Abstract
Concentrations and vertical distributions of total nitrogen (TN), total phosphorus (TP) and their different forms in sediments obtained from nine locations of Lake Dalinouer in September 2008 were analyzed. The results demonstrated that TP in surface sediments ranged from 0.493 to 0.904 g/kg, and inorganic phosphorus was the main fraction of total phosphorus, ranging from 335 to 738 mg/kg. Simultaneously, the autogenetic calcium phosphorus (ACa-P) was the main fraction of inorganic phosphorus, ranging from 145.4 to 543.2 mg/kg. Vertical distribution of different phosphorus forms in different sediment cores was distinguishing, and most of them tended to increase toward the surface sediment, indicated that the phosphorus concentration was related to the humanity with a certain extent. The relationships between TP and occluded phosphorus and ACa-P were significant. Nitrogen in the sediment was composed mainly of organic nitrogen, accounting for grater than 80 % of TN. NO3 −-N was the dominate fraction of inorganic nitrogen in the surface sediment, ranging between 51 and 346 mg/kg (151.1 ± 104.4 mg/kg), and accounting for between 2.2 and 17.7 % of total sediment nitrogen (6.2 ± 5.6 %). The ratio of organic carbon and TN in sediment was in range of 6.0–25.8 and presented a tendency of lake centre >lake sides, indicating that nitrogen accumulated in the sediments from lake sides came mainly from terrestrial source and nitrogen was mainly autogenetic in lake centre. Ratio of N:P in all sampling sites was below 14, indicated that N was the limiting nutrient for algal growth in this lake.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Lakes’ eutrophication has become one of the most serious environmental problems since 1970s in China. It has been widely recognized that phosphorus and nitrogen are two critical elements in the biogeochemical cycles due to their roles as the essential nutrient (Zhou et al. 2001; Kaiserli et al. 2002; Lü et al. 2005a, b; Wang et al. 2006). Consequently, the contents of total phosphorus and total nitrogen in the lake are always the most important indexes in the assessment of trophic status of lake (Hu et al. 2006). In general, nutrients reach surface water from many sources, and the main sources of phosphorus and nitrogen in lakes are external point and non-point sources such as runoff, industrial and municipal effluents. Moreover, the release of phosphorus and nitrogen from lake sediments is also an important internal source for overlying water of lakes (Ruban et al. 1999; Perkins and Underwood 2001; Balcerzak 2006). Bottom sediments which act as a source or become the sink in the exchange of nitrogen and phosphorus between overlying water and suspended particles under certain conditions, play vital roles in nitrogen and phosphorus cycle which involves physical, chemical, and biological processes (Wang et al. 2008; Liu et al. 2009). Surface sediment is the most active part in the interaction between water column and sediments, and it can release nitrogen and phosphorus into the water body to satisfy the growth of phytoplankton, on the other hand, when nutrients in the lake water are oversupplied, phytoplankton would bloom generating more fecal pellet and remains into sediments and boosting the organic matter content (Beretson 2001; Lü et al. 2005a, b; Li et al. 2007). Nitrogen and phosphorus in the sediment can be divided into organic and inorganic forms, both of which are needed by phytoplankton, zooplanktons, and microorganisms. However, not all of the phosphorus and nitrogen can be released from the sediments. In fact, the amount of phosphorus and nitrogen released from sediments to overlying water depends more on their contents of available fractions than on their total contents in sediments (Kaiserli et al. 2002). More efficient information for predicting potential ecological danger of nitrogen and phosphorus can be provided by the investigation of available fraction of them than by that of their total contents in lake sediments (Ruban et al. 1999; Zhou et al. 2001). Thus, it is necessary to know not only the contents of total phosphorus and nitrogen but also the contents of different fractions, which will help elucidate trends of these nutrients release into lake water (Wang et al. 2006).
The fractionation of phosphorus in lake sediments is complicated (Ribeiro et al. 2008), and the distribution and behavior of various forms of phosphorus in freshwater zone sediments have been studied by many researchers (Pettersson et al. 1988; Benitez-Nelson 2000; Wang et al. 2009; Zhu et al. 2013). Hosomi et al. (1982) distinguish five forms of phosphorus in the sediments of the lake of Kasumigaura: inorganic phosphate adsorbed on clay particles (Ads-P), inorganic phosphates precipitated with aluminum (Al-P), iron (Fe-P), calcium (Ca-P), and organic phosphorus. The changes that some phosphorus forms undergo during various biotic and abiotic processes cause phosphate ions to be released to interstitial and near-bottom waters (Emeis et al. 2000; Yurkovskis 2004). Accordingly, studies on the bioavailability of these phosphorus forms are most important for assessing their influences on eutrophication. There are many factors which may affect the concentration of phosphorus and its speciation in sediments such as the depth of water column, the magnitude of primary production, the oxygen conditions at the water–sediment interface, the hydrodynamic conditions, the chemical composition of the water and the sediments, the physical properties of sediments, and so on (Zabel et al. 1998; Gomez et al. 1999; Nilsson and Jansson 2002; Virtasalo et al. 2005; Łukawska-Matuszewska and Bolałek 2008; Wang et al. 2012). The content of total nitrogen in sediments is generally composed of three fractions: nitrate, ammonium, and organically fixed nitrogen proportion. Nitrogen fractions (NO3 −, NH4 +, Norg) in sediments are also important to study its release capacity, bioavailability and assess lake eutrophication levels, therefore the nitrogen fraction in lake sediments have been studied widely (Balzer 1984, Song et al. 2002, Wu et al. 2012). The ratio of N and P is important for the species of phytoplankton in lake and it directly indicates the nature of nutrient limitation on a community level (Koerselman and Meuleman 1996). A lot of studies has revealed that an N:P ratio >16 indicates P limitation on a community level, while an N:P ratio <14 is indicative of N limitation. At N:P ratios between 14 and 16, either N or P can be limiting or plant growth is limited by N and P together (Stelzer and Lamberti 2001; Elser et al. 2009).
Evaluation of the forms and distribution characteristics of phosphorus and nitrogen in sediments seems particularly important in the context of the eutrophication of lake water. Therefore, this study was conducted to investigate the content of total P, total N, and their various forms, as well as to analyze the spatial and vertical distribution characteristics and environmental significance of phosphorus in the sediments of Lake Dalinouer. Knowledge of various forms of phosphorus in the Dalinouer Lake sediments is useful for the evaluation of the environment effects of phosphorus, which can be used to provide theories regarding the significance of sediments as internal sources releasing into the overlying water.
