995). Therefore, an evaluation of the factors impacting groundwater contamination with pesticides is directly required. These factors are land use, depth to water, rainfall intensity, groundwater recharge, soil type and ground surface slope, pesticide mobility, adsorption, absorption and preferential flow characteristics (Tiryaki and Temur 2010; Hosseini 2012; Milhome et al. 2015). Pesticide and toxic persistent chemical drift have been a significant environmental problem in rural areas, and it may result in damage to human health (Crowe and Milburn 1995; Cohen et al. 1986; Trautmann et al. 1998; Reimer and Prokopy 2012; Youssef et al. 2015; Xiong et al. 2015). Herrero-Hernández et al. (2013) detected forty compounds among fifty-eight pesticides analyzed in water samples of the Spanish wine region. USEPA (1994) have imposed an upper limit of 2–3 µg/l for concentrations of Atrazine in drinking water. Once contaminated, the groundwater may take a long time to clear and there is always the danger of bioaccumulation (Premazzi and Ziglio 1995). The presence of pesticides in waters for human consumption is regulated by national and international rules that establish the maximum concentration of each pesticide at 0.1 µg/L and the total amount of pesticides at 0.5 µg/L in drinking water (European Community 1998; USEPA 2012).
The current pesticide concerns include their widespread usage, high toxicity and environmental persistence. In Saudi Arabia, pesticide applications have increased rapidly over the past four decades. The rapid development of agriculture required the use of a wide number of pesticides on crops (Al-Wabel et al. 2011). There is little environmental monitoring of pesticides. Pesticide consumption in Saudi Arabia has grown significantly from 5000 tons (12.3 million $) in 1976 to 20,000 tons (88.4 million $) in 1990 (Al-Saleh et al. 1999). This consumption rate jumped to 323,000 tons in 2010. In the study area, there are different crop groups accounting for the pesticide consumption, such as cereals, dates, watermelons, citrus fruits, tomatoes, potatoes and maize (Al Majathoub 2012). Atrazine and other pesticides are present in the groundwater of Saudi Arabia at levels greater than what is allowed by USEPA MCL standards (El-Saeid et al. 2011). Chronic health effects are associated with pesticide exposure, and the local farm workers are being at particular risk due to unsafe application methods and adverse living and working conditions (Maroni and Fait 1993; Dalvie et al. 1999).
The Al-Kharj area has fertile soil and is an important area for agricultural activities. The lack of proper management and excessive use of chemical fertilizers and pesticides have caused groundwater resources to become contaminated. Assessment of residual pesticides in groundwater can help in decision making for groundwater management in this area, where the groundwater is utilized as a drinking water supply. The main research focus is the evaluation of groundwater quality through the determination of pesticide residues. However, the overall goals are to improve our understanding of pesticide contamination risk and explore its implications for human health. The results obtained can be useful in developing strategies to minimize the impact of pesticides to humans and the environment. This will help to identify high-risk zones to decision makers for better risk assessment and management.
Materials and methods
Study area
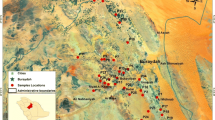
The area of Al-Kharj lies between the latitudes 23°35′–24°35′N and the longitudes 46°55′–48°10′E in the eastern part of the Najd, central Saudi Arabia (Fig. 1). An arid desert climate prevails. The average temperature is around 29 °C (minimum 10 and maximum 44 °C) and is rarely below 6 or above 46 °C. The relative humidity typically ranges from 6 to 65 % over the course of the year. There is a high annual evaporation rate (3070 mm), which is more than 32 times the annual rainfall. Annual rainfall is low, rarely exceeding 100 mm (94.6 mm). It varies throughout the year. This precipitation usually comes in the form of moderate rain, thunderstorms and light rain (PME 2014; Weather Spark 2015). These climatic factors have an impact on the water resources of the study area, and they are the cause of surface water scarcity.
Geology and hydrogeology
The study area grades upward from 526 meters in the low-lying area (in the south eastern part) to 684 m in the southwestern part. There are two series of escarpments, namely the Najd plateau and Tuwayq. They exist as a succession of northwest-trending cuestas of late Jurassic to Cretaceous formations, gently dipping eastward (Fig. 1). Several wadis such as Nisah, Hanifa, Raghib Al-Ayn and As-Sabha run from the Tuwayq escarpments across the plateau in a generally west–east-oriented course. The Tuwayq mountains are dissected by a system of parallel major faults and grabens (Wadi Nisah) running in an East–West direction.
