Abstract
This study was designed to survey the reservoir sediment properties, assess the phosphorus (P) sorption isotherm, and analyze the relationship between sediment properties and sorption parameters. Physicochemical analysis indicated that sediment from the FUSHI reservoir in Zhejiang Province, China, has similar physical and chemical properties and has been contaminated by P. Sorption isotherm experiments showed that the sorption process could be described by Langmuir and Freundlich models. The parameters of Q max (Phosphorus sorption maximum) and K (Freundlich adsorption isotherm constant) ranged from 618.98 to 825.70 mg kg−1 and 114.18 to 170.74 l kg−1, respectively. EPC0 (zero P equilibrium concentration) ranged from 0.14 to 0.24 mg l−1, more than the total P concentration in the water of the reservoir. Thus, the reservoir sediment releases P into the water and acts as a “P resource”. The clay, Feo, Alt, and Fet + Alt content were the main active components in P sorption. Q max had a highly significant positive relationship with some properties and could be estimated by a combination of these.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nutrients from chemical fertilizers or animal manures are beneficial if retained in the soil for uptake by plants; however, they become environmental pollutants if leached into watercourses, groundwater, lakes, and reservoirs (McGechan and Wu 1998; McGechan and Lewis 2000). Phosphorus (P) is an essential element in the biogeochemical cycle, but excessive utilization in agriculture as well as detergents will lead to environmental and ecological problems. In recent years, attention has shifted from nitrogen as a nutrient and pollutant (McGechan et al. 1997; Wu et al. 1998) toward the less soluble and non-volatile contaminating nutrient, P. Numerous studies have indicated that heavy use of P results in its accumulation in natural aquatic ecosystems and in sediments acting as P sinks (Jin et al. 2005; Zhang et al. 2007). The consequences of this accumulation will include serious problems for aquatic ecosystems and water environments, and even risks to human health. Developing more efficient nutrient application management strategies has been an important goal of various environmental agencies and farmers to prevent excessive phosphate buildup and protect the ecological integrity of sensitive watersheds (McDowell et al. 2001). The efficiency of these strategies relies on better understanding of the transformation processes of P at the soil–water interface. Pollutants such as P may be transferred from the water column to the sediment layer through biochemical, chemical, and physical reactions such as ion exchange, adsorption, and precipitation (Stumm and Morgan 1996). Therefore, pollutants may be adsorbed into sediments and accumulated in the bottom of rivers, lakes, or reservoirs (Kim et al. 2003). P may be re-released from the sediment if the overlying water quality changes (Furumai et al. 1989). Adsorption and desorption processes are universal phenomena existing on different interfaces in nature. These phenomena affect not only the characteristics of sediment and water, but also the surrounding environment.
Research into P sorption has been carried out in diverse sediment and soil types, but largely in temperate regions (Appan and Wang 2000; Zhao and Stanforth 2001; Kim et al. 2003; Oguz 2005; Auxtero et al. 2008; Derrick and Kin 2009). These studies have almost exclusively focused on soil and lake sediment, while information about P sorption/desorption of sediment in a subtropical reservoir is limited or nonexistent. However, sediment has become a serious problem in reservoirs in recent decades, as it both reduces the effective capacity of reservoirs and releases nutrients into the water (Sallade and Sims 1997). Understanding of the P adsorption/desorption in this system is vital as reservoirs are often used as a source of drinking water.
To understand the characteristics and processes of P sorption and the relationship between sediment characteristics and sorption parameters in aquatic sediment of a subtropical reservoir, the sediment characteristics and sorption isotherm were investigated. The objectives of the study were (1) to measure and analyze the characteristics of the sediment samples, (2) to identify the sorption isotherm characteristics and calculate the relevant parameters, and (3) to analyze the relationship between sediment characteristics and sorption parameters.
