Abstract
Tailings deposited over the Castanheira, a stream which flows through the old Ag–Pb–Zn Terramonte mine area, showed a great potential environmental risk due to sulphide weathering, facilitated by the tailings–water interaction. The high concentrations of Al, Fe, Pb and Zn in the tailings are associated with the exchangeable, reducible and sulphide fractions and suggest sphalerite and pyrite occurrences. Oxidation of pyrite is responsible for the low pH values (3.38–4.89) of the tailings. The water from the Castanheira stream is not suitable for human consumption due to high concentrations of SO4 2−, Mn, Al, Cd, Ni, and Pb. The lowest concentrations of metals and metalloids were detected in downstream stretches of the Castanheira. However, As, Fe and Zn in deeper sediments tend to increase downstream. Significant concentrations of trivalent forms of arsenic were detected in water samples. In downstream stretches of the Castanheira, some free ions (Fe2+, Mn2+ and Zn2+) also predominate and the water is saturated with ferrihydrite, goethite, hematite, lepidocrosite and magnetite.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Tailings are an important source of potentially toxic metals and metalloids which can easily be dispersed and be of significant environmental concern due to their toxic effect in aquatic systems, high enrichment factor and slow removal rate (Alloway and Ayres 1997). The chemistry and potential hazard of tailings depends on ore mineralogy, processing methods and thus particle size distribution, climate and the period after the closure of mines (Lottermoser 2010). Since the mine closure, different impacts associated with distinct mine dumps have occurred in the environment (Carvalho et al. 2009, 2012; Flores and Rubio 2010). In general, high concentrations of metals and metalloids in tailings are due to sulphide oxidation and the subsequent redistribution of trace metals and metalloids by secondary Fe precipitates and phases formation, as well as adsorption on the clay fraction (Carlsson et al. 2002; Heikkinen and Räisänen 2009). As the pH decreases due to sulphides weathering, caused by O2 and water infiltration, the metals and metalloids are easily mobilised in streamwater (Heikkinen and Räisänen 2009). Tailings with a low organic matter content and without buffering capacity tend to easily release metals into the environment (Heikkinen and Räisänen 2009; Wang and Mulligan 2009).
Metals and metalloids released from tailings into a streamwater are distributed between the aqueous phase and bed sediments. Adsorption, hydrolysis and co-precipitation cause significant retention of metals and metalloids in sediments and consequently only a small portion of free metal ions remain dissolved in water (Macklin 1992). Therefore, stream sediments can provide much information on aquatic environmental quality. Metals and metalloids can be present in variable solid phases of stream sediments, which control their mobility and bioavailability, or they can return into solution if physico-chemical conditions are favorable. The total metal and metalloid concentrations in tailings and sediments are not enough to assess the potential environmental impact as they do not indicate the metal and metalloid contents that can easily be mobilised in water. The solid-phase speciation and physical–chemical processes existing at the water–solid interface is very important for predicting potential mobility and metal availability in the environment (Bogush and Lazareva 2011; Li et al. 2011; Larios et al. 2013). Their distribution into different solid phases is determined by the sequential extraction procedure, which is a useful technique to characterise the solid-phase carrier of elements (Hass and Fine 2010). The BCR three-step sequential extraction procedure is generally used nowadays (Janoš et al. 2010; Rauret et al. 1999). Moreover, the sequential extraction technique has been used to predict the oxidation degree of sulphides and secondary Fe precipitates in tailings (Carlsson et al. 2002; Dold 2003; Heikkinen and Räisänen 2009), as well as in stream sediments (Cappuyns et al. 2007; Charriau et al. 2011).
Water is the most significant pathway for the transport of metals and metalloids derived from the chemical weathering of rock and ore minerals. The water quality depends on the physico-chemical conditions, as the dissolution/adsorption of metals and metalloids is mainly controlled by the redox potential and pH. The physico-chemical conditions determine the ionic speciation of metals and metalloids that control their degree of mobility and adsorption facility to be integrated into the solid phases of sediments (Kartal et al. 2006). Therefore, the interest in quantifying the individual species, in particular the arsenic speciation, because of its toxic properties, has increased in environmental studies (Jain and Ali 2000; Sharma and Sohn 2009; Wilson et al. 2010). Arsenite is usually more toxic than arsenate. The toxicity of trivalent arsenic is related to its high affinity for many enzymes, inhibiting their activities (Aposhian and Aposhian 2006). But it is also related to its mobility and the availability due to its high solubility (Van Herreweghe et al. 2003).
The main purposes of this research are: (1) to determine the geochemistry, mineralogy and chemical partitioning of selected metals and metalloids in tailings, surface sediments and deeper sediments; (2) to characterise the water geochemistry throughout a year, and (3) to obtain the speciation of metals and metalloids in waters. Those results will provide the degree of mobilisation in water and stream sediments of metals and metalloids from tailings and make it possible to discuss the bioavailability implications for the dispersion of metals and metalloids in the environment.