Materials and methods
Study area
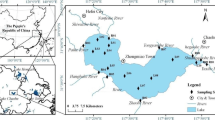
Lake Dalinouer (E116°26′–116°45′, N43°13′–43°23′, Fig. 1), which is the seventh largest inland freshwater lake in China, is located in the Inner Mongolia Plateau of northwest China. It covers a surface area of 238 km2 with a maximum water depth of 11 m and an elevation of 1,226 m above sea level. The lake is a typical closed-basin lake and is surrounded by hills of basaltic rocks to the west, north, and east, and the regional zonal vegetation was mainly steppe with localized sedge meadows developed in the humid river bank and lake shore. In the lake region, the annual mean temperature ranged from −1 to +2 °C (Wang et al. 2001; Reiche et al. 2011), with a low mean monthly temperature of −23 °C in January, and a high mean monthly temperature of 17 °C in July. Climatically, Dali Lake is located at the transition from semi-humid to semi-arid areas in the middle temperate zone of China, the strong seasonal influence of the East Asian monsoon reaches the area during the summer (Wang et al. 2004), the mean annual precipitation, estimated for many years, is about 350–400 mm (Wang et al. 2001; Xiao et al. 2008), concentrated between July and September. However, the annual average evaporation is 1,600–1,800 mm, mostly between April and August. The major sources of water to lake are river water and rainwater, two permanent rivers (Gongger River and Salin River) from the northeast and two intermittent streams (Liangzi River and Holai River) from the southwest feed the lake, but no rivers drain the lake. Due to the influence of climate and human activity, the lake water has degenerated into a Mg2+-Cl−-HCO3 − type (Zhai et al. 2010) with high pH value ranged from 9.1 to 9.8, and the mean alkalinity is up to 53.6 mmol L−1 high. In addition, the Dalinouer Lake has a great ecological value. Due to its morphological and brackish water characteristics, it is the best suitable habitat for breeding and nursing ground for fishes, and it is also the home site for nearly 40 different species of water birds. And now it is being designated as a national nature reserve by China. However, in recent years, the environmental and ecological problems of the Lake Dalinouer such as declining water level, shrinking area, increasing salinity and the intensifying eutrophication, is getting more and more concern and becomes a focus area in the global change studies.
Sample collection and chemical analysis
Location of sampling sites in the Lake Dalinouer was set according to the book entitled “Lake Ecosystem Observation Method” and the sampling sites were given in Fig. 1. Nine surface sediment samples (0–10 cm) were collected by grab bucket from Dalinouer Lake in September of 2008. Sediment cores collected from the sites DLNE-3, DLNE-5, and DLNE-9 were sectioned to 2 cm slices immediately on the boat. After sampling, the sediment samples were taken to the laboratory in sealed plastic bags, stored in iceboxes (−20 °C), and were then freeze-dried; part of the samples were ground and sieved with a standard 150-μm sieve for phosphorus experimental study, and the others were conserved without grind for nitrogen fraction. Water samples were collected just above the sediment surface (50 cm) with acid washed 1-L polyethylene bottles, and kept in a cooler at 4 °C until analysis. The analyses were performed within 24 h. The locations of the sampling points in the lake were surveyed by GPS.
Total nitrogen was measured using alkaline potassium persulfate digestion (Ebina et al. 1983) by converting all nitrogen forms to nitrate and subsequent analysis of nitrate by 2,6-dimethylphenol method using a spectrophotometer (Qian and Fu 1987). NO3 −-N was determined using a standard spectrophotometric method with phenol disulfonic acid. NH4 +-N was determined using colorimetric method (Strickland and Parson 1972). Total phosphorus was determined colorimetrically after wet digestion with H2SO4 plus HClO4 (Parkinson and Allen 1975). Phosphorus fractions were determined using a sequential extraction procedure described in a previous study (Ruttenberg 1992). The operationally defined scheme was composed of seven steps which separate the major reservoirs of sedimentary phosphorus into seven pools: exchangeable phosphorus, aluminum-bound phosphorus, iron-bound phosphorus, occluded phosphorus, authigenic carbonate fluorapatite + biogenic apatite + CaCO3-associate phosphorus, detrital apatite phosphorus of igneous or metamorphic origin, and organic phosphorus. The analysis of soluble reactive phosphorus in each fraction was made by molybdenum blue method (Murphy and Riley 1962).
Quality control and statistical analysis
All analytical operations were conducted under strict quality control guidelines, including the use of standard operating procedures, calibration with standards, analysis of reagent blanks, and analysis of replicates. All experiments were carried out in triplicate, and the results were expressed as the mean. Pearson correlation coefficients were employed for the better understanding of relationship among sediment phosphorus, nitrogen, and environmental factors. Statistical analyses were conducted using SPSS 16.0 statistical package and Origin 8.0, and differences were considered significant if P < 0.05. One-way ANOVA analysis was used to test the differences of nutrient contents between the sampling sites and between sediment depth increments.
Results and discussions
General physicochemical properties of water and sediment
The general physical–chemical features and the chemical component contents of sediment and water of Dalinouer Lake were presented in Table 1. The pH of water varied from 9.01 to 9.80, which indicated the lake water was slight basic. The changes of pH in lake water significantly influenced hydroxide, carbonate, and silicate equilibria, and these equilibria could regulate the precipitation and dissolution, the sorption and desorption of phosphorus. Secchi disk measurements in the studied lake ranged between 0.5 and 0.8 m, and the euphotic zone was at a depth of 2.4 m. Dissolved oxygen concentrations, measured 0.5 m from the lake bottom and varied from 3.98 to 5.06 mg/L. The concentrations of total nitrogen and total phosphorus in lake water exhibited a range of 4.06–27.3 and 2.06–3.45 mg/L, respectively. Sediment samples were organic rich, dark brown or black in color, and showed no visible signs of stratification. The distinct sulfur odor from most samples suggested that reducing conditions existed at the sediment–water interface due to long-term impoundment. All samples contained a water content of approximately 60 % decreased significantly with the depth of sediment, and the texture of the Dalinouer Lake sediment ranged from very fine silt to clay (Xiao et al. 2008). Calcite and dolomite concentrations were less than 0.8 %. Organic carbon contents ranged from 6.25 to 65.5 g/kg, with a mean of 36.4 g/kg.
The distribution characteristics of phosphorus and its environmental significances
Spatial characteristics of phosphorus fractions in surface sediments
In general, phosphorus in surface sediments primarily consisted of inorganic phosphorus species, including Fe-P, Ca-P, Al-P, and De-P, as well as organic phosphorus species such as phosphate monoesters, phosphate diesters and organic polyphosphates. The concentrations of total phosphorus and different phosphorus form in surface sediments of Lake Dalinouer were shown in Table 2, and it could be known that the concentrations of total P and different phosphorus forms in surface sediments obvious varied, which might be attributed to differences in the sedimentation environments. The total phosphorus in surface sediments ranged from 0.493 to 0.904 g/kg with a mean of 0.73 g/kg which indicated that the lake sediments had a great potential to supply phosphorus to the overlying water, and its spatial distribution presented an increasing tendency from the northeast to southwest part of the lake (Fig. 2a). The high concentration of total phosphorus was found in site of DLNE-4 to -6, largely due to domestic sewage dischargers and anthropogenic input. The lowest phosphorus content was shown in DLNE-1 site, which might be related to the low water depth and unstable sedimentary environment. About 68.0–81.6 % of total phosphorus was in form of inorganic phosphorus, which comprised a major constituent in the surface sediments, and its contents ranged from 335 to 738 mg/kg with a mean of 522 mg/kg. The organic phosphorus concentrations in surface sediments ranged from 21.3 to 292.7 mg/kg with a mean value of 122.5 mg/kg, and the ratios of organic phosphorus/total phosphorus were all less than 32.3 %. The organic phosphorus content was influenced by inputs, sediment characteristics, early disgenesis and organisms (Liu et al. 2001; Xiang and Zhou 2011). The concentrations of total phosphorus in Lake Dalinouer sediments were relatively low when compared to some other eutrophic lakes in China such as Taihu Lake (420–3,408 mg/kg, Jin et al. 2006) and Danchi Lake (1.01–6.66 g/kg, Gao et al. 2004).