Al-Kharj Plain consists of eastward-dipping alluvium deposits of sand and gravel. Depending on the location, these alluvium deposits will be either hydraulically connected with Wasia-Bayiadh sandstone or with Sulaiy-Arab limestone, in both cases forming a good shallow aquifer. The wadis flow into Al-Kharj Plain, creating a large alluvial fill within a wide valley which recharges groundwater from precipitation and runoff. Toward the center of the Al-Kharj area, these wadis have brought considerable amounts of alluvial deposits with thickness varying between 30 and 65 m. In this area, the shallow aquifer ranges from being unconfined to semiconfined, where the water level responds significantly to the amount of precipitation and runoff. Groundwater flows from upstream areas in the western part to downstream area in the east. According to location, the depths to groundwater vary between 30 and 120 m. There are several springs and marshes, but due to the heavy pumping by wells, some of them are now dry. Chemical dissolution of limestone by groundwater has led to the formation of up to 150-m-deep karst sinkholes south of Al-Kharj (Vaslet et al. 1991).
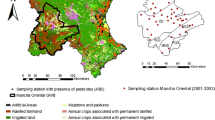
Different types of land uses are recognized within the study area. The northern and western parts are represented by an agricultural development area, while the central and southern parts are dominated by Al-Kharj city (urban built-up and inhabited rural areas). The main pollution sources are as follows: (1) the excessive application of fertilizers and pesticides of cultivated lands, and (2) the prevalence of unsewered rural and urban lands of long-term influence. There are large numbers of drilled wells pumping out vast quantities of groundwater, because of good groundwater quality in the study area, and it is one of the most productive agricultural areas in Saudi Arabia. Unfortunately, due to the intensive use of pesticides and fertilizers, the limited groundwater resources face the danger of contamination. Agricultural modernization relies on the concept of maximum productivity, with intense exploitation of natural resources, and this generates new risks and environmental vulnerabilities. The pesticides applied to farmland can move downward with deep percolation from the root zone to the underlying groundwater. Consequently, the maintenance of groundwater quality is a major water management concern.
The salinity of the sampled groundwater varied from 957 to 3366 mg/L, with an average value of 1848 mg/L, with a standard deviation of 539. According to Chebotarev (1955), there are three main classes of groundwater. The samples represent two of these classes, fresh and brackish, and four subclasses (Table 1). The spatial salinity map of this alluvium aquifer is plotted in Fig. 2. Generally, there is an increasing trend in salinity from east to west, and from the center of the wadi courses to the embankments. This is because the modern groundwater recharge from rainfall and runoff is remarkably restricted to wadi networks, while to the east, the old fresh groundwater of the Wasia-Bayiadh sandstone and Sulaiy-Arab limestone was recharged in the Pleistocene. The high peaks of salinity in the western part are related to over-exploitation areas, irrigation return flows, and upward movements of saline water.
Sampling and chemical analysis
In April 2014, two-week field trips were carried out, where the geology, hydrogeology and land use of the study area were investigated and verified. Forty groundwater samples were collected from the wells after pumping water for about 10 min (Fig. 1). The variables recorded were as follows: water level, pH, water temperature, dissolved oxygen and other hydrochemical parameters. One-liter groundwater samples were collected and stored in amber bottles, and kept under refrigeration (4 °C). The sampling and cleaning of the bottles and glassware were carried out as described by Standard Methods for the Examination of Water and Wastewater (APHA 2005).
Chemical analyses were conducted by the Analytical Chemistry Laboratories of the Soil Department at King Saud University. A gas chromatograph mass spectrometer (Agilent model 6890 N) was used. It is attached with a quadrupole mass spectrometer (model 5975B) with a GC column HP-5MS 5 % phenyl and 95 % methyl siloxane, and 30 × 0.25 mm id × 0.25 µm film thickness (El-Saeid and Selim 2013). The GC operating settings were as follows: splitless injection, injector temperature 250 °C, helium carrier gas (99.9999 purity) at a flow rate of 0.9 mL/min with a column head pressure of 7.4 psi, oven temperature from 70 °C (2 min hold), then raised to 130 °C at the rate (25 °C/min) afterward raised to 220 °C at (2 °C/min) and then raised to 280 °C at (10 °C/min) and eventually (4.6 min hold) (El-Saeid and Selim 2013). An appropriate aliquot (2 µl) of samples was injected in the gas chromatography mass spectrometer under these conditions. Then, pesticide residues were identified by comparing retention time values with the reference standard (Table 2). Results were confirmed using the selected ion monitoring (SIM) mode, and one is the quantifier and the qualifying ion (El-Saeid and Selim 2013).