Materials and methods
Study area description
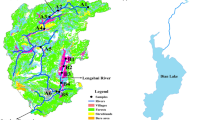
The FUSHI reservoir is a subtropical reservoir located at 30°38′10.96″N and 119°30′14.60″E in the Zhejiang Province, China. It was formed by damming the Xixi River in 1980. The reservoir has a maximum storage capacity of 218 × 106 m3 and 331 km2 upstream areas. It provides 25 × 103 m3 d−1 drinking water, irrigates 8 × 103 ha fields, and generates 5.4 × 103 kW/y power. The area upstream of the FUSHI reservoir consists of low mountains and hills covered with bamboo and Chinese chestnut tree plantations. Soils in these hills are impoverished and easily eroded. Large volumes of fertilizers are used in this area to improve crop growth, leading to heavy nutrient loss and soil erosion. The area experiences a typical subtropical marine-monsoon climate. The mean annual precipitation is 1344.1 mm and the mean annual temperature is 16.6°C. The concentration of P in the reservoir and river which flows into the reservoir ranges from 0.012 to 0.07 mg l−1.
Sediment samples
Four sediment samples, S1, S2, S3 and S4, were taken from the FUSHI reservoir using a special messenger-activated core sampler (ETC-200) in August 2009 (Fig. 1). Water depths of the sample stations were 6 m for S1 and S3, and 8 m for S2 and S4. Collected samples were stored in an icebox and transported immediately to the laboratory, where they were centrifuged for 10 min at 5,000 rpm to remove most of the water. Subsequently the sample was air dried, passed through a 100-mesh sieve and stored at 4°C.
Sediment analysis
Sediment pH was measured in distilled water (w/v ratio of 1:2.5) and 1 M NaF (pH 8.2) (w/v ratio of 1:50). Particle size distribution of the sediment was determined using the hydrometer method (Bouyoucos 1951). Total nitrogen (TN) was measured by the Kjeldahl apparatus (Kjelflex k-360) after digestion with sulfuric acid (H2SO4). Total phosphorus (TP) content was determined using the molybdenum-blue complex method following destruction of P with HClO4/H2SO4 at 370°C (Krom and Berner 1981). Available phosphorus (Avi-P) was extracted with 0.5 mol/L NaHCO3 (Bray and Kurtz 1945). The organic matter (OM) was measured by the dichromate volumetric method. Cation exchange capacity (CEC) was measured by ammonium acetate exchanging method. Fe and Mn were measured by flame atomic absorption spectroscopy (SHIMADZU A-A6300) and Al was measured by xylenol-orange photometric method (Bao 2005). Total Fe, Al, and Mn (Fet, Alt, and Mnt) were measured after digestion of the sediment with 30 ml mixture of HF, HNO3, and HClO4 in the ratio of 10:15:5. Crystalline and non-crystalline forms of Fe, Al, and Mn were extracted with dithionite-citrate-bicarbonate solution (DCB) (Fed, Ald, Mnd) and 0.2 M ammonium-oxalate solution at pH 3.0 (Feo, Alo, Mno), respectively (Wisawapipat et al. 2009; Mckeague and Day 1966). Inorganic P in the forms of aluminum phosphate (Al–P), iron phosphate (Fe–P), and calcium phosphate (Ca–P) were measured using the molybdenum-blue method after extraction with 0.5 M ammonium fluoride solution at pH 8.2, 0.1 M sodium hydroxide solution and 0.5 M sulfuric acid, respectively (Bao 2005).
Sorption isotherm
For the P sorption study, 0.5 g sediment samples were placed in a series of 50 ml polyethylene centrifuge tubes with 25 ml distilled water containing P at various concentrations. The tubes were shaken at 200 rpm on a rotary shaker at 25°C for 24 h. The initial concentrations (C o) prepared by analytical pure anhydrous potassium phosphate (K2HPO4) were 0, 0.05, 0.1, 0.15, 0.2, 0.4, 0.6, 0.8, 1.0, 2.0, 5.0, 10, 15, and 20 mg l−1, respectively. Two drops of toluene were added to restrict microbial activity. After shaking, suspensions were centrifuged at 3,500 rpm for 10 min. Then supernatants were filtered through a 0.45-μm filter membrane. Phosphorus equilibrium concentration (C eq) of the supernatant was measured by the molybdate blue method using a UV/VIS (UV-2550) spectrophotometer. The amount of P adsorbed by the sediment was calculated by the difference between C o and C eq. The formula was:
where Q is the amount sorbed (mg kg−1), C o is the initial P concentration (mg l−1), C eq is the equilibrium concentration (mg l−1), w is the sample weight (g), and v is the solution volume (ml) of K2HPO4.