The spatial distribution of different phosphorus fraction in surface sediment from the Lake Dalinouer. a Total phosphorus (g/kg), b exchangeable phosphorus (mg/kg), c aluminum phosphorus (mg/kg), d ferrum phosphorus (mg/kg), e occluded phosphorus (mg/kg), f auto-calcium phosphorus (mg/kg), g debris phosphorus (mg/kg), h organic phosphorus (mg/kg)
Inorganic phosphorus was the most important mass fraction of total phosphorus in surface sediments, which had reported as an important source of bioavailable phosphorus in eutrophic sediments (Ruban et al. 2001). The content of exchangeable phosphorus (Ex-P) ranges from 7.92 to 55.31 mg/kg, phosphorus bound to Al (Al-P) ranged from 0.78 to 1.61 mg/kg, phosphorus bound to Fe (Fe-P) ranged from 1.27 to 3.95 mg/kg, occluded phosphorus (Oc-P) ranged from 1.91 to 7.83 mg/kg, autogenetic calcium-bound phosphorus(ACa-P) ranged from 145.37 to 543.21 mg/kg, debris phosphorus (De-P) ranged from 110.14 to 218.29 mg/kg. The concentrations of different phosphorus fractions in surface sediments all varied greatly, indicating that the content of phosphorus was affected by the changing of environmental factors and that the problem of increasing phosphorus input might be serious. These differences mainly were caused by different geographical locations, anthropogenic phosphorus sources and sediment types. The relative contributions of each fraction of the inorganic phosphorus were shown in Fig. 3, the Ex-P, Al-P, Fe-P, Oc-P, ACa-P, and De-P concentrations accounted for about 1.10–6.12 %, 0.10–0.21 %, 0.28–0.55 %, 0.38–1.13 %, 29.4–67.7 %, and 15.1–34.7 % of total phosphorus, respectively. As a whole, the decreasing order of the inorganic phosphorus was ACa-P > De-P > Ex-P > Oc-P > Fe-P > Al-P. At all the sampling sites, more than 50 % of inorganic phosphorus would be in the form of Ca-P. Golterman (2004) thought that high pH and Ca2+ concentration in water column could reduce the phosphorus adsorption by Fe(OOH) particles, and Ca2+ in turn would tend to form Ca5(PO4)3OH precipitate with orthophosphate. This would be the main reason why the sediment in Lake Dalinouer had more phosphorus bound to Ca and less phosphorus bound to Fe/Al than those in other lakes of South China, which had a Ca2+ concentration 20 times lower than that in Lake Dalinouer. Ca-P had a detrital origin and was not available to algae. High portions of calcium mineral phosphorus were also observed in different sampling sites with varying trophic status. The Pearson correlation coefficient between ACa-P and total phosphorus was 0.683, which was relatively higher than that between Fe-P and total phosphorus, Al-P and total phosphorus (Table 3), and the RSD (relative standard deviation) was 30.4 % at nine sampling sites, this could indicate that the Ca-P content changed not so much as the total phosphorus at the sampling sites, which was because the Ca-P is autogenetic and had little relevance to the eutrophic status. Consequently, ACa-P was considered as a relatively stable fraction of sedimentary phosphorus and contributed to a permanent burial of phosphorus in sediment (Kaiserli et al. 2002).
There were intercorrelations among various phosphorus fractions (Table 3). Inorganic phosphorus and Ca-P contents had the most significant relationship. The relationships between Org-P content and Ex-P content were also significant. Therefore, inorganic phosphorus was caused by Ca-P contents, and the origin of Org-P was the same to the Ex-P or Ex-P originated from Org-P decomposition. In surface sediment, Ex-P represented the loosely sorbed phosphorus, and this fraction might include dissolved phosphorus in the pore water (Kaiserli et al. 2002) and released from Ca-P or leached from decayed cells of bacterial biomass in deposition of phytodetritus (Pettersson 2001), therefore, the Ex-P was considered to be immediately bioavailable. In the sediment from aquatic macrophyte dominant parts of the lake (DLNE-8 and DLNE-9), the concentration of Ex-P was relatively low with a mean value of 9.64 and 7.92 mg/kg, respectively. All these results indicated that macrophytes possibly restrained algal blooms by effectively decreasing the Ex-P in the bottom sediment, thereby reducing large scale releases of phosphorus from the sediment to the overlying water. In addition, the water quality of the lake strongly affected the contents of Ex-P. In Lake Dalinouer, pollution became more serious with an order of DLNE-9 > DLNE-8 > DLNE-1 > DLNE-3 > DLNE-7 > DLNE-4 > DLNE-2 > DLNE-6 > DLNE-5, but the content of Ex-P in sediment showed an opposite tendency. The relatively insignificant linear correlation between total phosphorus concentration in the overlying water and the Ex-P of the sediment supported this (R 2 = 0.0174, n = 9, Fig. 4a). Org-P in the surface sediments ranged from 21.3 to 292.7 mg/kg, with a mean of 123.0 mg/kg. Org-P demonstrated a similar distribution pattern to Ex-P (Fig. 2) because Org-P was strongly associated with Ex-P (r = 0.902, P < 0.01, Table 3).
The Al-P and Fe-P fractions were bound up with the pollution status. Apatite in sediments was the only phosphorus mineral, which, to a large extent, was conserved in its original form; therefore, Al-P and Fe-P generally might be formed in sediment by diagenetic processes (Wang et al. 2009). The anthropogenic phosphate ion and the phosphorus compounds would first bind to aluminum and iron oxides/hydroxides in the surface sediments for the reason that active Fe and Al had been deemed to be the main sorbent for adsorbing phosphorus in natural sediments (Danen-Louwerse et al. 1993), and which had been verified by Moturi et al. (2005). In all the sediments from the Lake Dalinouer, Al-P was relative low (<0.25 % of total phosphorus), which means that the internal load of Al-P for this studied lake was unimportant. The content of Al-P was higher at sites DLNE-4 and DLNE-6 than at the other sites, which might relate to the anthropogenic origin (agricultural activities) of Al-P. The content of Fe-P in surface sediment of Lake Dalinouer ranged from 1.27 to 3.95 mg/kg, and the Fe-P content was higher at sites DLNE-5, 9 and lower at site DLNE-7. The reason for the relatively low content of Fe-P and Al-P was that the surface sediment in Lake Dalinouer was low lever of iron and aluminum. The Fe-P fraction which had been shown to be a potential variable component of sedimentary phosphorus, and it could also be used to determine the source of phosphorus and indicated the extent of environmental pollution (Zheng et al. 2004). In addition, Fe/Al-P was a bioavailable source of phosphorus for phytoplankton growth (Mesnage and Picot 1995), and it was usually considered a redox sensitive phosphorus fraction due to the reductive dissolution of Fe(OH)3(S) (Hupfer and Lewandowski 2008). Meanwhile, higher water pH would make dissolved phosphorus releasing into water from the Fe(OOH)-P pool through ion exchange of OH− with PO4 3− (Golterman 2004). The Oc-P fractions which referred to phosphorus present within the mineral matrix of discrete mineral phase were not easily fixed, hence they were easily detached physically. The entrained Oc-P fractions enter the water bodies where they caused eutrophication. The insignificant linear relation (R 2 = 0.0065, n = 9, Fig. 4b) between total phosphorus in overlying water and Oc-P in surface sediment might support this point. Oc-P content in surface sediment of Lake Dalinouer was relatively higher than other phosphorus fractions (e.g., Al-P and Fe-P), and it had a lowest value in the centre of lake.