Materials and methods
Study area and sampling
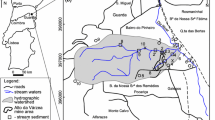
The Ag–Pb–Zn Terramonte mine is located about 18 km east of Porto in northern Portugal (Fig. 1a), close to the south side (left bank) of the River Douro (Fig. 1b). The Cambrian schist–graywacke complex predominates in the area and consists of alternating metapelites and metagraywackes containing metaconglomerate and marble lenses and is overlain, with angular discordance by the Arenigian quartzites with intercalated clay schists. The Ag–Pb–Zn quartz veins cut the schist–graywacke complex at the core of a large Variscan fold, the Valongo anticline. They fill the N60°E; 82°NW faults, and their thickness ranges between 5 and 10 m (Fig. 1b). The Ag–Pb–Zn quartz veins consist of quartz, arsenopyrite, pyrite, pyrrhotite, sphalerite, chalcopyrite, freieslebenite, semseyite, galena, jamesonite, polybasite, owyheeite, bournonite, boulangerite, freibergite, pyrargyrite, dolomite, ankerite, scorodite, anglesite, plattnerite, covellite, cerussite and pyromorphite. The exploration was carried out underground and the mine was active between 1866 and 1973 (Parra et al. 2002). About 3,200 tonnes of galena containing 70 % Pb and 3,300 g/t of Au, 3,200 tonnes of sphalerite containing 50 % Zn and 800 g/t of Ag were exploited (Mitel-Minas de Terramonte 1966). While the mine was active, three dumps (E1, E2 and E3) were deposited along the Castanheira stream and its tributaries (Fig. 1b). This stream is a tributary of the River Douro and a fourth deposit (E4) was formed in the River Douro bank, by the remobilization of tailings (Fig. 1b).
a Location of the Terramonte mine area on the map of Portugal; b Geological map of the Terramonte mine and location of water, surface sediments and deeper sediments samples. 1 Schist–graywacke complex, 2 Arenigian quartzites with intercalated clay schists, 3 Ag–Pb–Zn quartz veins, 4 mine dumps, 5 streams, 6 locations, 7 spring, 8 water + surface sediment + deeper sediment, 9 water + deeper sediment, 10 water + surface sediment, 11 water
The climate of this area is characterised by a maritime temperate climate with temperatures ranging between 5 and 30 °C in dry, warm summers. The wet season starts in October and lasts until May; the mean annual temperature is 14.6 °C and annual precipitation ranges from 1,148 to 1,771 mm/year (SNIRH 2012). The relief is rough and the altitude reaches 300 m at the upstream mine dumps, but the altitude of the River Douro bank is about 10 m. The mine dumps (E1, E2 and E3) are composed of tailings and rejected ore minerals and now they have a very sparse natural vegetation cover, whereas the E4 dump is composed of fine tailings.
In order to study the effect of mine dumps on the Castanheira stream, nine surface water samples were collected downstream of the mine area (TER 1, TER 2, TER 3, TER 4, TER 5, TER 6, TER 7, TER 10 and TER 11) and two samples were collected from springs upstream of the mine area (TER 8 and TER 9) (Fig. 1b). The water samples were collected in September 2007 and January, May and August 2008 to control the seasonal variation of the physico-chemical composition. September and August represent the dry season, and January and May the rainy season. The parameters temperature, pH, Eh, electrical conductivity and alkalinity were measured in situ. Tailings resulted from processing ore minerals in the old Terramonte mine and were deposited over the Castanheira stream. Four tailing samples were collected from two mine dumps [TAI E2 (20), TAI E2 (40), TAI E4 (20) and TAI E4 (70)]. These tailing samples were collected along a vertical profile of the mine dumps, at depths of 20, 40 and 70 cm (indicated in brackets). In addition, five deeper sediment samples (TER 6S, TER 10S, TER 11S, TER 1S and TER 4S) were collected from the Castanheira stream, its tributaries and the River Douro, and six surface sediments (TER 6-surf, TER 5-surf, TER 7-surf, TER 10-surf, TER 1-surf, TER 1-surf-a) were also collected from the Terramonte mine area (Fig. 1b). The deeper sediment samples were collected at a depth of 10 cm and the surface sediment samples were taken from the surface layer of sediments (0–1 cm). Some of the surface sediments and deeper sediment samples were collected from exactly the same locations to investigate the effect of acid mine drainage on the mobilisation of metals and metalloids.
Sample processing
All sediment samples were carried to the laboratory in polyethylene bags and were dried at 40 °C, disaggregated and sieved (2 mm mesh size). The 2-mm mesh size sample fraction was used to measure the pH, electrical conductivity, carbon content, cation exchange capacity and grain size distribution. The same fraction (2-mm mesh size) was used for pseudo-total concentration and BCR sequential chemical extraction.
The BCR sequential extraction procedure was used on the tailing and sediment samples. This method consists of three extraction steps, the first fraction (F1: exchangeable-easily soluble) was extracted with 0.11 M acetic acid, the second fraction (F2: reducible) was extracted with 0.1 M hydroxylamine hydrochloride (pH 2.0) and the third fraction (F3: oxidizable) was extracted with 8.8 M H2O2 followed by extraction with ammonium acetate at pH 2.0 (Rauret et al. 1999). A fourth step (F4) was added, which consists of digestion of the final residue by aqua regia (3:1 HCl–HNO3). An independent aqua regia digestion was performed on separate samples, which gives the recovery of the method.