Statistical analysis
All data presented were the mean values of three replicates. The softwares of Office Excel and SPSS 13.0 were used for calculation and statistical analysis, and Origin 8.0 was used to fit curves and calculate parameters. Pearson’s correlation was used to examine the individual relationship between the P sorption parameters (Q max and k) and between each physicochemical variable of the sediment. Multivariate stepwise regression was used to analyze and calculate the weights of physicochemical parameters on Q max.
Results and discussion
Sediment characteristics
The physical/chemical properties of four sediment samples are summarized in Table 1. Sediment from the FUSHI reservoir generally had a silt clay texture, with a silt content greater than 40%. A considerable portion of the sediment may be derived from decomposed loamy fine-texture and farmland soil upstream of the reservoir. All sediment samples were slightly alkaline with a pH (water) ranging from 7.28 to 7.52. Near neutral pH was typically found in flooded anaerobic sediment, owing to a reduction of FeCO3 in acid soil and production of CO2 in alkaline soil (Vepraskas and Faulkner 2001). The pH value measured by the NaF solution ranged from 9.69 to 10.18. Higher values indicated that the soil contained mineral constituents with surface hydroxyl ions, which were exchanged by the fluoride ion (Perrott et al. 1976).
Crystalline iron oxides, estimated by DCB extraction (Fed), were found to be the major forms of iron oxide in these sediment, though there were large differences between the four sediment samples (3055.73–6312.48 mg kg−1). The value of Feo greater than Fed indicated that most Fe existed in the form of crystalline Fe-oxide. While the higher value of Feo indicated that there was a substantial amount of both non-crystalline iron oxide and microcrystalline iron oxide in this sediment (Fontes et al. 1992).
The ratio of different types of sediment P varies at the four sample sites. The TP content in the sediment ranged from 510.13 to 842.84 mg kg−1. Percentages of TP constituted by Ca–P ranged from 31.62 to 51.29%, accounting for around half of TP. Percentages of Al–P and Fe–P ranged from 2.35 to 3.70% and 16.52 to 23.43%, respectively. The Avi-P value ranged from 29.34 to 40.29 mg kg−1, accounting for 4.73–5.75% of TP. There are two possible explanations for the difference in P fraction. Firstly, the four substrate samples, S1–S4, were collected from different rivers (Fig. 1). Secondly, the rinse action during the sediment movement and transportation by flood or undercurrent almost certainly differs at the four sites.
Sorption isotherm
Sorption isotherms are useful for summarizing sorption data and illustrating the relationship between aqueous concentration of the adsorbate and the amount of adsorption by the solid phase sorbent at constant temperature (Stumm and Morgan 1996). House and Denison (2000) and Zhou et al. (2005) called the P included in sediment as native adsorbed exchangeable phosphorus (NAP) and the NAP in sediment cannot be neglected in sorption (Zhou et al. 2005).
Figure 2a shows the P sorption isotherm of NAP-polluted adsorbent. It can be divided into two sections where “D” denotes the desorption area and “A” denotes the adsorption area. As shown in the figure, when adsorbed P fell to zero, and C eq (equilibrium P concentration) was equal to EPC0 (concentration at which no net adsorption or desorption of P occurs). When C eq was less than EPC0, the net effect of sorption was the release of NAP from the natural sediment (denoted by D). Reversely, when C eq was greater than EPC0, the net effect of sorption was P uptake by the sediment (denoted by A). When C eq equaled EPC0, the system was at equilibrium, with no net adsorption or desorption.
Figure 2b shows the P sorption isotherm of sediment in the FUSHI reservoir. From this figure it can be concluded that the P sorption isotherm was similar to that of some reports on soil and lake sediment (Appan and Wang 2000; Derrick and Kin 2009). Moreover, the P adsorption isotherms were generally L-shaped, indicating that P adsorption increased with a greater magnitude at low C eq than at high P loadings where P sorption sites were mostly saturated.