The resulting data were subjected to strict quality control measures, including the analysis of procedural and instrumental blanks. Blanks were used to avoid laboratory contamination and analytical interferences. All the laboratory blanks were below the detection limit.
Recoveries were determined for all samples by spiking with a standard mix of pesticides prior to extraction. This resulted in confirming a high level of precision and high recoveries of the pesticide residues. Analytical method recoveries for the given set of pesticides were also determined by repeating the whole analytical procedure with the reference water enriched by the standard mix of pesticides of the known concentration. The recovery of targeted pesticides tested in spiked groundwater samples ranged from 89.9 to 99.2 % as shown in Table 2. The target pesticide quantification based on the peak area ratio of the target ion divided by the internal standard. Reproducibility was calculated based on the replicate analyses, with relative standard deviations (RSD, %) less than 3.5 %. Further details of the method have been previously published (Kouzayha et al. 2012). The limit of detection (LOD) was determined by performing a GC–MS quantification using a four-point calibration curve. The peak areas of 32 pesticides were plotted versus concentrations using dilution levels ranging from 0.0001 to 5.0000 mg/L.
Results and discussion
Occurrence and spatial distribution of pesticide residues
The natural flow of groundwater promotes the formation of pores or openings which facilitate water infiltration and carry the contaminants to the groundwater. The quantification limits (2 × SD) of the groundwater samples are presented in Table 2. Thirty-two pesticides from different chemical families were investigated using the GC method, of which a total of twenty-two were detected. The remaining ten were not detected in any sample.
All forty groundwater samples were positive for at least one pesticide residue. The hydraulic conductivity of the alluvium sediments is good. This is one of the important parameters influencing groundwater movement and contamination, because the high hydraulic conductivity and surface recharge make the transport of pesticides fast, which in turn makes the concentration of the pesticides high. Twenty-one compounds were above the quantification limit, and ten of them were even above the legal limit of 0.1 mg/L. Table 2 shows the detection frequency (%), recovery (%) and the range of concentrations detected for each pesticide. Carbaryl was the most frequently detected pesticide in the sampled groundwater (82.5 %). Lindane (γ–HCH) (75 %), atrazine and dimethoate (72.5 %), endosulfan (70 %), chlorpyrifos (57.5) and heptachlor (52.5 %) were also frequently detected. Other pesticides had a moderate detection frequency, such as cypermethrin (45 %), chlorpyrifos methyl (40 %), deltamethrin (27.5 %) and fenoxycarb (22.5 %). The spatial distributions, uses, concentrations, and contamination risks of these pesticides’ residue will be discussed in the following paragraphs.
Carbaryl is an insecticide used frequently by farmers on a broad spectrum of crops. The concentration in the samples varies from ND to 0.94 μg/L, with an average value of 0.12 μg/L (Table 2; Fig. 3). It is common in the sampled groundwater (82.5 %), in spite of the fact that it is has a moderate affinity to be soluble in water. However, its solubility increases with increasing temperature and the amount of organic solvents in water (USEPA 2003). Twenty-five percent of the samples analyzed were above the legal limit (>0.1 μg/L). Carbaryl has a half-life in soil ranging from 4 to 72 days. It breaks down faster in sandy, flooded or well-aerated soils. It does not accumulate in mammals and is rapidly metabolized to nontoxic substances which are eliminated in the urine and feces.
Lindane (γ–HCH) is an insecticide used on fruit and vegetable crops, seed treatment and in forestry (ATSDR 1989a; IPCS 1991). In spite of the fact that lindane is rarely leached to the groundwater, it was detected in about 75 % of the samples taken from agricultural areas. Its concentrations range from ND to 0.89 μg/L, with an average value of 0.09 μg/L (Table 2; Fig. 4). Of the samples, 22.5 % were above the legal limit (>0.1 μg/L), and lindane is a chemical which potentially causes nervous system effects from short-term exposures at levels above the MCL (USEPA 2012). Lindane degrades faster at lower soil depths, since anaerobic degradation is more rapid than aerobic degradation. Its half-life under aerobic conditions ranges from 88 to 1146 days, and under anaerobic conditions from 12 to 174 days. Due to the absence of genotoxicity and the weight of the evidence from studies of carcinogenicity, it is concluded that lindane is not likely to pose a carcinogenic risk to humans (WHO 2004a).