Sediment pH was measured in a 1:2.5, w:v sediment to water suspension and the electrical conductivity (EC) was measured in the supernatant liquid of a 1:5, w:v sediment to water suspension. The cation exchange capacity (CEC) was determined as the sum of extractable bases and extractable acidity by the ammonium acetate solution (pH 7) method (Thomas 1982). The carbon content was determined by the loss of mass on ignition at 1,100 °C, multiplied by 1.724, on the assumption that the organic matter contains 58 % organic carbon (Nelson and Sommers 1996). The particle size distribution was determined by sieving and by sedimentation process for particles <63 μm, with an accuracy of up to 10 %.
The mineralogical study of the samples from tailings and sediments was obtained by X-ray diffraction (XRD), using a Philips PW3710 diffractometer. The clay mineralogy was determined in orientated aggregates of the <2 μm fraction obtained by sedimentation from an aqueous suspension into glass slides. The orientated aggregates were subjected to thermal treatment at 550 °C for 2 h and dissolved with ethylene glycol.
Metal and metalloid analysis
Metal and metalloid digests and extractions of tailing and sediment samples were analysed using a Perkin Elmer Optima 3000 inductively coupled plasma optical emission spectrometer (ICP-OES). The quality of the analytical data was assessed by carrying out analyses of the certified reference materials, BCR-701 for the sequential extraction procedure and BCR 143R for aqua regia digestion. BCR-701 is a lake sediment certified for extractable metal contents in the three steps of the modified BCR sequential extraction procedure. BCR-143R is a sewage sludge amended soil used for certification of the metal contents of the aqua regia digestion. The recovery ranges from 90 to 110 % for 90 % of elements. Each extraction step was performed in duplicate, using 0.5 g of the original material. The certified reference materials and a blank sample were included in duplicate in each batch of 30 samples. A good agreement was observed between the obtained and the certified values for the metals and metalloids (Al, As, Co, Cd, Cr, Cu, Fe, Mn, Ni, Pb, Sb and Zn) analysed. Calibration and check standards were made up in the same matrix as the extractable solution. The reproducibility for the analysis of standard solutions has consistently been <5 % for all elements and matrices.
The water samples were filtered through 0.45-μm cellulose nitrate membrane filter. The water samples’ anions were determined by ion chromatography using a Dionex ICS 3000 Model, whereas cations were determined in acidified waters (pH 2) by ICP-OES using a Horiba Jovin Hyvon JY 2000-2 Model. The detection limits were 1 g/l for cations and 0.01 μg/l for As and Sb. The precision was better than 5 %. Duplicate blanks and a laboratory water standard were analysed for quality control. The electroneutrality is up to 10 % for 87 % of the samples and higher than 10 % for 13 % of the samples. The water samples with the highest As contents were collected at the driest season for the arsenic speciation. These waters were filtered in situ with 0.45-μm polytetrafluoroethylene membrane filters, preserved with 0.25 M Na2-EDTA, stored in opaque polyethylene bottles and kept cool. They were analysed by anion-exchange chromatography–anion-self-regenerating suppressor–inductively coupled plasma–dynamic reaction cell–mass spectrometry (AEC-ASRS-ICP-DRC-MS) (Planer-Friedrich et al. 2007). One water sample was analysed in duplicate. Precision was <3 % for total As, <2 % for total Sb, <5 % for As species and <4 % for Sb species. Accuracy was checked by analysing the certified reference material TMDA-61 (National Water Research Institute, Burlington, ON). Recoveries were 98.3 % for total As and 105.5 % for total Sb. The geochemical modelling program PHREEQC (Parkhurst and Appelo 1999) and MINTEQ.V4.dat database (Allison et al. 1991) were used to predict aqueous speciation and the thermodynamic equilibrium conditions of water related to the main mineral phases.
The risk assessment code (RAC) is used by several authors to assess the ability of sediments to release metals and metalloids into water (Perin et al. 1985; Rodríguez et al. 2009; Singh et al. 2005). The RAC is the percentage of metals associated with the exchangeable and carbonate fraction. The risk assessment was calculated for tailings, surface sediments and deeper sediments from Terramonte using the metal and metalloid percentages extracted in fraction F1 of surface and deeper sediments \(\left( {\frac{F1}{(F1 + F2 + F3 + R)}} \right) \times 100 \,\%\).
Results and discussion
Mineralogy and geochemistry of tailings, surface sediments and deeper sediments
The results of the X-ray diffraction of the tailings, surface sediments and deeper sediments are given in Table 1. Quartz is the most abundant mineral followed by illite, in all samples. The chlorite, kaolinite and montmorillonite are the other clay minerals identified in most samples. Sulphate minerals (jarosite, plumbojarosite, natrojarosite, argentojarosite, beudantite and Al–H sulphate) are present in small amounts, mainly in the tailings and surface sediments. Sulphides (pyrite, miargyrite and argentite) are rare, except in the deeper sediment sample TER 4S.