P sorption data could be fitted to the nonlinear equation of the Langmuir isotherms. The Langmuir adsorption isotherm is expressed here as Eq. 1 (Voice and Weber 1983; Weber et al. 1991):
where Q is the amount of net adsorbed P at the equilibrium stage (mg kg−1). Q max is the maximum P adsorption capacity (mg kg−1), k is the half-saturation concentration (mg l−1), and C eq is the P adsorption equilibrium concentration (mg l−1). This model has been used widely to describe anion sorption by soil and sediment (Del et al. 2003; Tian and Zhou 2008). However, it is not appropriate to use Eq. (1) when native adsorbed exchangeable phosphorus (NAP) plays a significant role in the adsorption process (Aminot and Andrieux 1996; Zhou et al. 2005). Therefore, the modified Langmuir adsorption isotherm equations (Eqs. 2, 3, 4, 5) (Zhou et al. 2005) were used to model the P adsorption of sediments in the FUSHI reservoir.
C add is C o (mg kg−1) and \( C_{\text{eq} }^{0} \)is the equilibrium concentration (mg l−1), while C o is equal to 0 mg l−1.
The P sorption data of the sediments can be described by Langmuir and Freundlich equations. The parameters are listed in Table 2.
The Langmuir adsorption isotherm assumes the occurrence of monolayer adsorption, while the Freundich adsorption isotherm assumes the presence of chemosorption processes in addition to surface adsorption (Rhue and Harris 1999; Sundareshwar and Morris 1999). The values of R 2 in Table 2 suggested that more complex mechanisms other than surface adsorption were involved in P sorption in the FUSHI sediment.
The Langmuir adsorption isotherm provided a good estimate of both theoretical adsorption maxima and the capacity of the sediment–water system to remove P. Langmuir adsorption maxima (Q max) of the FUSHI sediment ranged from 618.98 to 825.70 mg kg−1. These values are much lower than the 4,770–10,3400 mg kg−1 reported in sediment from the Kranji reservoir (Appan and Wang 2000), and the 930–3,751 mg kg−1 reported from Balearic Islands (Spain) (Lopez et al. 1996). However, the values were higher than 150–260 mg kg−1 and 196–281 mg kg−1 reported in constructed wetland sediments in Florida (Gale et al. 1994) and Korea (Yoo et al. 2006), respectively. This could be attributed to the concentrations of amorphous Fe and Al in the FUSHI reservoir sediment providing abundant specific surface area for P adsorption to take place (Paludan and Jensen 1995). Thus, increasing the proportion of completely decomposed granite in the substrate is expected to enhance the P sorption capacity considerably, as it contributed to the majority of the non-crystalline Fe and Al in the sediment. The EPC0 value in FUSHI reservoir sediment ranged from 0.14 to 0.24 mg l−1 with an average of 0.19 mg l−1, and more than 0.012 mg l−1 (the concentration of P in the FUSHI reservoir water). According to the adsorption/desorption mechanism, this suggests that soluble P can easily be re-released into the reservoir water. Thus, the FUSHI reservoir sediment acts as a P source.
Phosphorus sorption coefficients in relation to sediment properties
The relationship between the Q max and k were evaluated and sediment properties were selected to obtain information about main sediment properties responsible for P retention.
Phosphorus sorption maximum Q max had a highly significant (P = 0.05) positive relationship with clay, Fet, Alt, Fet + Alt, Feo, Feo + Alo, Al–P, and Fe–P (Table 3). Some bivariate plots for Q max illustrating the relationships are shown in Fig. 3.
The P sorption coefficient, k, was the half-saturation concentration (mg l−1) and had a significant (P = 0.05) negative relationship with silt, Fet, and Feo + Alo + Mno. Table 3 illustrates the correlation between sediment properties and Q max and k separately. Some properties of sediment correlated negatively with Q max, but had positive relationship with k. For example, the coefficient between silt and Q max was −0.99, but with k it was 0.91. Similarly, if k had a negative relationship with other sediment properties, Q max had a positive relationship, for example, with clay. These results also showed a similar pattern when using the Langmuir formula to calculate Q max and k.