Atrazine is a widely applied herbicide used to control weeds in maize and sorghum. It has mutagenic effects on aquatic organisms (Ventura et al. 2008; Vryzas et al. 2009). It is moderately persistent in the environment and degrades in water within 3 months. Atrazine was detected in 72.5 % of the samples. Its concentrations range between ND and 1.3 μg/L, with an average value of 0.26 μg/L (Table 2; Fig. 5). The contamination of groundwater by atrazine in agricultural areas can be increased by fast transport processes which are controlled by topography, soil properties, and vegetation type. Contamination of well water may be caused by leaching near the wellhead due to atrazine loading. A total of 37.5 % of the samples were above the legal limit (>0.1 μg/L). Atrazine has the potential to cause weight loss, cardiovascular damage, retinal and some muscle degeneration, and mammary tumors from lifetime exposure at levels above the MCL (USEPA 2012). Ocular and dermal irritation, chest pain, dizziness and nausea have been reported following dermal, oral or inhalation exposures to contaminated well water from adjacent fields sprayed with atrazine (WHO 1996).
Dimethoate is an organophosphate insecticide used to kill a wide range of insects on a wide range of crops. Dimethoate has been used to livestock for control of botflies. Dimethoate is highly toxic to honey bees, livestock and other wildlife (Clarke et al. 1981). It is highly soluble in water, it has very weak adsorption affinity to soil particles, and therefore, it may be subject to considerable leaching to the groundwater. Dimethoate is detected in about 72.5 % of the samples, with concentrations ranging from ND to 0.09 μg/L, and an average value of 0.04 μg/L (Table 2; Fig. 6). All of the samples were below the legal limit (>0.1 μg/L). Under anaerobic conditions and hydrolysis, microbe activities represent the major degradation pathway.
Endosulfan is found to be widespread in 70 % of the samples, with concentration values varying from 0.02 to 0.33 μg/L, and an average value of 0.07 μg/L (Table 2; Fig. 7). The slightly higher frequency of endosulfan might be explained by the higher levels of spraying during the irrigation period. Twenty percent of the samples are above the legal limit (>0.1 μg/L). Endosulfan in the soil has a half-life of 120 days and a sorption coefficient of 17.52 L/g (McGregor 1999). Its chronic effect value is 0.01 μg/L, and its acute effect value is 0.02 μg/L (DWAF 1996).
Chlorpyrifos is an organophosphorus insecticide, used for the control of mosquitos, flies and various crop pests. It is also used as a soil treatment (preplanting and at planting). It is detected in nearly 57.5 % of the samples with concentrations varying between ND and 0.17 μg/L and an average value of 0.04 μg/L (Table 2; Fig. 8). Ten percent of the samples are above the legal limit (>0.1 μg/L). Although chlorpyrifos has low leachability, it still has a high contamination potential because of its intensive use in the area, which has been confirmed by personal communications with local farmers. This causes it to infiltrate the groundwater by way of irrigation return flows.
Chlorpyrifos then persists in the soil for 60–120 days and degrades primarily through microbial action. WHO (1999) has classified chlorpyrifos as moderately hazardous. Organophosphates such as chlorpyrifos cause neurobehavioral disorders, including anxiety and depression. This may even lead to suicide cases in individuals exposed to pesticides of this group. This also applies to other organophosphates such as dimethoate (Dell’Omo and Shore 1996). Assuming a 10 % allocation of the acceptable daily intake (ADI) of 0.01 mg/kg of body weight to drinking water, the guideline value for chlorpyrifos is therefore 30 µg/L (WHO 2004b).
Heptachlor is an organochlorine compound that acts as a nonsystemic stomach and contact insecticide. It is used on seed grains and crops to kill termites. Although heptachlor is almost insoluble in water and is therefore expected to be unlikely to contaminate groundwater (Hartley and Kidd 1983), it is nevertheless detected in about 52.5 % of the samples, with a concentration varying from ND to 0.09 μg/L, and an average value of 0.05 μg/L (Table 2; Fig. 9). The legal limit (>0.1 μg/L) was exceeded in 12.5 % of the samples. The prevalence of this insoluble pesticide in the groundwater is due to its intensive use in the area, causing it to infiltrate the groundwater by way of irrigation return flows. Heptachlor is highly toxic to humans, since it can be absorbed through the skin, lungs and gastrointestinal tract. It causes hyper-excitation of the central nervous system, and damage to the liver (OHS 1991). The USEPA has established a lifetime health advisory level for heptachlor of 17.5 μg/L. This means that a person may drink water containing heptachlor at or below these levels daily over the course of a lifetime with no health effects. The US Public Health Service has published a health advisory which is based on cancer risk and which recommends a 0.0104 μg/L limit for long-term exposure in drinking water (ATSDR 1989b). In 1988, the USEPA stopped all commercial uses of heptachlor in the USA except for the control of fire ants in power transformers, though it is still available for agricultural uses outside the USA (WHO 1984; ATSDR 1989b; Meister 1992).