Tailings showed low pH and high EC and CEC in samples from the mine dump TAI E4 mine dump (Table 2). The low pH in the tailings is due to abundant oxidation of pyrite, which is confirmed by the Fe content (up to 1,024.70 mg/kg) extracted from the fraction F3 of the sequential chemical extraction (supplemental electronic Table 3), suggesting that some Fe is linked to sulphides, mainly pyrite, as the organic matter (OM) content is very low in the tailing samples (≤0.17 %). However, the most pyrite is weathered to jarosite and beudantite (Table 1). Jarosite is a secondary weathering mineral associated with the oxidation of pyrite and pyrrhotite in acid mine drainage (Dutrizac and Jambor 2000). Some of this pyrite from the tailings was transported to the sediments, as the TER 4S has a high pyrite content. In general, surface sediments have higher EC and CEC and also have higher clay size contents than deeper sediments (Table 2). Therefore, cation exchanges between surface sediments and waters are expected. The pH values of the surface sediments (4.05–5.40) are lower than those of the deeper sediments (5.51–6.50), which suggest sulphide oxidation in the surface layer of stream sediments, due to the oxidant conditions of the surface environment. Moreover, the high EC values confirm the high metals contents in the surface sediments. In addition, OM content is up to (0.28–0.66 %) in the surface and stream sediments, compared with (0.10–0.36 %) in the deeper sediments (Table 2), suggesting it contributes little to the retention of metals.
The presence of Pb sulphates in the sediments (plumbojarosite) suggests the rapid alteration of galena under acid conditions (Moncur et al. 2005). Galena usually alters to anglesite and the latter to plumbojarosite, as anglesite is stable over a narrow range of Eh–pH (Forray et al. 2010). This can explain why Pb does not occur in the sulphide fraction (F3) of most samples (supplemental electronic Table 3). Plumbojarosite occurs in some tailings [TAI E2 (20) and TAI E4 (70)] and surface sediments (TER 10-surf and TER 10-surf-a) as its dissolution took place above pH 6 (Hochella et al. 1999), which is higher than the pH values of these samples from Terramonte. In general, the surface sediments and deeper sediments tend to have higher Pb concentrations in the fraction F1 than the tailings do, because the tailings generally have a low pH. Moreover, plumbojarosite is stable in conditions close to neutral pH (Forray et al. 2010).
The high Zn concentrations were found in fractions F1, F2 and F3 of the surface and deeper sediment samples compared to the tailings (supplemental electronic Table 3, Fig. 2). The Fe-poor sphalerite is less susceptible to weathering (Kossoff et al. 2011). However, the sphalerite from quartz veins of Terramonte contains up to 12.98 wt% of Fe (Carvalho 2010). Therefore, the Fe-rich sphalerite may be responsible for the high Zn concentrations in fractions F1, F2 and F3. The element mobility varies greatly in the profile of the sulphide tailings and Zn is one of the most mobile metals, as reported in other studies (Bogush and Lazareva 2011). Similar chemical fractionation was observed for Cu and Mn. This indicates that the weathering of Cu and Mn solid phase in the tailings incorporates their available forms in the stream sediments (Ettler et al. 2007; Beauchemin et al. 2012). After BCR fractionation, the As and Sb looked entirely different and were mainly retained in the oxyhydroxide (F2) and residual (R) fractions of the tailings, surface and deeper sediments (Fig. 2, supplemental electronic Table 3), which could be associated with a significant weathering of arsenopyrite (for As), present in the sediments (e.g., Flakova et al. 2012).
Most Sb secondary minerals are stable under a narrow Eh–pH range, whereas others are extremely rare and there is not enough information about their stability (Roper et al. 2012; Vink 1996). The metalloids Sb and As are strongly adsorbed on Fe–Mn oxyhydroxides, mainly at pH below 7 (Belzile et al. 2001) and are attenuated by dilution and by adsorption on ferric iron minerals in stream sediments (Flakova et al. 2012). The pH values of tailings, surface and deeper sediments, are lower than seven, which explains the detection of Sb and As in fraction F2 of the tailings (Table 2, supplemental electronic Table 3). At the Pezinok mining site (Slovak Republic), the most solid phase for As and Sb is also in the reducible fraction (F2) of the mine tailings (Flakova et al. 2012). Moreover, in this pH range, As and Sb tend to occur as oxyanionic forms that are adsorbed on clay minerals (Wilson et al. 2010), detected in the residual fraction (R). The adsorption of As on clay minerals mainly occurs at a pH lower than five, but decreases significantly with increasing pH (Kim 2010). Therefore, the As concentration is lower in the deeper sediments with high pH (5.51–6.50), than in the tailings and surface sediments (Tables 2, 3).
The Al, Cu, Fe, Mn, Pb and Zn concentrations in the exchangeable fraction of the surface and deeper sediments (supplemental electronic Table 3) suggest the high potential of those elements to be easily mobilised to waters. The surface sediments have the highest exchangeable metal concentrations, which is in agreement with their high CEC values (Table 2). Moreover, the percentage of the particle clay size is higher in surface sediments (8.58–11.88 %), than in deeper sediments (3.33–7.64 %, Table 2); metals and metalloids are mainly associated with finer particles (Salomons and Förstner 1984). Despite the dispersion of mine dumps (E1, E2, E3 and E4) in the Castanheira stream, the higher pH of the downstream water samples (from TER6 to TER4) causes Fe hydroxides precipitate and metals and metalloids are consequently retained in the sediments. Jarosite, beudantite, plumbojarosite and aluminium hydrogen sulphate were formed in the downstream surface sediments (Table 1). The surface sediments have higher metal concentrations than the deeper sediments probably because of the direct interaction with the water and the particles’ size composition. The high contents of metals and metalloids in the surface and deeper sediments compared to the tailings (Table 3) clearly indicate the precipitation on the streambed, which can be seen from the red-brownish surface sediments due to ferric precipitation.