The relationships of Q max with clay, Fet, Alt, Feo, Fet + Alt + Mnt, and Feo + Alo + Mno were close, and the sediment properties which are routinely determined by sediment survey could be used with confidence to predict Q max for a subtropical reservoir sediment in China. Multivariate stepwise regression analysis indicated that 98% of the variation in Langmuir P sorption maximum for these sediments can be explained by combinations of clay, Feo, Alt, Fet, and the sum Fet + Alt (Fig. 4) as follows:
The close relationship of Feo with Q max indicates that amorphous or poorly crystalline Fe oxyhydroxides that have a small bulk and big surface provide much of the P sorption, consistent with the findings of a number of studies looking at highly weathered soils (Maguire and Sims 2002; Agbenin 2003; Penn et al. 2005; Li et al. 2007; Lair et al. 2009). As Feo was nearly one-quarter of Fet and had a significant positive relationship with Q max, whereas Fed had no correlation with Q max, Feo had an important effect on the relationship between Fet and Q max.
Alt had a significant positive relationship with Q max, while Ald and Alo nearly had no effect on Q max (Table 3). The content of Ald and Alo were lower (Table 1) in comparison to Alt, suggesting that other Al fractions, such as organic Al, alumina silicate and oxyhydroxide Al, affected the relationship between Q max and Alt, Alo and Mno did not show significant correlation with P retention. However, a strong correlation was found between the sum of Feo, Alo, Mno, and Q max, as well as between the sum of Fet, Alt, Mnt, and Q max.
The positive correlation between Q max and clay suggested that there was an association between the colloidal components of the sediment causing the increase of effective surface area of P-sorbing. Organic matter in the soil did not show a significant correlation with Q max, reflecting its low affinity for anions due to negative surface charges in the pH range of the studied sediment.
This study revealed that the clay, Feo, Alt and Fet + Alt in the FUSHI reservoir sediment were the mainly active components in P sorption. Hence, P adsorption parameter Q max could be estimated by the combinations of clay and Feo, clay and Alt, Feo and Alt, and Feo and Fet + Alt, respectively.
Conclusions
These experiments revealed that the sediment in the FUSHI reservoir had similar physical and chemical properties, and that it has been contaminated by P. There was no obvious difference in the capacity for P sorption in the separate samples. Both the Langmuir and Freundlich models can be used to describe and calculate P sorption data, with R 2 ranging from 0.91 to 0.97. The Q max and K ranged from 618.98 to 825.70 and 114.18 to 170.74 l kg−1 for Langmuir and Freundlich models, respectively. EPC0 ranged from 0.14 mg l−1 to 0.24 mg l−1, more than the TP concentration in the FUSHI reservoir waters. Hence, it can be concluded that sediment in the FUSHI reservoir has not only been contaminated by P, but also releases P into the water and thus acts as a “P resource”.
The adsorption capacity, Q max, had a highly significant positive relationship with clay, Feo, Alt, Fet + Alt, Feo, Feo + Alo + Mno, Al–P and Fe–P, while the P sorption coefficient, k, had a negative relationship with these sediment properties. Multivariate stepwise regression analysis indicated that Q max can be estimated by the combination of clay with Feo, clay with Alt, Feo with Alt, and Feo with Fet + Alt. Thus, clay, Feo, Alt, and Fet + Alt contents were the main active components contributing to the P sorption maxima. This indicates that poorly crystalline Fe and Al oxyhydroxides provide much of the P sorption capacity and sorption energies.
As the sediments had similar physical and chemical properties, they did not show obvious sorption trends. The P released from sediment into the reservoir water could reduce the TP content in the sediment. Further studies are needed to calculate the amount of P released into the reservoir, and to analyze and evaluate the effects of this sediment behavior on the reservoir ecosystem and surrounding environment.