Generally, Ex-P, Al-P, and Fe-P were easily desorbed from sediments and released to the overlying water. Therefore, they were referred to as the bioavailable forms of phosphorus in sediment in this study. Of this, Ex-P was the main existing forms of bioavailable phosphorus, which would have a significant impact on the eutrophication process of Dalinouer Lake. Mobility of different phosphorus fractions extracted from sediments usually decreased from Ex-P to Org-P; thus, mobility of bioavailable phosphorus was higher than other parts of phosphorus fractions. The more seriously polluted the sampling site was, the higher the bioavailable phosphorus in the sediments, therefore, the phosphorus in the sediment, especially bioavailable phosphorus, had a close relationship with water quality and the type of lake ecosystem.
Vertical distribution of phosphorus fraction in sediment cores
Total phosphorus and different phosphorus fractions contents in sediment profiles of the Lake Dalinouer were shown in Fig. 5. The concentration of total phosphorus in sediments decreased gradually with depth increasing in the sediment column. Of all the three sediment cores, the highest content of total phosphorus was found in DLNE-5, and there was an accumulative peak in the 8–16 cm depth increment in DLNE-5, which was 1.08–1.28 times higher than that of the surface value, which is mainly because the DLNE-5 site located in deep water region where sedimentary environments were relatively stable and grain size of sediments were relatively fine, resulting in the phosphorus adsorption capacity of sediments in this site was strong. Compared to the site DLNE-5, the total phosphorus levels were lower in the sites of DLNE-3 and DLNE-9, and with lower variability for the two sediment profiles. In descending order of importance, total phosphorus concentration in the three sediment cores amount to 758.59, 479.31, and 383.78 mg/kg in the site of DLNE-5, DLNE-3, and DLNE-9, respectively. In the three sediment cores, the form of De-P was the main fraction of the phosphorus from the top to the bottom of the cores, accounting for, on an average, 35.8 % of the total phosphorus. The De-P generally decreased with depth in the three sediment cores, but small accumulative peaks appeared in deeper sediment in the site of DLNE-5. The secondary major component of phosphorus in sediment was ACa-P, followed by Org-P, amounting to 21.6–37.2 % and 18.1–33.7 % with an average of 30.6 and 26.9 % of total phosphorus, respectively. Vertical change of ACa-P concentration was not observed (except DLNE-5), and it was mainly because that Ca-P was a relatively stable fraction of sedimentary phosphorus. In all the layers, Al-P was lower than 1.92 mg/kg, with an average of 0.3 % of total phosphorus, which implied that Al-P plays an unimportant role on the internal loading of phosphorus in the studied lake. The concentrations of Al-P and Fe-P fluctuated greatly in deeper layer of the sediment column, which might be related to early diagenetic processes. Low values of Al-P and Fe-P below Ca-P were possibly due to higher alkalinity in sediment of the studied lake. Under acid environment condition, most of the solid phase phosphorus was associated with Fe and Al, while phosphorus was associated with Ca under more basic condition. Oc-P was one of the most abundant of phosphorus forms in the sediment cores and this could be due to greater adsorption of this unreactive form of phosphorus. The vertical distribution of Oc-P in the sediment cores was somewhat different from those of Al-P and Fe-P, the concentration of Oc-P did not change remarkably in the deeper of the sediment column (Fig. 5). Generally, the mean contents of Oc-P in the sediment profiles from 0 to 26 cm deep were significantly higher in site DLNE-5 with higher vertical variation coefficients than those in sites DLNE-3 and DLNE-9 with lower variation coefficients (P < 0.05, Table 4). The changing trends of the Ex-P fraction in the three sediment profiles were similar, and the average contents of Ex-P were significantly enriched in the surface sediments, and had a tendency to decrease toward the bottom of the sediment core. Generally, phosphorus in the top layers of sediments was considered to participate in the whole lake metabolism, while the release of phosphorus was believed to occur simultaneously, resulting in a decrease in phosphorus contents in the top sediment with time (Li et al. 2006; Xiang and Zhou 2011). These longitudinal distribution characteristic of phosphorus in sediments was the net result of the history of phosphorus sedimentation as well as many transformation processes.
Of all the phosphorus species, Org-P was present in high concentrations with a mean of 143.76, 181.27, and 87.81 mg/kg in DLNE-3, DLNE-5, and DLNE-9, respectively. But it was less in abundance than inorganic phosphorus. Frossard et al. (1989) reported that Org-P such as adenosine triphosphate, choline phosphate, and glucose-6-phosphate were less strongly adsorbed onto soil particles than inorganic phosphorus. Because of the influence and constriction of a number of factors, the vertical distribution of Org-P in sediment core was complicated. Previous study had shown that high clay content and high deposition rate were the main reasons caused high content of Org-P (Zhang et al. 2012). In addition, the input of terrestrial organic matters might also increase the level of organic phosphorus in sediment.
Though each phosphorus form had its own vertical changing tendency in different sites, most of them tended to increase toward the surface sediment and the concentration gradients were very large in top layer at some sites. This vertical distribution characteristic indicated that the phosphorus load of Dalinouer Lake had been aggravated with the intensification of human activities or rapid development of industry and agriculture in recent years.
Environmental significances of different phosphorus forms
Phosphorus is often a growth-limiting element for aquatic organisms and plays a significant role in the eutrophication process of a reservoir. In addition, phosphorus is the key element responsible for eutrophication in many lakes. Excessive phosphorus in the water body can accelerate freshwater primary productivity, leading to eutrophication and negative impact on ecosystem function. The mobile phosphorus (Ex-P, Fe/Al-P, and Org-P) in sediment can be used to estimate the potential release of sediment phosphorus to the overlying water (Rydin 2000; Gao et al. 2004). Generally, the bioavailable phosphorus can be easily desorbed from sediments and release into the overlying water when environmental conditions changed; therefore, it is important to clarify the relations between these mobile forms of phosphorus and environment factors, which may provide valuable information to better understand the eutrophication processes of lake.