Cypermethrin is a synthetic pyrethroid insecticide used to control many pests, including moth pests of cotton, fruit and vegetable crops (Meister 1992). It is a moderately toxic material by dermal absorption or ingestion. Long-term exposure to cypermethrin may cause liver changes (OHS 1993). It is detected in about 45 % of the samples, with concentrations varying between 0.02 and 0.82 μg/L, and an average value of 0.09 μg/L (Table 2; Fig. 10). Ten percent of the samples are above the legal limit (>0.1 μg/L).
Chlorpyrifos methyl is an organophosphorus insecticide used in agriculture and not registered for residential use. It demonstrates moderate acute toxicity in animal studies. It has been widely used to control insects on food crops such as corn. It also has been applied directly on animals to kill mites, applied to structures to kill termites, and sprayed to kill mosquitoes. It is detected in about 40 % of the samples, with concentrations varying between 0.03 and 0.86 μg/L and an average value of 0.05 μg/L (Table 2; Fig. 11). Five percent of the samples are above the legal limit (>0.1 μg/L).
Deltamethrin is a pyrethroid insecticide and veterinary treatment. It has a low aqueous solubility, is nonvolatile and has a low potential to leach to groundwater. It is a fast-acting insecticide used to control a wide range of pests including Coleoptera, Heteroptera and Homoptera. It is detected in about 27.5 % of the samples with concentrations varying between 0.02 and 0.68 μg/L and an average value of 0.06 μg/L (Table 2; Fig. 12). The legal limit (>0.1 μg/L) is exceeded in 12.5 % of the samples. Deltamethrin is highly toxic to humans and other mammals and is a neurotoxin. It is relatively nontoxic to birds and earthworms although it presents a high risk to most aquatic organisms and honeybees.
Fenoxycarb is a carbamate insect growth regulator and is a slightly toxic compound. It is used as fire ant bait and for flea, mosquito and cockroach control, especially on stored products. Fenoxycarb is readily broken down in soil by hydrolysis. Residues in soil are no longer detectable 3 days after application. The compound also has a low potential for leaching from the soil and has a moderate to strong soil binding tendency (USEPA 1983–1985). These characteristics of fenoxycarb in soil indicate that it is should be unlikely to contaminate groundwater. However, it is detected in about 22.5 % of the samples with concentrations between 0.03 and 0.17 μg/L and an average value of 0.02 μg/L (Table 2; Fig. 13). Five percent of the samples are above the legal limit (>0.1 μg/L). This could be explained by heavy use coupled with the shallow water table and high soil permeability in the study area.
Nitrate concentrations of the groundwater samples were also evaluated in order to make correlations with the pesticide residues, since groundwater contamination with both of them are derived from the agricultural activities. One important source of nitrates is fertilizers, which are used to increase the availability of nitrogen, phosphorus and potassium to plants. Other sources of nitrate are the discharge of effluent from intensive livestock units, leachate from manure stores, leaking slurry pits, and slurry or manure spreading on land. Nitrate concentration values in the samples vary between 0 and 59 mg/L with an average value of 13 mg/L (Fig. 14; Table 3). Table 3 shows that nitrate values exceed the maximum contaminant level for drinking water in 7.5 % of the samples. While 30.0 % of the samples indicate elevated concentrations, this certainly results from human activities (Madison and Brunett 1985; USEPA 2012). Nitrate is the main nutrient leached to groundwater, and this is because it is highly soluble, mobile and not readily degraded under aerobic conditions. Nitrite and organic species are metastable compounds in aerated water, whereas ammonium is strongly sorbed on mineral surfaces. The risk of groundwater pollution by nitrate depends on the interaction of the nitrogen loading and the vulnerability of the aquifer. The alluvium aquifer in the study area is composed of sand and gravel, and there are no clay strata separating the saturated aquifer from the land surface activities, so the aquifer is adversely affected by pollution loads applied at the agriculture lands. Consequently, the potential for attenuation of pollutants by processes such as sorption, degradation, ion exchange, filtration and precipitation is small.
Health risk assessment
In the samples, the total pesticide levels range from 0.18 to 2.21 μg/L (Fig. 15). In 68 % of the samples, total pesticide levels are above the legal limits established by the European Community (>0.5 μg/L). All of the samples show the presence of pesticides at total levels ranging from 0.18 to 2.21 μg/L. The most contaminated sample is sample no. 36, with seven pesticides (2.21 μg/L). The high concentration of pesticide residues in the samples from the study area could be explained by intensive use of pesticides, a shallow water table and high soil hydraulic parameters. Table 4 shows the values of acceptable daily intake (ADI), acceptable daily consumption, and mutagenicity and carcinogenicity of the pesticides detected.