References
Agbenin JO (2003) Extractable iron and aluminum effects on phosphate sorption in a savanna alfisol. Soil Sci Soc Am J 67:589–595
Aminot A, Andrieux F (1996) Concept and determination of exchangeable phosphate in aquatic sediments. Water Res 30:2805–2811
Appan A, Wang H (2000) Sorption isotherms and kinetics of sediment phosphorus in a tropical reservoir. J Environ Eng-ASCE 126:993–998
Auxtero E, Madeira M, Sousa E (2008) Phosphorus sorption maxima and desorbability in selected soils with andic properties from the Azores, Portugal. Geoderma 144:535–544
Bao SD (2005) Soil and Agricultural Chemistry Analysis, 3rd edn. China Agriculture Press, Beijing, pp 231–232 and 90–93 (in Chinese)
Bouyoucos GJ (1951) A recalibration of the hydrometer method for making mechanical analysis of soil. Agron J 43:434–438
Bray RA, Kurtz LT (1945) Determination of total organic and available form of phosphorus in soil. Soil Sci 59:39–45
Del BM, Arias CA, Brix H (2003) Phosphorus adsorption maximum of sands for use as media in subsurface flow constructed reed beds as measured by the Langmuir isotherm. Water Res 37:3390–3400
Derrick YF, Kin CL (2009) Phosphorus sorption by sediments in a subtropical constructed wetland receiving stormwater runoff. Ecol Eng 35:735–743
Fontes MR, Weed SB, Bowen LH (1992) Association of microcrystalline goethite and humic acid in some oxisols from Brazil. Soil Sci Soc Am J 56:982–990
Furumai H, Konda T, Ohgaki S (1989) Phosphorus exchange kinetics and exchangeable phosphorus forms in sediments. Water Res 23(6):685–691
Gale PM, Reddy KR, Graetz DA (1994) Phosphorus retention by wetland soils used for treated waste-water disposal. J Environ Qual 23:370–377
House WA, Denison FH (2000) Factors influencing the measurement of equilibrium phosphate concentrations in river sediments. Water Res 34:1187–1200
Jin XC, Wang SR, Pang Y, Zhao HC, Zhou XN (2005) The adsorption of phosphate on different trophic lake sediments. Colloid Surf A 254:241–248
Kim LH, Choi E, Stenstrom MK (2003) Sediment characteristics, phosphorus types and phosphorus release rates between river and lake sediments. Chemosphere 50:53–61
Krom MD, Berner RA (1981) The diagenesis of phosphorus in a nearshore marine sediment. Geochim Cosmochim Ac 45(2):207–216
Lair GJ, Zehetner F, Khan ZH, Gerazbek MH (2009) Phosphorus sorption desorption in alluvial soils of a young weathering sequence at the Danube River. Geoderma 149:39–44
Li M, Hou YL, Zhu B (2007) Phosphorus sorption–desorption by purple soils of China in relation to their properties. Aust J Soil Res 45:182–189
Lopez P, Lluch X, Vidal M, Morgui JA (1996) Adsorption of phosphorus on sediments of the Balearic islands (Spain) related to their composition. Estuar Coast shelf S 42:185–196
Maguire RO, Sims JT (2002) Measuring agronomic and environmental soil phosphorus saturation predicting phosphorus leaching with Mehlich 3. Soil Sci Soc Am J 66:2033–2039
McDowell RW, Sharpley AN, Condron LM, Haygarth PM, Brookes PC (2001) Processes controlling soil phosphorus release to runoff and implications for agricultural management. Nutr Cycl Agroecosys 59:269–284
McGechan MB, Lewis DR (2000) Watercourse pollution due to surface runoff following slurry spreading, Part 2: decision support to minimize pollution. J Agric Engng Res 75:429–447
McGechan MB, Wu L (1998) Environmental and economic implications of some slurry management options. J Agric Engng Res 71:273–283
McGechan MB, Graham R, Vinten AJA, Douglas JT, Hooda PS (1997) Parameter selection and testing the soil water model SOIL. J Hydrol 195:312–334