Samples from tailings, surface sediments and deeper sediments are classified according to the RAC (Table 4). This classification is only based on the proportion of metals in the labile fraction and does not consider the aqua regia concentration of elements (concentration of elements obtained by aqua regia digestion), which is also shown in Table 4. In most tailings and stream sediments samples Al, Cu and Zn have low to medium risk. Sample TAI E4 (70) has a high risk for Al, Cu, and a very high risk for Zn. Surface sediments TER1-surf and TER10-surf has a high risk for Zn and TER10-surf for Pb. In general, the deeper sediments show a medium risk for Cu and Zn, but they show a high risk for Pb in most samples, except in TER4S.
Geochemistry of waters
The physico-chemical parameters of waters from Terramonte are shown in supplemental electronic Table 6. The pH and total dissolved solids (TDS) values indicate a clear distinction between the water upstream and downstream of the mine dumps. The pH of samples TER 8 and TER 9, upstream of the mine dumps, ranges from 5.17 to 6.01, whereas the pH of the water located up to 900 m downstream (TER 6, TER 5, TER 7 and TER 10) of the mine dump E1 tends to have values lower than four (Fig. 3). However, the pH gradually increases lower down the Castanheira stream. Generally, water samples have the lowest pH values in the dry season (September 2007 and August 2008).
The samples TER 5, TER 6, TER 7 and TER 10 exhibited high EC values (667–1,940 μScm−1), as they are close to the E1 mine dump. Furthermore, EC was high in the dry season (August and September) because the concentration of major anions and cations increases in this season, due to evaporation. Dissolved oxygen (DO) is higher in the wet season (January) than at other sampling times and the lowest values occur in the dry season (August), because oxygen solubility in water deceases with increasing temperature. The samples upstream of mine dumps have the lowest DO, as they are groundwaters. The DO concentration tends to fall from upstream to downstream in the Castanheira stream, probably because the flow velocity lowers downstream and this reduces the oxygen exchanges between the atmosphere and water.
There is a gradual decrease in F−, SO4 2−, Na, Ca, Mg, Al, Cu, Fe, Mn, Ni, Pb and Zn concentrations (supplemental electronic Table 6) from the E1 mine dump to lower down the Castanheira stream (samples TER6, TER5, TER7, TER10, TER11, TER1 and TER2), which can be explained by the rising pH, while NO3 −, NO2 − and HCO3 − contents tend to increase downstream.
The Al, Cu, Fe and Pb tend to have higher concentrations in the wet season (January and May). The occurrence of beudantite, corkite, plumbojarosite and jarosite in the tailings, of aluminium hydrogen sulphate in the surface sediments and of aluminium phosphate in the deeper sediments can explain why the concentrations of Al, Cu, Fe and Pb in the water are higher in the rainy season than in the dry season, because those minerals are easily dissolved during rainy periods. The Cu is incorporated in minor amounts in minerals of the jarosite group (Scott 1987). The higher availability of water in the raining season also promotes higher dissolution rates. Arsenic tends to decrease lower down the Castanheira due to the formation of secondary minerals in the tailings (Tables 1, 4, Fig. 3). However, As concentrations increase in samples TER 3 and TER 4 of the River Douro (Fig. 3). The tailing sample TAI E4 (70) showed a significant As concentration (90.18 mg/kg) in the fraction F1 (supplemental electronic Table 3) and the highest As concentration in F2 (538.4 mg/kg), which explains the highest mobilisation of As from the mine dump E4 in the closest water samples. The concentrations of Sb tend to increase lower down the Castanheira, because this metalloid is kept in solution, since it occurs as oxyanion; however, in samples from Douro river TER3 and TER4, the Sb concentrations decrease (supplemental electronic Table 6) due to the dilution effect caused by this river.
The results of the analytical arsenic speciation of selected water samples from Terramonte (Table 5) show that the As (V) species predominates in sample TER 4, whereas the As (III) predominates in the TER 7 and TER 11 samples. The difference between total As concentration and the sum of measured As (III) and As (V) is due to the existence of unidentified As species that cannot be detected by the analytical method used. The As (III) is more toxic and more mobile than the As (V) species (Sharma and Sohn 2009). The As (V) may be incorporated in jarosite and can be sorbed onto iron minerals (Courtin-Nomade et al. 2005).
The Eh and pH values are the most important parameters that control arsenic speciation. Therefore, water samples were plotted in the Eh–pH diagram (Fig. 4). Most water samples from Terramonte plot in the field were arsenate H2AsO4 − predominates. However, some of those collected in the dry season fall close to the transition between arsenite and arsenate species fields, particularly those collected in August (Fig. 4). The water sample TER 4 plots in the arsenate field, whereas TER 7 and TER 11 plot very close to the limit of the arsenite and arsenate species (Fig. 4), which agrees with the speciation results for those samples because As (V) predominates in the TER 4 sample and As (III) was not detected (Table 5). The fact that the pH value of the TER 4 is higher than the pH of TER 7 and TER 11 (supplemental electronic Table 6) can justify the As (V) predominance in the TER4. Most water samples collected in August tend to plot close to the arsenite field, because Eh decreases in summer (Fig. 4).