In order to determine the health risks posed by the pesticide contamination, two scenarios were modeled: a worst-case scenario where drinking water concentrations are characterized at the highest concentrations detected in the area; and an average scenario where the mean pesticide concentrations are used. Both of the two scenarios are then used to estimate total daily human intake of pesticides, which is then compared to published acceptable daily intakes (ADIs), to calculate a percentage of ADI derived from water consumption. Estimates are based on an average daily water consumption of 2 L of water per day and a person weighing 70 kg.
The actual daily intake for most of the pesticides is relatively low when compared to WHO acceptable daily intake values. However, drinking water intake is thought to pose a health risks if it exceeds 1–10 % of ADI, because chronic exposures to low levels of pesticides are able to affect human health. Also, regular use of pesticides in agriculture can cause their dispersal into the environment, affecting other organisms in the ecosystem (Cid et al. 2007). In the worst-case scenario, with the exception of fenoxycarb and cypermethrin, the rest of pesticide residues have DIP % in excess of 10 % of ADI. In the average scenario, the mean estimates of four pesticides (DIM %) still exceed the 10 % level, these being lindane, atrazine, dimethoate and heptachlor. Therefore, it is reasonable to infer that these levels reveal a serious problem of groundwater contamination with pesticides. It should be noted that the calculations in Table 4 do not take account of vulnerable groups, such as children who have a higher consumption per kg body weight.
Conclusion
Groundwater contamination with pesticides is a critical issue, especially in arid areas, since groundwater is the main drinking water resource. The rapid increase in pesticide usage has led to greater concern that pesticide residues could reach groundwater at problematic concentrations. Thin permeable soils, permeable aquifers and shallow water tables are especially vulnerable to pesticides. Irrigation and runoff waters are considered to be an important trigger for pesticides leaching. Thirty-two pesticides from different chemical families were investigated by the GC–MS method, and 22 pesticides were detected in the groundwater of Saudi Arabia’s Al-Kharj area. All 40 samples were positive for at least one pesticide residue. Since water quality is controlled by national and international standards, 68 % of the analyzed samples are above tolerable pesticide levels established by the WHO, USEPA and European Community, and they are characterized as being unfit for human consumption. The comparison of DIP and DIM to ADI shows that pesticide residue levels in groundwater pose a serious health problem, considering the high risks caused by prolonged exposure to these residues. The study has shown the need for continuous monitoring of pesticide residue levels in the area’s groundwater, as well as the development of drinking water quality standards for specific pesticides. Further studies investigating the health effects of pesticides should be undertaken. This is because the effects of the combined presence of more than one pesticide in drinking water might be different than the effects of each individual pesticide alone. Policies aimed at reducing the potential for groundwater contamination by pesticides need to be developed and implemented.
References
ADI List (2014). Acceptable daily intakes for agricultural and veterinary chemicals. The Office of Chemical Safety Department of Health. ISSN 1446-1412, 114 p.
Al Majathoub, M. (2012). Market access and registration procedure Saudi Arabia. International Crop Science Conference & Buyer-Seller Meet 2012. Pesticides Manufacturers & Formulators Association of India. Crowne Plaza Hotel Dubai, Sheikh Zayed Road, Dubai- UAE.
Al-Saleh, I., Al-Doush, I., Echeverria-Quevedo, A. (1999). Residues of pesticides in grains locally grown in Saudi Arabia. Bulletin of Environmental Contamination and Toxicology, 63, 451–459.
Al-Wabel, M., El-Saied, M., Al-Turki, A., & Abdel-Nasser, G. (2011). Monitoring of pesticide residues in Saudi Arabia agricultural soils. Research Journal of Environmental Sciences, 5, 269–278.
APHA (2005). American Public Health Association, Standard Methods for the Examination of Water and Wastewater, Method 1020.
ATSDR (1989). Toxicological profile for alpha-, beta-, gamma- and delta-hexachlorocyclohexane. Atlanta, GA, US Department of Health and Human Services, Public Health Service, Agency for Toxic Substances and Disease Registry.
ATSDR (1989). Toxicological Profile for Heptachlor/Heptachlor Epoxide, ATSDR/TP-88/16. ATSDR, US. Public Health Service, Washington, DC.
Chebotarev, I. (1955). Metamorphism of natural water in the crust of weathering. Geochimica Cosmochim Acta, 8, 22.
Cid, F., Anton, R., & Caviedes-Vidal, E. (2007). Organochlorine pesticide contamination in three bird species of the Embalse La Florida water reservoir in the semiarid midwest of Argentina. Science of the Total Environment, 385(1), 86–96.