Principal component analysis (PCA) is used to identify the main factors which influenced the contents of different phosphorus forms in sediment. The initial set of factors was usually transformed by varimax rotation, and then their identity was assigned. The sum of the five factors accounts for 92.9 % of the variance of data in the sediments (Table 5). The first factor accounts for 37.9 % of all variance and is mainly correlated with water content, electric conductivity, pH, total P, ACa-P, and Fe-P. It indicates that water content, pH, and electric conductivity are the control factors to total P, ACa-P, and Fe-P. It also shows that ACa-P and Fe-P is significantly associated with total P in sediments. The second factor accounts for 22.4 % of total variance and is associated with Ex-P, water content, dissolved oxygen, Org-P and total phosphorus, suggesting that water content and dissolved oxygen have an impact in Ex-P and Org-P. The third factor accounts for 16.8 % of all variance, is correlated with organic carbon and Org-P, suggesting that terrestrial organic matter from streams and rivers may be one of the most important sources of Org-P in sediment. The four and five factors account for 8.7 and 6.9 % of total variance, respectively. These factors which mainly showed the fraction of De-P is not sensitive with lake properties.
The distribution characteristics of nitrogen in sediment of Lake Dalinouer
Horizontal changes of total nitrogen and all forms of nitrogen in surface sediment
Nitrogen is an incredibly versatile element, existing in both inorganic and organic forms as well as many different oxidation states in the sediments. Concentrations of total nitrogen and different nitrogen fractions in surface sediment are presented in Fig. 6. Total nitrogen concentration in the surface sediment from Dalinouer Lake is high, ranging from 371 to 8,353 mg/kg with a mean of 3,492 mg/kg, which indicates that the sediment has a great potential to supply nitrogen to the water. Comparing different sampling sites in the lake, total nitrogen in sediments is higher in the south-western part of the lake than in the north-eastern. Distribution of nitrogen concentration in sediment is partly dependent on geographical location and land-use type in the Dalinouer catchment and higher nitrogen concentrations usually occur in areas of urban and agricultural activities (Carpenter et al. 1998; Li et al. 2009). Much higher total nitrogen concentrations are observed in the site of DLNE-5, therefore a large amount of nitrogen came into the lake through the two tributaries that fed the lake from southwest, which carry large amounts of agriculture irrigation backwater, urban sewage, and industrial waste, indicating that the nitrogen contents in this site is disturbed to a certain degree by humans.
Ammonium (NH4 +), nitrite (NO2 −), and nitrate (NO3 −) are the most common ionic (reactive) forms of inorganic nitrogen in the sediment, NH4 +-N and NO3 −-N are also important influencing factors of water quality assessment. The inorganic nitrogen in lake sediment is primarily NH4 + and NO3 −; the content of NO2 − is small and can be left out of total nitrogen. Concentration of nitrate nitrogen in the surface sediment ranges between 51 and 346 mg/kg (mean 151.1 ± 104.4 mg/kg), accounts for between 2.2 and 17.7 % of total sediment nitrogen (mean 6.2 ± 5.6 %). The relatively high value of NO3 −-N is found in DLNE-8 mainly due to this site located in tourist locale, and presents high sensitivity to any disturbance caused by humans. Besides, the strong nitrification of NH4 +-N in this site greatly increases NO3 −-N. In addition, relatively high water depth in DLNE-8 may reduce the possibilities for nutrient retention through aquatic plant uptake and nitrogen removal through denitrification. NH4 +-N contents in the surface sediment ranges from 10 to 149 mg/kg with a mean of 60 mg/kg. In horizontal distribution, NH4 +-N in sediments is higher in the centre than near the shore. The anaerobic conditions in the central lake inhibit nitrifying activity and favor increasing NH4 +-N contents; moreover, the high alkaline condition (9.0 < pH < 9.8) greatly favors ammonia volatilization and the transformation from NH4-N to NH3-N is regulated by the pH of the water column, and large amount of transformation between NH4-N and NH3-N occurred at pH values from 8 to 10 (Reddy 1983). Organic nitrogen in surface sediment is ranged from 297 to 8,020 mg/kg with an average value of 3,281 mg/kg. Organic nitrogen is the dominate fraction of nitrogen in sediment, accounts for 80.1–97.6 % of total nitrogen, and it presents a falling tendency on the whole from southwest to northeast of the lake, which may be the fact the south-western lake located in deep water region where the productivity is higher than that in the northeastern lake.
The inorganic nitrogen in sediment is controlled by its redox condition and microorganism. The intensive N transformation occurs at sediment–water interface. Organic N can be transformed into NH4 +-N by the process of ammonification, and parts of NH4 +-N in sediment can be transformed into NO3 −-N only when sediment is under aerobic condition. Therefore, the NO3 −-N content in surface sediments remains high and its relative contribution to inorganic carbon is high.
Vertical distribution of nitrogen along sediment profiles
Figure 7 shows the vertical distribution of total nitrogen, organic nitrogen, NO3 −-N and NH4 +-N in three sediment cores from Lake Dalinouer. It can be found that the concentration of total nitrogen on the whole takes on a decreasing tendency with depth. The average content of total nitrogen decreases rapidly from 0 to 8 cm layers, and then decreases slightly in 8–22 cm. This vertical distribution characteristic indicates that the nitrogen load of the studied lake also be ascribed to the intensification of human activities and rapid development of industry and agriculture in recent years. The distribution of organic nitrogen is similar to that of total nitrogen. NO3 −-N is the predominant form of inorganic nitrogen in the sediment cores. At the site DLNE-3, the content of NO3 −-N decreases slightly with depth, while in DLNE-5 and DLNE-9, NO3 −-N content decreases with a big fluctuation from depths of 0–16 cm and then stabilized below 16 cm. In general, the changes with depth differ due to different sedimentation rates. The contents of NH4 +-N at DLNE-5 and DLNE-9 increases slightly with depth, but at DLNE-3, decreased with 0–8 cm, fluctuating increases between 8 and 20 cm, and the decreases below 20 cm. This is possibly related to the shallow plant system, with the roots and rhizomes of reed in these areas concentrating on the sediment depths above 20 cm, since they can reduce ammonium nitrogen contents in the upper sediment by plant uptake. Besides, ammonia volatilization is also a mechanism of nitrogen reduction in upper sediment layers (Bai et al. 2007), and ammonia volatilization can occur under alkaline conditions with higher sediment pH values. High NH4 +-N contents in deep sediment layers are most likely caused by less plant uptake and nitrification. In addition, the NH4 + release of parent material can also increase NH4 +-N contents in deeper sediment layers. Variations of NO3 −-N contents are not consistent with those of NH4 +-N contents in sediment profiles. Denitrification is the main mechanism leading to N loss of top sediment layers; NO3 −-N can be denitrified, and thus left the sediment in gaseous forms under anaerobic conditions. In addition, NO3 −-N content in upper sediment layers can also be reduced by plant uptake, but it does not respond to this procees, which is likely to be balanced through nitrification of NH4 +-N.