Eh-pH diagram with water samples plotted; diagram adapted from Vink (1996). Samples TER 4, TER 7 and TER 11 were analysed for arsenic speciation
The main aqueous chemical forms of metals and metalloids were predicted considering the most common inorganic ligands in the water (OH, Cl, F, SO4, HCO3, CO3, NO2, NO3) (Table 6). Cu, Fe, Ni, Mn and Zn occur predominantly as free aqueous ions (Table 6). Sulphate complexation is also important for Fe, Ni, Pb, Mn and Zn in samples TER7 and TER 11. But in the sample TER4, from River Douro, sulphate complexation is important only for Fe, as the pH becomes neutral and SO4 2− concentration decreases (supplemental electronic Table 6). Arsenic occurs predominantly as arsenite form (H3AsO3) in TER 11 and as arsenate in TER 7 (H2AsO4 −) and in TER 4 (HAsO4 2−), which agrees with the Eh–pH diagram plot (Fig. 4). Antimony is pentavalent in TER 7 and TER 4, but trivalent in TER 11 (Table 6). Therefore, the free aqueous species and sulphate species are common in polluted waters, downstream of the E1 mine dump. Carbonate species occur only in TER4, due to its circumneutral pH. Saturation is only reached for SbO2 and hematite in TER7 and SbO2 in TER11. The TER4 is saturated in ferrihydrite, goethite, hematite, lepidocrosite and magnetite due to the higher pH.
Conclusions
The complete weathering of most sulphides in tailings, surface and deeper sediments was confirmed by the absence or very small extractable concentrations of As, Cr, Cu, Mn, Ni and Sb in the oxidizable fraction. The very low Zn and Pb concentrations associated with the sulphide fraction clearly indicate that most of the main sulphides contained in the tailings have been weathered since the Terramonte mine closure. The risk assessment is low to medium for Al, Cu and Zn in most tailing and stream sediments samples. Deeper sediments have high risk for Pb. However, aqua regia metal and metalloid concentrations decrease from the tailings to surface sediments and to deeper sediments. The high Al, As, Pb and Zn contents in the most labile fraction of the tailings suggest a continuous leaching of those elements into the environment.
The fact that there is more than one mine dump along the Castanheira stream has no cumulative contamination effect on the surface water, as most element concentrations decrease downstream. Dilution by tributaries contributes to reducing the water contamination and the higher pH downstream causes the subsequent precipitation of metals and metalloids precipitation or their adsorption by the streambed sediments. Al, Cu, Fe and Pb tend to have higher concentrations in waters collected in the rainy season due to the dissolution of beudantite, corkite, plumbojarosite, jarosite and aluminium hydrogen sulphate from the tailings. According Portuguese law (Decree law 236/98 and Decree law 306/207), water from the Castanheira stream is not fit for human consumption because the SO4 2−, Mn, Al, Ni, Pb contents are above the limit for human consumption (VP) (Fig. 3, supplemental electronic Table 6). The As and Sb contents are above VP in some samples. Furthermore, the Mn and Zn contents from this stream are higher than the values recommended for agriculture. Arsenic tends to occur in pentavalent forms. However, trivalent forms, which are more toxic and mobile than pentavalent forms, were also detected. Free ions and a sulphate complex dominate in polluted waters. Lower down the Castanheira stream, some free ions (Fe2+, Mn2+ and Zn2+) also predominate and water becomes saturated in oxyhydroxides. Therefore, a decrease in the concentration of most metals in water more than 900-m downstream of the E1 mine dump suggests transport of metals in suspension. The sedimentation of suspended particles and the subsequent high contents of metals in the stream’s sediments constitute a potential environmental risk, because a significant part of the metals in the surface and deeper sediments can be released into the surface water. The tailings have low amounts of sulphides, although they still have a significant potential to release metals and metalloids into the environment.