Clarke, M., Harvey, D., & Humphreys, D. (1981). Veterinary toxicology (2nd ed., p. 153). London: Bailliere Tindall.
Cohen, S., Eiden, C., & Lorber, M, (1986). Monitoring groundwater for pesticides. In W. Y. Garner, Honeycutt, R. C. & H. N. Nigg (Eds.), Evaluation of pesticides in groundwater. ACS Symposium Series 315, American Chemical Society, Washington, DC, pp. 170–196.
Crowe, A., & Milburn, P. (1995). The contamination of groundwater in Canada from pesticides. Water Quality Research Journal of Canada, 30(3), 363–561.
Dalvie, M., White, N., Raine, R., et al. (1999). Long-term respiratory health effects of the herbicide, paraquat, among workers in the Western Cape. Occupational and Environmental Medicine, 56, 391–396.
Dell’Omo, G., & Shore, R. (1996). Behavioral and physiological effects of acute sublethal exposure to dimethoate on wood mice Apodemus sylvaticus. Archives of Environmental Contamination and Toxicology, 31(1), 91–97.
DWAF (Department of Water Affairs and Forestry) (1996). South African water quality guidelines. Pretoria, 1–7.
El-Saeid, M., & Selim, M. (2013). Multiresidue analysis of 86 pesticides using gas chromatography mass spectrometry: II-Nonleafy vegetables. Journal of Chemistry, 2013, 1–10.
El-Saeid, M., El-Turki, A., Al-Wable, M., et al. (2011). Evaluation of pesticide residues in Saudi Arabia ground water. Research Journal of Environmental Sciences, 5(2), 171–178.
European Community (1998). Council Directive 98/83/EC. Retrieved October 1, 2015, http://faolex.fao.org/docs/pdf/eur18700.pdf.
Hartley, D., & Kidd, H. (1983). The agrochemicals handbook. Nottingham, England: Royal Society of Chemistry.
Herrero-Hernández, E., Andrades, M. S., Álvarez-Martín, A., et al. (2013). Occurrence of pesticides and some of their degradation products in waters in a Spanish wine region. Journal of Hydrology, 486, 234–245.
Hosseini, A. (2012). Modeling field-scale vulnerability to pesticide runoff. Dissertation, University of Nebraska.
IPCS (1991). Lindane. Geneva, World Health Organization, International Programme on Chemical Safety (Environmental Health Criteria 124).
Kouzayha, A., Rabaa, A. R., Al Iskandarani, M., Beh, D., Budzinski, H., & Jaber, F. (2012). Multiresidue method for determination of 67 pesticides in water samples using solid-phase extraction with centrifugation and gas chromatography mass spectrometry. American Journal of Analytical Chemistry, 3, 257–265.
Madison, R., & Brunett, J. (1985). Overview of the occurrence of nitrate in groundwater of the United States. Water-Supply Paper 2275. Reston, Virginia: U.S. Geological Survey.
Maroni, M., & Fait, A. (1993). Health Effects in man from long-term exposure to pesticides. A review of the 1975–1991 literature. Toxicology, 78, 1–174.
McGregor, F. (1999). The mobility of endosulfan and chlorpyrifos in the soil of the Hex River Valley. Dissertation, University of Cape Town.
Meister, R. (1992). Farm chemicals handbook ’92. Willoughby, OH: Meister Publishing Company.
Milhome, M., Sousa, P., Lima, F., & Nascimento, R. (2015). Influence the USE of pesticides in the quality of surface and groundwater located IN irrigated areas of Jaguaribe, Ceara Brazil. International Journal of Environmental Research, 9(1), 255–262.
OHS (Occupational Health Services). (1991). MSDS for heptachlor. Secaucus, NJ: OHS Inc.
OHS (Occupational Health Services). (1993). MSDS for cypermethrin. Secaucus, NJ: OHS Inc.
Pimentel, D. (1995). Amounts of pesticides reaching target pests: environmental impacts and ethics. Journal of Agricultural and Environmental Ethics, 8, 17–29.
PME. (2014). Metrological data of Al-Kharj area. Riyadh, KSA: Presidency of Meteorology and Environment.
PPDB (2014). Pesticide Properties Data-Base, Retrieved April 23, 2014. http://sitem.herts.ac.uk/aeru/ppdb/en/atoz.htm.
Premazzi, G., & Ziglio, G. (1995). Regulations and Management. In M. Vighi & E. Funari (Eds.), Pesticide Risk in Groundwater (pp. 203–240). Boca Raton: CRC Lewis Publishers. (Chapter 10).