Relationships among total N, NO3 −-N, organic N, NH4 +-N and environmental factors
Table 6 shows Pearson correlation coefficients among total N, NO3 −-N, organic N, NH4 +-N and environmental factors. A correlation analysis revealed that there is significantly positive correlation between total N and organic N (P < 0.01). Total N, NO3 −-N, and organic N are positively correlated with sediment properties such as sediment organic matter, total phosphorus, and water depth. But total N, NH4 +-N, organic N and NO3 −-N are negatively correlated with water pH and temperature in all nine sampling sites. Of these, NH4 +-N is significantly negatively correlated with water pH, which is in good agreement with the fact ammonia volatilization occur under alkaline conditions. The total nitrogen shows a significantly positive correlation with sediment organic matter, which may be due to the electrostatic attraction of nitrate anions to the positively charged sites of organic matter. Additionally, organic matter may promote the metabolic activities of microorganisms to indirectly influence the absorption or release of nitrogen in sediment. Furthermore, organic matter mineralization results in the changes in redox and pH, thus further affecting the biogeochemical processes of various nitrogen compound. Phosphorus is one of the main limiting factors impacting the rates of sediment organic nitrogen mineralization, and high levels of sediment carbon and phosphorus can enhance organic nitrogen mineralization because additions of a nutrient can transform an ecosystem. Similarly, organic nitrogen mineralization can increase the contents of phosphorus and organic matter in sediment. Although total nitrogen content and different nitrogen forms in sediment are associated with dissolved oxygen, electric conductively and water content of sediment but no significant correlations are observed, indicating that these environment factors are not an important factor influencing sediment nitrogen content in this lake. NH4 +-N content is significantly (P < 0.05) and negatively correlated with water pH. This is likely related to the process of ammoniation.
C:N:P ratio in sediment of Lake Dalinouer
C:N ratio in sediment
The C/N ratio of sediment is a sensitive indicator of sediment quality and it is also widely used to reflect the balance of carbon and nitrogen of sediments (Martinez-Soto and Martínez 2012). The source of sediment organic matter whether is autogenetic or allochthonous also can be expressed by C/N ratio. If all the organic matters in the sediment come from phytoplanktons, the ratio of C:N:P will be close to the Redfield Value (108:16:1), and the ratio of organic C: total N will be about 6.6. If they are from a terrestrial source, the ratio of organic C: total N will generally be >20. The higher the percentage of terrestrial organic matter, the greater the ratio of organic C: total N (Li et al. 2007). Figure 8a illustrates the variations of sediment C/N ratio along the sampling sites of Lake Dalinouer. The obvious differences of C/N ratios are observed in different sites, and the mean values of the organic C: total N ratios in the surface sediment ranges from 6.1 to 25.8 with a mean of 14.3, indicating that the source of organic matter are land derived and lake autogenetic. The former comes form agricultural activities along the lake and its surrounding districts or form domestic sewage which is released into lake along with the tributary; the latter is mainly from primary production in the lake. In the aspect of horizontal distribution, the overall variation of the ratio shows a decreasing trend from the centre to the sides of lake, this is mainly because there is more terrestrial matter intake of the sediment from rivers. The ratio of organic C: total N in Dalinouer Lake sediment increases with depth (Fig. 9), which reflects, to some extent, the changes in the organic matter source in recent years. During the past decades, rapid urbanization and development of industry have increasingly resulted in pollution, the large amount of suspend particles from city discharges is more than that of natural sources, and has become the main source of sediment as indicated by the constantly decreased organic C/total N ratio of the sediment.
Mean nutrients concentrations and their ratios in surface sediment of Lake Dalinouer. a Organic C, total N concentrations, and C:N ratio in surface sediment of Lake Dalinouer, b Total N and total P concentrations and N:P ratio in overlying water of Lake Dalinouer, c Total N and total P concentrations and N:P ratio in surface sediment of Lake Dalinouer
N:P ratio in water and sediments
Nitrogen and phosphorus are the important variables for classification of trophic state because they are the nutrients most likely to limit aquatic primary producers in rivers and lakes. Lake water total N (TN) to total P (TP) ratio is commonly used as an index that represents the nutrient limitation for algal growth. Elser et al. (2009) reports that P is limiting when TN:TP by weight is >16, N is limiting when TN:TP is <14, and either N or P, or both are limiting when TN:TP is between 14 and 16. This study shows that the TN:TP ratio in water column is ranged from 1.5 to 14.2 with a mean of 4.5 (Fig. 8b), suggesting that the limiting nutrient for the algal growth in the Lake Dalinouer is N. Nitrogen is a limiting nutrient for the algal growth, mainly due to this lake receives wastewater discharges with low N:P ratio and/or agricultural runoff. The ratio of TN:TP at site DLNE-7 is between 14 an 16, implying that limiting nutrient is either N or P, or both.
The TN:TP ratio in surface sediments of the Lake Dalinouer ranges from 2.4 to 9.2 with an average of 4.6 (Fig. 8c). The highest value of TN:TP ratio (9.2) is observed at site DLNE-5, while the lowest value of TN:TP ratio (2.4) is observed at site DLNE-8. Compared with water column, sediments show a similar median ratio, which is due to the exchange processes that occur between sediments and the water column. In vertical direction, the ratio of TN:TP is decreased with depth, indicating that the degree of eutrophication is aggravated year by year in Dalinouer Lake.
There is limited information on quality guidelines for the TN and TP concentration of sediment in aquatic environments. According to Ontario sediment quality guidelines, sediment which has TN and TP content at or above 4,800 and 2,000 mg/kg, respectively, is defined as sediment that is considered to be heavily polluted and likely to affect the health of sediment-dwelling organisms (Persaud et al. 1993). In this study, TN and TP contents of sediments are found to be over the severely effective levels, suggesting that harmful effects on sediment-dwelling organisms should be concerned sufficiently.
Conclusions
In this study, fractions, spatial distribution, and variations of phosphorus and nitrogen in sediments of the Lake Dalinouer were investigated. From the data presented in this study, the following conclusions can be drawn:
-
1.
Total phosphorus concentration was high in the Lake Dalinouer, and decreased gradually with increasing depth in the sediment column. Almost 68.0–81.6 % of total phosphorus comprised inorganic phosphorus, which was a major constituent in the surface sediment. ACa-P was the major component of the inorganic phosphorus in the sediment, the second being De-P. Al-P and Fe-P concentrations only accounted for 0.10–0.21 % and 0.28–0.55 %, respectively, and the vertical distribution of them markedly changed in the deeper layer of the sediment column, whereas that of De-P did not, and decreased gradually along the sediment profiles. A correlation analysis revealed that there were significantly positive correlations among Org-P, total P, Oc-P, ACa-P and Ex-P, indicated that the distribution of them could be affected each other.
-
2.
The distribution of total nitrogen in surface sediments presented a decreasing tends from west to east part of the studied lake, and its content ranged from 371 to 8,353 mg/kg with a mean of 3,492 mg/kg. Org-N was the main fraction in the sediments, accounted for over 80 %of total nitrogen. The ratio of organic carbon and TN in sediment was in range of 6.0–25.8 and presented a tendency of lake centre >lake sides, indicating that the nitrogen accumulated in the sediments from lake sides came mainly from terrestrial source and nitrogen was mainly autogenetic in lake centre.
-
3.