References
Allison JD, Brown DS, Novo-Gradac KJ (1991) MINTEQA2, a geochemical assessment model for environmental systems. Report EPA/600/3-91/0-21. USEPA, Athens, Georgia
Alloway J, Ayres DC (1997) Chemical principles of environmental pollution. Chapman and Hall, UK
Aposhian HV, Aposhian MM (2006) Arsenic toxicology: five questions. Chem Res Toxicol 19:1–15
Beauchemin S, Kwong YTJ, Desbarats AJ, Mackinnon T, Percival JB, Parsons MB, Pandya K (2012) Downstream changes in antimony and arsenicspeciation in sediments at a mesothermal gold deposit in British Columbia, Canada. Appl Geoch 27:1953–1965
Belzile N, Yu-Wei Chen, Wang Z (2001) Oxidation of antimony III/by amorphous iron and manganese oxyhydroxides. Chem Geol 174:379–387
Bogush AA, Lazareva EV (2011) Behavior of heavy metals in sulfide mine tailings and bottom sediment (Salair, Kemerovo region, Russia). Environ Earth Sci 64:1293–1302
Cappuyns V, Swennen R, Niclaes M (2007) Application of the BCR sequential extraction scheme to dredged pond sediments contaminated by Pb–Zn mining: a combined geochemical and mineralogical approach. J Geochem Explor 93:78–90
Carlsson E, Thunberg J, Öhlander B, Holmström H (2002) Sequential extraction of sulphide-rich tailings remediated by the application of till cover, Kristineberg mine, northern Sweden. Sci Total Environ 299:207–226
Carvalho PCS (2010) As antigas explorações mineiras de Sb–Au, As–Au e Ag–Pb–Zn da região de Valongo (norte de Portugal): seu impacte ambiental. Unpublished PhD Thesis, Portugal: University of Coimbra, p 468
Carvalho PCS, Neiva AMR, Silva MMVG (2009) Geochemistry of soils, stream sediments and waters close to abandoned W-Au-Sb mines at Sarzedas, Castelo Branco, central Portugal. Geochem Explor Environ Anal 9:341–352
Carvalho PCS, Neiva AMR, Silva MMVG (2012) Assessment of the potential mobility and toxicity of metals and metalloids in soils contaminated by old Sb–Au and As–Au (NW Portugal). Environ Earth Sci 65:1215–1230
Charriau A, Lesven L, Gao Y, Leermakers M, Baeyens W, Ouddane B, Billon G (2011) Trace metal behaviour in riverine sediments: role of organic matter and sulphides. Appl Geoch 26:80–90
Courtin-Nomade A, Grosbois C, Bril H, Roussel C (2005) Spatial variability of arsenic in some iron-rich deposits generated by acid mine drainage. App Geoch 20:383–396
Dold B (2003) Speciation of the most soluble phases in a sequential extraction procedure adapted for geochemical studies of copper sulfide mine waste. J Geochem Explor 80:55–68
Dutrizac JE, Jambor JL (2000) Jarosites and their application in hydrometallurgy. Rev Min Geochem 40:405–453
Ettler V, Mihaljevič M, Šebek O, Nechutný Z (2007) Antimony availability in highly polluted soils and sediments: a comparison of single extractions. Chemosphere 68:455–463
Flakova R, Zenisova Z, Sracek O, Krcmar D, Ondrejkova I, Chovan M, Lalinska B, Fendekova M (2012) The behavior of arsenic and antimony at Pezinok mining site, southwestern part of the Slovak Republic. Environ Earth Sci 66:1043–1057
Flores AF, Rubio LMD (2010) Arsenic and metal mobility from Au mine tailings in Rodalquilar (Almería, SE Spain). Environ Earth Sci 60:121–138
Forray FL, Smith AML, Drouet C, Navrotsky A, Wright K, Hudson-Edwards KA, Dubbin WE (2010) Synthesis, characterization and thermochemistry of a Pb-jarosite. Geochim Cosmoch Acta 74(1):215–224
Hass A, Fine P (2010) Sequential selective extraction procedures for the study of heavy metals in soils, sediments and waste materials: a critical review. Critical Rev Environ Sci Technol 40:365–399
Heikkinen PM, Räisänen ML (2009) Trace metal and As solid-phase speciation in sulphide mine tailings: Indicators of spatial distribution of sulphide oxidation in active tailings impoundments. Appl Geochem 24:1224–1237
Hochella MF, Moore JN, Golla U, Putnis A (1999) A TEM study of samples from acid mine drainage systems: metal—mineral association with implications for transport. Geochim Cosmoch Acta 63:3395–3406
Jain CK, Ali I (2000) Arsenic: occurrence, toxicity and speciation techniques. Wat Res 34(17):4304–4312
Janoš P, Vávorová J, Herzogová L, Pilarová V (2010) Effects of inorganic and organic amendments on the mobility (leachabilty) of heavy metals in contaminated soil: a sequential extraction study. Geoderma 159:335–341
Kartal S, Aydin Z, Tokalioglu S (2006) Fractionation of metals in street sediment samples by using the BCR sequential extraction procedure and multivariate statistical elucidation of the data. J Hazard Mater 132:80–89
Kim MJ (2010) Effects of pH, adsorbate/adsorbent ratio, temperature and ionic strength on the adsorption of arsenate onto soil. Geochem Explor Environ Anal 10:407–412
Kossoff D, Hudson-Edwardsa KA, Dubbin WE, Alfredsson MA (2011) Incongruent weathering of Cd and Zn from mine tailings: a column leaching study. Chem Geol 281:52–71
Larios R, Fernández-Martínez R, Silva V, Rucandio I (2013) Chemical availability of arsenic and heavy metals in sediments from abandoned cinnabar mine tailings. Environ Earth Sci 68:535–546
Li XH, Tang ZL, Chu FY, Yang LY (2011) Characteristics of distribution and chemical speciation of heavy metals in environmental mediums around Jinchang mining city, Northwest China. Environ Earth Sci 64:1667–1674
Lottermoser BG (2010) Mine wastes: characterization, treatment environmental impacts. Springer, Berlin, 315 p