Mckeague JA, Day JH (1966) Dithionite and oxalate-extractable Fe and Al as aids in differentiating various classes of soils. Can J Soil Sci 46:13–22
Oguz E (2005) Sorption of phosphate from solid/liquid interface by fly ash. Colloid Surf A 262:113–117
Paludan C, Jensen HS (1995) Sequential extraction of phosphorus in freshwater wetland and lake sediment: significance of humic acids. Wetlands 15:365–373
Penn CJ, Mullins GL, Zealazny LW (2005) Mineralogy in relation to phosphorus sorption and dissolved phosphorus losses in runoff. Soil Sci Soc Am J 69:1532–1540
Perrott KW, Smith BFL, Inkson RHE (1976) Reaction of fluoride with soils and soil minerals. J Soil Sci 27:58–67
Rhue RD, Harris WG (1999) Phosphorus sorption/desorption reactions in soils and sediments. In: Reddy KR, O’connor GA, Schelske CL (eds) Phosphorus biogeochemistry in subtropical ecosystems. CRC Press, Boca Raton, pp 187–206
Sallade YE, Sims JT (1997) Phosphorus transformations in the sediments of Delaware’s agricultural drainage ways: 1. Phosphorus forms and sorption. J Environ Qual 26:1571–1579
Stumm W, Morgan JJ (1996) Aquatic chemistry: chemical equilibria and rates in natural waters. Wiley, New York, p 975
Sundareshwar PV, Morris JT (1999) Phosphorus sorption characteristics of intertidal marsh sediments along an estuarine salinity gradient. Limnol Oceanogr 44:1693–1701
Tian JR, Zhou PJ (2008) Phosphorus fractions and adsorption characteristics of floodplain sediments in the lower reaches of the Hanjiang River, China. Environ Monit Assess 137:233–241
Vepraskas MJ, Faulkner SP (2001) Redox chemistry of hydric soils. In: Richardson JL, Vepraskas MJ (eds) Wetland soils: genesis, hydrology, landscapes, and classification. Lewis Publishers, Boca Raton, pp 85–106
Voice TC, Weber WJ (1983) Sorption of hydrophobic compounds by sediments, soils and suspended solids. 1. Theory and background. Water Res 17:1433–1441
Weber WJ, Mcginley PM, Katz LE (1991) Sorption phenomenon in subsurface systems: concepts, models and effects on contaminant fate and transport. Water Res 25:499–528
Wisawapipat W, Kheoruenromne I, Suddhiprakarn A, Gilkes RJ (2009) Phosphate sorption and desorption by Thai upland soils. Geoderma 153:408–415
Wu L, McGechan MB, Lewis DR, Hooda PS, Vinten AJA (1998) Parameter selection and testing the soil nitrogen dynamics model SOILN. Soil Use Manage 14:170–181
Yoo JH, Ro HM, Choi WJ, Yoo SH, Han KH (2006) Phosphorus adsorption and removal by sediments of a constructed marsh in Korea. Ecol Eng 27:109–117
Zhang TX, Wang XR, Jin XC (2007) Variations of alkaline phosphate activity and P fractions in sediments of a shallow Chinese eutrophic lake (Lake Tainhu). Environ Pollut 150:288–294
Zhao HS, Stanforth R (2001) Competitive adsorption of phosphate and arsenate on goethite. Environ Sci Technol 35:4753–4757
Zhou AM, Tang HX, Wang DS (2005) Phosphorus adsorption on natural sediments: modeling and effects of pH and sediment composition. Water Res 39:1245–1254
Acknowledgments
This research was supported by the project: Effect of water loss and soil erosion on FUSHI watershed environment (No. H20092404). Our warmest thanks are also expressed to Miss Colette Matthewman in the Department of Genetics and Biotechnology, Aarhus University, for her assistance in the English presentation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, X., Zhang, L., Zhang, H. et al. Phosphorus adsorption characteristics at the sediment–water interface and relationship with sediment properties in FUSHI reservoir, China. Environ Earth Sci 67, 15–22 (2012). https://doi.org/10.1007/s12665-011-1476-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12665-011-1476-z