The median TN:TP ratio in water column was 4.5, suggesting that the limiting nutrient for the algal growth in the Lake Dalinouer is N. The median TN:TP ratio in surface sediment was 4.6, which was similar with that in water column, mainly due to the exchange processes that occur at water–sediment interface. According to Ontario sediment quality guidelines, the contents of TN and TP in sediments were over the severely effective levels (TN ≤ 4,800 mg/kg; TP ≤ 2,000 mg/kg); therefore, their harmful effects on sediment-dwelling organisms should be concerned sufficiently.
References
Bai JH, Deng W, Wang QG, Cui BS, Ding QY (2007) Spatial distribution of inorganic nitrogen contents of marsh soil in a river floodplain with different flood frequencies from soil-defrozen period. Environ Monit Assess 134:421–428
Balcerzak W (2006) The protection of reservoir water against the eutrophication process. Pol J Environ Stud 15(6):837–844
Balzer W (1984) Organic matter degradation and biogenic element cycling in a near shore sediment (Kiel Bight). Limnol Oceanogr 29(6):1231–1246
Benitez-Nelson CR (2000) The biogeochemical cycling of phosphorus in marine systems. Earth Sci Rev 51:109–135
Beretson WM (2001) The flux of particulate organic carbon into the ocean interior: a comparison of four U.S.JGOFS regional studies. Oceanography 14(4):59–67
Carpenter SR, Caraco NF, Correll DL, Howarth RW, Sharpley AN, Smith VH (1998) Nonpoint pollution of surface waterswith phosphorus and nitrogen. Ecol Appl 8:559–568
Danen-Louwerse H, Lijklema L, Coenraats M (1993) Iron content of sediment and phosphate adsorption properties. Hydrobiologia 253(1):311–317
Ebina J, Tsutsui T, Shirai T (1983) Simultaneous determination of total nitrogen and total phosphorus in water using peroxodisulfate oxidation. Water Res 17:1721–1726
Elser JJ, Andersen T, Baron JS, Bergstrom AK, Jansson M, Kyle M (2009) Shifts in Lake N:P stoichiometry and nutrient limitation driven by atmospheric nitrogen deposition. Science 326(5954):835–837
Emeis KC, Struck U, Leipe T, Pollehne F, Kunzendorf H, Christiansen C (2000) Changes in the C, N, P burial rates in some Baltic Sea sediments over the last 150 years-relevance to P regeneration rates and the phosphorus cycle. Marine Geol 167:43–59
Frossard E, Stewart JWB, Amaud RJSt (1989) Distribution and mobility of phosphorus in grassland and forest soils of Saskatchewan. Can J Soil Sci 69:401–416
Gao L, Yang H, Zhou JM, Lü JJ (2004) Lake Sediments from Dianchi Lake: a phosphorus sink or source. Pedosphere 14(4):483–490
Golterman HL (2004) The chemistry of phosphate and nitrogen compounds in sediments. Kluwer, Dordrecht, pp 57–59
Gomez E, Durillon C, Rofes G, Picot B (1999) Phosphate adsorption and release from aerobic sediments of brackish lagoons: pH, O2 and loading influence. Water Res 33:2437–2447
Hosomi M, Okada M, Sudo R (1982) Release of phosphorus from lake sediments. Environ Int 7:93–98
Hu J, Liu YD, Liu JT (2006) The comparison of phosphorus pools from the sediment in two bays of Lake Dianchi for cyanobacterial bloom assessment. Environ Monit Assess 121(1–3):1–14
Hupfer M, Lewandowski J (2008) Oxygen controls the phosphorus release from lake sediments: a long-lasting paradigm in limnology. Int Rev Hydrobiol 93(4–5):415–432
Jin XC, Wang SR, Pang Y, Wu FC (2006) Phosphorus fractions and the effect of pH on the phosphorus release of the sediments from different trophic areas in Taihu Lake, China. Environ Pollut 139:288–295
Kaiserli A, Voutsa D, Samara C (2002) Phosphorus fractionation in lake sediments, lakes Volvi and Koronia, N. Greece. Chemosphere 46:1147–1155
Koerselman W, Meuleman AFM (1996) The vegetation N:P ratio: a new tool to detect the nature of nutrient limitation. J Appl Ecol 33(6):1441–1450
Li J, Liu CQ, Wang SL, Zhu ZZ, Zhou ZH, Xiao HY (2006) Vertical variation of phosphorus forms in surface sediments from Wuli Bay, Taihu Lake, China. Chin J Geochem 25(3):279–284
Li XG, Song JM, Yuan HM, Dai JC, Li N (2007) Biogeochemical characteristics of nitrogen and phosphorus in Jiaozhou Bay sediments. Chin J Oceanol Limnol 25(2):157–165
Li S, Liu W, Gu S, Cheng X, Xu Z, Zhang Q (2009) Spatial-temporal dynamics of nutrients in the upper Han River basin, China. J Hazard Mater 162:1340–1346
Liu M, Xu SY, Hou LJ, Ou DN (2001) Phosphorus forms in sediments and their distribution in the Yangtze Estuary and coastal areas. Mar Sci Bull 3(2):55–62
Liu JY, Wang H, Yang HJ, Ma YJ, Cai OC (2009) Detection of phosphorus species in sediments of artificial landscape lakes in China by fractionation and phosphorus-31 nuclear magnetic resonance spectroscopy. Environ Pollut 157(1):49–56
Lü JJ, Yang H, Gao L, Yu TY (2005a) Spatial variation of P and N in water and sediments of Dianchi Lake, China. Pedosphere 15(1):78–83
Lü XX, Song JM, Li XG, Yuan HM, Zhan TR, Li N, Gao XL (2005b) Geochemical characteristics and early diagenesis of nitrogen in the Northern Yellow Sea sediments. Acta Geol Sinica 79(1):114–123
Łukawska-Matuszewska K, Bolałek J (2008) Spatial distribution of phosphorus forms in sediments in the Gulf of Gdańsk (southern Baltic Sea). Cont Shelf Res 28:977–990
Martinez-Soto MC, Martínez G (2012) Organic carbon, phosphorus and nitrogen in surface sediments of the marine-coastal region north and south of the Paria Peninsula, Venezuela. Environ Earth Sci 65(2):429–439
Mesnage B, Picot V (1995) The distribution of phosphate in sediments and its relation with eutrophication of a Mediterranean Coastal Lagoon. Hydrobiologia 297(1):29–41
Moturi MC, Rawat M, Subramanian V (2005) Distribution and partitioning of phosphorus in solid waste and sediments from drainage canals in the industrial belt of Delhi, India. Chemosphere 60(2):237–244
Murphy J, Riley JP (1962) A modified single solution method for the determination of phosphate in natural waters. Anal Chim Acta 27:31–36
Nilsson P, Jansson M (2002) Hydrodynamic control of nitrogen and phosphorus turnover in an eutrophicated estuary in the Baltic. Water Res 36:4616–4626
Parkinson JA, Allen SE (1975) A wet oxidation procedure suitable for determination of nitrogen and mineral nutrients in biological material. Commun Soil Sci Plant Anal 6:1–11
Perkins RG, Underwood GJ (2001) The potential for phosphorus release across the sediment-water interface in an eutrophic reservoir dosed with ferric sulphate. Water Res 35(6):1399–1406