Macklin MG (1992) Metal pollution of soils and sediments: a geographical perspective. In: Newson MD (ed) Managing the human impact of the natural environment. Belhaven Press, London, pp 172–195
Mitel-Minas de Terramonte (1966) Minas de Terramonte, nota descritiva. Explorações mineiras: Terramonte, Portugal: Castelo de Paiva. (Unpublished Repport)
Moncur MC, Ptacek CJ, Blowes DW, Jambor JL (2005) Release, transport and attenuation of metals from an old tailings impoundment. Appl Geochem 20:639–659
Nelson DW, Sommers LE (1996) Total carbon, organic carbon, and organic matter. In: Page AL et al (ed) Methods of soil analysis, part 2, 2nd edn. Agronomy. Am Soc Agron., Inc. vol 9. Madison, WI, pp 961–1010
Parkhurst DL, Appelo CAJ (1999) User’s guide to PHREEQC (version 2): a computer program for speciation, reaction-path, 1D-transport, and inverse geochemical calculations. US Geol Surv Water Resour Invest Rep, pp 99–4259 (online version)
Parra A, Filipe A, Falé P (2002) Sistema de Informação de Ocorrência e Recursos Minerais Portugueses -SIORMINP. Instituto Geológico Mineiro, Lisboa
Perin G, Craboledda L, Lucchese M, Cirillo R, Dotta L, Zanette ML, Orio AA (1985) Heavy metal speciation in the sediments of Northern Adriatic Sea: a new approach for environmental toxicity determination. In: Lekkas TD (ed) Heavy metal in the environment, pp 454–456
Planer-Friedrich B, London J, McCleskey RB, Nordstrom DK, Wallschläger D (2007) Thioarsenates in geothermal waters of Yellowstone National Park: determination, preservation and geochemical importance. Environ Sci Technol 41:5245–5251
Portuguese Law (1998) Decree 236/1998: Portuguese legislation on water quality. Diário da República I-A, pp 3676–3722
Portuguese Law (2007) Decree 306/2007: Portuguese Legislation on Water Quality. Diário da República I-A, pp 5747–5765
Rauret G, López-Sánches JF, Sahuquillo A, Rugio R, Davidson C, Ure A (1999) Improvement of the BRC three-step sequential extraction procedure prior to the certification of new sediment and soil reference materials. J Environ Monit 1:57–61
Rodríguez L, Ruiz E, Alonso-Azcárate J, Rincón J (2009) Heavy metal distribution and chemical speciation in tailings and soils around a Pb–Zn mine in Spain. J Environ Manag 90:1106–1116
Roper AJ, Williams PA, Filella M (2012) Secondary antimony minerals: phases that control the dispersion of antimony in the supergene zone. Chem Erde 72(4):9–14
Salomons W, Förstner U (1984) Metals in the Hydrocycle. Springer-Verlag, Berlin
Scott KM (1987) Solid-solution in, and classification of, gossan-derived members of the alunite-jarosite family, Northwest Queensland, Australia. Am Mineral 72:178–187
Sharma V, Sohn M (2009) Aquatic arsenic: toxicity, speciation, transformations, and remediation. Environ Intern 35:743–759
Singh KP, Mohan D, Singh VK, Malik A (2005) Studies on distribution and fractionation of heavy metals in Gomti river sediments: a tributary of the Ganges India. J Hydrol 312:14–27
SNIRH (2012) Sistema de Nacional de Informação dos Recursos Hídricos. http://www.snirh.pt. Accessed July 2012
Thomas GW (1982) Exchangeable cations. In: Page AL (ed) Methods of soil analysis, Part 2: chemical and microbiological properties. 2nd edn. vol 9 Agron. pp 159–165
Van Herreweghe S, Swennen R, Vandecasteele C, Cappuyns V (2003) Solid phase speciation of arsenic by sequential extraction in standard reference materials and industrially contaminated soil samples. Environ Pol 122:323–342
Vink BW (1996) Stability relations of antimony and arsenic compounds in the light of revised and extended Eh–pH diagrams. Chem Geol 130:21–30
Wang S, Mulligan C (2009) Enhanced mobilization of arsenic and heavy metals from mine tailings by humic acid. Chemosphere 74:274–279
Wilson S, Lockwood PV, Ashley PM, Tighe M (2010) The chemistry and behaviour of antimony in the soil environment with comparisons to arsenic: a critical review. Environ Pol 158:1169–1181
Acknowledgments
This paper represents a part of the PhD thesis of P.C.S. Carvalho. Thanks are due to Prof. Andrew Parker, Dr. Martin Heaps and Dr. Anne Stephanie Dudley for use of facilities in the Department of Soil Sciences of the University of Reading, UK.; Prof. Dirk Wallschläger of Trent University, Canada for arsenic speciation in water samples; Carlos Maia of the Department of Earth Sciences, University of Coimbra, Portugal for his help with XRD identifications. Funding was provided to P. C. S. Carvalho by the SFRH/BD/21373/2005 Grant from Fundação para a Ciência e a Tecnologia (FCT), Portugal. Financial support was also provided by the Portuguese Government for the project PEST-OE/CTE/UI0073/2011 of the Geosciences Centre through FCT. Very helpful and thorough journal reviews were provided by Prof. Anshumali. We are also grateful to Prof. Gunter Doerhoefer and Prof. Olaf Kolditz for their helpful comments.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Carvalho, P.C.S., Neiva, A.M.R., Silva, M.M.V.G. et al. Metal and metalloid leaching from tailings into streamwater and sediments in the old Ag–Pb–Zn Terramonte mine, northern Portugal. Environ Earth Sci 71, 2029–2041 (2014). https://doi.org/10.1007/s12665-013-2605-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12665-013-2605-7