Reimer, A., & Prokopy, L. (2012). Environmental attitudes and drift reduction behavior among commercial pesticide applicators in a U.S. agricultural landscape. Journal of Environmental Management, 113, 361–369.
The World Bank (2012). Policy Options for a National Water Strategy. Part One: Water Resources Assessment, Challenges and Options. Ministry of Water and Electricity, Riyadh, Saudi Arabia, 153 p.
Tiryaki, O., & Temur, C. (2010). The fate of pesticide in the environment. Journal of Biological and Environmental Sciences, 4(10), 29–38.
Trautmann, N., Porter, K., & Wagenet, R. (1998). Pesticides and groundwater: A guide for the pesticide user. Cornell University, Ithaca, New York. http://psep.cce.cornell.edu/facts-slides-self/facts/pest-gr-gud-grw89.aspx.
USEPA (1983–1985). Chemical Information Fact Sheet. Office of Pesticides and Toxic Substances, Office of Pesticide Programs (TS-766C), United Stated Environmental Protection Agency Washington, DC.
USEPA (1994). National primary drinking water standards. Washington, DC.
USEPA (2003). Revised EFED Risk Assessment of Carbaryl in Support of the Reregistration Eligibility Decision (RED). Office of Pesticide Programs, Environmental Fate and Effects Division, U.S. Environmental Protection Agency Washington, DC.
USEPA (2004). Chemicals Evaluated for Carcinogenic Potential. Science Information Management Branch Health Effects Division Office of Pesticide Programs U.S. Environmental Protection Agency, 22 p.
USEPA (2012). Edition of the Drinking Water Standards and Health Advisories. EPA 822-S-12-001 Office of Water U.S. Environmental Protection Agency Washington, DC, p. 9.
Vaslet, D., al-Muallem, M., Maddah, S., et al. (1991). Geologic Map of the Ar Riyadh Quadrangle, Sheet 24 I, Kingdom of Saudi Arabia [1:250.000] & Explanatory Notes to the Geologic Map of the Ar Riyadh Quadrangle, Kingdom of Saudi Arabia. Riyadh: Ministry of Petroleum and Mineral Resources.
Ventura, B., Angelis, D., Maria, A., & Morales, M. (2008). Mutagenic and genotoxic effects of the Atrazine herbicide in Oreochromis niloticus (Perciformes, Cichlidae) detected by the micronuclei test and the comet assay. Pesticide Biochemistry and Physiology, 90, 42–51.
Vryzas, Z., Vassiliou, G., Alexoudis, C., & Papadopoulou-Mourkidou, E. (2009). Spatial and temporal distribution of pesticide residues in surface waters in northeastern Greece. Water Research, 43, 1–10.
Weather Spark (2015). https://weatherspark.com/averages/32768/Al-Kharj-Riyadh-Saudi-Arabia.
WHO (1984). Environmental Health Criteria 38: Heptachlor. WHO, Geneva, Switzerland.
WHO (1996). Atrazine in Drinking-water: Guidelines for drinking-water quality, 2nd ed. Vol. 2. Health criteria and other supporting information. World Health Organization, Geneva, 1996.
WHO (1999). Recommended classification of pesticides by hazard and guidelines to classification 1998–1999. Geneva, (WHO/PCS/98.21/Rev. 1).
WHO (2004). Chlorpyrifos in Drinking-water “Background document for development of WHO Guidelines for Drinking-water Quality” WHO/SDE/WSH/03.04/87: 6 p.
WHO (2004). Lindane in Drinking-water “Background document for development of WHO Guidelines for Drinking-water Quality” WHO/SDE/WSH/03.04/102: 6 p.
Xiong, J., An, T., Zhang, C., & Li, G. (2015). Pollution profiles and risk assessment of PBDEs and phenolic brominated flame retardants in water environments within a typical electronic waste dismantling region. Environmental Geochemistry and Health, 37, 457–473.
Youssef, L., Younes, G., Kouzayha, A., & Jaber, F. (2015). Occurrence and levels of pesticides in South Lebanon water. Chemical Speciation and Bioavailability,. doi:10.1080/09542299.2015.1023092.
Acknowledgments
The authors wish to express their gratitude to Dr. David Jalajel for his valuable comments and manuscript revision. This project was financially supported by King Saud University, Vice Deanship of Research chairs.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
El Alfy, M., Faraj, T. Spatial distribution and health risk assessment for groundwater contamination from intensive pesticide use in arid areas. Environ Geochem Health 39, 231–253 (2017). https://doi.org/10.1007/s10653-016-9825-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10653-016-9825-1