Persaud D, Jaagumagi R, Hayton A (1993) Guidelines for the protection and management of aquatic sediment quality in Ontario. Water Resources Branch, Ontario Ministry of the Environment, Toronto
Pettersson K (2001) Phosphorus characteristics of settling and suspended particles in Lake Erken. Sci Total Environ 266:79–86
Pettersson K, Boström B, Jacobsen OS (1988) Phosphorus in sediments speciation and analysis. Hydrobiologia 170:91–101
Qian J, Fu L (1987) Simultaneous determination of total nitrogen and total phosphorus in waters by persulphate digestion. Environ Sci 8:9–14 (in Chinese)
Reddy KR (1983) Fate of nitrogen and phosphorous in a waste-water retention reservoir containing aquatic macrophytes. J Environ Qual 12:137–141
Reiche M, Funk R, Zhang ZD, Hoffmann C, Reiche J, Wehrhan M, Li Y, Sommer M (2011) Application of satellite remote sensing for mapping wind erosion risk and dust emission- deposition in Inner Mongolia grassland, China. Grassl Sci 58:8–19
Ribeiro DC, Martins G, Nogueira R, Cruz JV, Brito AG (2008) Phosphorus fractionation in volcanic lake sediments (Azores-Portugal). Chemosphere 70:1256–1263
Ruban V, Brigault S, Demare D, Philippe AM (1999) An investigation of the origin and mobility of phosphorus in freshwater sediments from Bort-Les-Orgues reservoir, France. J Environ Monit 1(4):403–407
Ruban V, López-Sánchez JF, Pardo P, Rauret G, Muntau H, Quevauville RP (2001) Harmonized protocol and certified reference material for the determination of extractable contents of phosphorus in freshwater sediments-a synthesis of recent works. Fresenius J Anal Chem 370(2–3):224–228
Ruttenberg KC (1992) Development of a sequential extraction method for different forms of phosphorus in marine sediments. Limnol Oceanogr 37(7):1460–1482
Rydin E (2000) Potentially mobile phosphorus in Lake Erken sediment. Water Res 34(34):2037–2042
Song JM, Ma HB, Lv XX (2002) Nitrogen forms and decomposition of organic carbon in the Southern Bohai Sea core sediments. Acta Oceanol Sinica 21(l):125–133 (in Chinese)
Stelzer RS, Lamberti GA (2001) Effects of N:P ratio and total nutrient concentration on stream Periphyton community structure, biomass, and elemental composition. Limnol Oceanogr 46(2):356–367
Strickland JD, Parson TR (1972) A practical book of seawater analysis, 2nd edn. Bull Fish Res Bd Can, p 167
Virtasalo JJ, Kohonen T, Vuorinen I, Huttula T (2005) Sea bottom in the Archipelago Sea, northern Baltic Sea-implication for phosphorus remineralization at the sediment surface. Mar Geol 224:103–122
Wang HY, Liu HY, Cui HT, Abrahamsen N (2001) Terminal Pleistocene/Holocene palaeo-environmental changes revealed by mineral-magnetism measurements of lakes sediments for Dali Nor area, southeastern Inner Mongolia Plateau, China. Palaeogeogr Palaeo Climatol Palaeoecol 170:115–132
Wang HY, Liu HY, Liu YH, Cui HT (2004) Mineral magnetism of lacustrine sediments and Holocene palaeoenvironmental changes in Dali Nor area, southeast Inner Mongolia Plateau, China. Palaeogeogr Palaeo Climatol Palaeoecol 208:175–193
Wang SR, Jin XC, Zhao HC, Wu FC (2006) Phosphorus fractions and its release in the sediments from the shallow lakes in the middle and lower reaches of Yangtze River area in China. Colloids and Surfaces A: physicochem. Eng Aspects 273:109–116
Wang C, Qian J, Guo ZY, Zhao L, Li XC (2008) Vertical distributions of phosphorus fractions in sediments of three typical shallow urban lakes in P.R. China. Pol J Environ Stud 17(1):155–162
Wang P, He MC, Lin CY, Men B, Liu RM, Quan XC, Yang ZF (2009) Phosphorus distribution in the estuarine sediments of the Daliao River, China. Estuar Coast Shelf Sci 84(2):246–252
Wang XY, Zhang LP, Zhang H, Wu XY, Mei DL (2012) Phosphorus adsorption characteristics at the sediment-water interface and relationship with sediment properties in FUSHI reservoir, China. Environ Earth Sci 67(1):15–22
Wu XY, Zhang LP, Yu XX (2012) Impacts of surface runoff and sediment on nitrogen and phosphorus loss in red soil region of southern China. Environ Earth Sci 67(7):1939–1949
Xiang SL, Zhou WB (2011) Phosphorus forms and distribution in the sediments of Poyang Lake, China. Int J Sedim Res 26:230–238
Xiao JL, Si B, Zhai DY, Itoh S, Lomtatidze Z (2008) Hydrology of Dali Lake in central- eastern Inner Mongolia and Holocene East Asian monsoon variability. J Paleolimnol 40:519–528
Yurkovskis A (2004) Long-term land-based and internal forcing of the nutrient state of the Gulf of Riga (Baltic Sea). J Mar Syst 50:181–197
Zabel M, Dahmke A, Schulz HD (1998) Regional distribution of diffusive phosphate and silicate fluxes through the sediment-water interface: the eastern South Atlantic. Deep Sea Res 45:277–300
Zhai DY, Xiao JL, Zhou L, Wen RL, Chang ZG, Pang QQ (2010) Similar distribution pattern of different phenotypes of limnocythere inopinata (Baird) in a brackish-water lake in Inner Mongolia. Hydrobiologia 651:185–197
Zhang ZB, Tan XB, Wei LL, Yu SM, Wu DJ (2012) Comparison between the lower Nansi Lake and its inflow rivers in sedimentary phosphorus fractions and phosphorus adsorption characteristics. Environ Earth Sci 66(5):1569–1576
Zheng LB, Ye Y, Zhou HY (2004) Phosphorus forms in sediments of the East China Sea and its environmental significance. J Geogr Sci 14(1):113–120
Zhou Q, Gibson CE, Zhu Y (2001) Evaluation of phosphorus bioavailability in sediments of three contrasting lakes in China and the UK. Chemosphere 42:221–225
Zhu YR, Zhang RY, Wu FC, Qu XX, Xie FZ, Du ZY (2013) Phosphorus fractions and bioavailability in relation to particle size characteristics in sediments from Lake Hongfeng, Southwest China. Environ Earth Sci 68(4):1041–1052
Acknowledgments
This research was financially supported by the National Natural Science Foundation (No. 40863003). We express our thanks to the members of the project who provided valuable support during the course of sediment collection and chemical analysis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hou, D., He, J., Lü, C. et al. Spatial variations and distributions of phosphorus and nitrogen in bottom sediments from a typical north-temperate lake, China. Environ Earth Sci 71, 3063–3079 (2014). https://doi.org/10.1007/s12665-013-2683-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12665-013-2683-6