Abstract
The chemical analysis of 19 water wells in Ferdows area, Northeastern Iran, has been evaluated to determine the hydrogeochemical processes and ion concentration background in the region. In the study area, the order of cation and anion abundance is Na+ > Ca2+ > Mg2+ > K+ and Cl− > SO −24 > HCO3 − > NO3 −, respectively, and the dominating hydrochemical types are Na–Cl. Most metal concentrations in water depend on the mineral solubility, and pH, Eh, and salinity of the solution. Their ΣREE concentrations showed excellent correlations with parameters such as TDS and pH. North American Shale Composite (NASC)-normalized REE patterns are enriched in the HREEs relative to the LREEs for all groundwaters. They all have positive Eu anomalies (Eu/Eu* = 0.752–3.934) and slightly negative Ce anomalies (Ce/Ce* = 0.019–1.057). Reduction–oxidation, complexation, desorption, and re-adsorption alter groundwater REE concentrations and fractionation patterns. The positive Eu anomalies in groundwaters are probably due to preferential mobilization of Eu2+ relative to the trivalent REEs in the reducing condition.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Water shortage has become an increasingly serious problem in Iran, especially in the arid and semi-arid regions of western Iran (Jalali 2006). Iran is located in a semi-arid area with an average annual precipitation of less than one-third of that of the world (Baghvand et al. 2010). Therefore, water is a rare and precious resource.
Intense agricultural and urban development has placed a high demand on groundwater resources, especially in the Ferdows region, and these resources are now at greater risk of contamination. Variation in groundwater chemistry is mainly a function through the interaction between the groundwater and the mineral composition of the aquifer materials through which it moves and is controlled by various factors, like evaporation, dissolution-precipitation, weathering of silicates, oxidation–reduction, sorption and exchange reactions, transformation of organic matter, and mixing processes (Carrilo-Rivera et al. 2007; Sharif et al. 2008). The interaction of all factors leads to various water types (Azaza et al. 2010). The importance of water quality to human health has recently attracted a great deal of interest (Pazand et al. 2011). The evaluation and management of groundwater resources require an understanding of hydrogeological and hydrochemical properties of the aquifer. Rare earth elements (REEs) are believed to be potential tracers for studying groundwater–aquifer rock interactions because of their generally coherent and predictable behavior (Tang and Johannesson 2006). This coherent behavior of the REEs, combined with their sensitivity to changes in pH, redox conditions and adsorption/desorption reactions, makes the REEs particularly useful in hydrogeochemical studies (Wood 1990). Because the REEs typically behave coherently, perturbations from the expected patterns can yield critical information about the types of processes occurring within a water–rock system. Increased knowledge of geochemical evolution of groundwater quality could lead to improved understanding of hydrochemical systems in such areas, leading to sustainable development of water resources and effective management of the groundwater resource (Azaza et al. 2010). Therefore, the objective of this study was to identify the processes controlling the geochemical evolution of groundwater quality and to characterize water–rock interaction and its effects on the chemistry of the water. Finally, to aid the management and future development of groundwater resources in the region, the hydrochemical approach and statistical methods were also applied to divide the territory into areas with distinct groundwater quality. This may help in the long run to support new and more efficient remedial measures to combat the deterioration of water quality.
Geology and hydrogeological setting
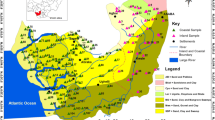
The study area lies in the northeastern Iran. The topography of the study area is the mixture of rugged mountains with flat plains (Fig. 1). The average elevation (altitude) for the study area is 2,000 m a.s.l. The area has a hot temperate climate, with mean maximum summer temperatures (July) of about 40 °C and minimum winter temperatures (January) of 15 °C. The climate of the study area is considered to be arid, the annual precipitation being approximately 100 mm (Pourlatifi 1995).
Geologic map of study area (after Pourlatifi 1995)
The Mountain region is part of the Alpine-Himalayan organizations that their shaping as a result of the convergence zone of Eurasia, Arabia and India continental. Most of the study area is formed by recent alluvium that consists of gravel. Volcanic Rocks consist of andesite and rhyolite in the Cenozoic period that was affected by granodiorite intrusions placed above sediment with Carboniferous and Jurassic age (Eftekhar Nezhad and Valeh 1977; Pourlatifi 1995).
Materials and methods
Field sampling and field parameter measurement
A total of 19 groundwater samples were collected from existing wells in the study areas in October 2010 (Fig. 2). The geographical locations of the wells were determined with a GARMIN handheld global positioning system (GPS). The selected wells are used for agricultural and domestic purposes. Groundwater samples were collected following the standard procedures (Halim et al. 2009) which included (1) filtered using 0.45 μm filters and acidified with supra pure 0.7 N HNO3 to pH ∼ 2 for the analysis of heavy and trace elements and (2) filtered, but not acidified, for the analysis of major ions. The samples were collected in 100 mL polyethylene bottles, after pumping continuously 15–30 min until the temperature, electrical conductivity (EC) and pH reading had stabilized. Immediately, after sampling, pH, temperature and electrical conductivity (EC) were measured in the field using a portable pH meter (Metrohum) (Table 1).
Analytical techniques
Samples were analyzed at the laboratory for the major ions employing standard methods. Calcium (Ca2+) and magnesium (Mg2+) was determined titrimetrically using standard EDTA. Chloride (Cl−) was determined by standard AgNO3 titration method. Carbonate CO3 2− and bicarbonates (HCO3 −) were determined by titration with HCl. Sodium (Na+) and potassium (K+) were measured by flame photometry, sulfate SO4 2− by spectrophotometric turbidimetry, and NO3 − by colorimetry with an UV–visible spectrophotometer. Total dissolved solids (TDS) were determined gravimetrically at 105–110 °C (Table 1). Major and trace elements were measured with inductively coupled plasma and mass spectrometry (ICP–MS) in the filtered and acidified water samples within 2 weeks after sampling (Table 2).
Results and discussion
Geochemistry of major ions
The pH of the groundwater in the study area ranged from 7.30 to 8.69 with an average value of 7.82, indicating an alkaline nature of the samples. Eh varies between −70 and 6.30 mV. The relationship between Eh and pH show that Eh is decreased with increasing pH; this relationship probably indicates the progressive reduction of Fe2+ with deeper circulation associated with pH increase. The relative content of a cation or an anion is defined as the percentage of the comparative amount of that ion to the total cations or anions, respectively (Pazand et al. 2012). The concentrations of Ca2+, Mg2+, Na+, and K+ represent on average 14.39, 5.18, 79.95, and 0.48 % of all the cations, respectively. Among the anions, the concentrations of HCO3 −, Cl−, NO3 −, and SO −24 represent on average 9.60, 51.37, 1.93, and 37.10 %, respectively. Thus, the order of cation and anion abundance is Na+ > Ca2+ > Mg2+ > K+ and Cl− > SO −24 > HCO3 − > NO3 −, respectively. If halite dissolution is responsible for sodium, the Na+/Cl− ratio should be approximately equal to 1, whereas the ratio greater than 1 is typically interpreted as Na released from silicate weathering reactions (Meyback 1987). Samples having Na+/Cl− ratio greater and less than 1 in the study area are nearly equal and hence sodium might have come from irrigation return flow, anthropogenic activity, and silicate weathering (Fig. 3). The chloro-alkaline index, CAI = [Cl − (Na + K)]/Cl, is suggested by Schoeller (1977), which indicates the ion exchange between the groundwater and its host environment. If there is ion exchange of Na+ and K+ from water with Mg2+ and Ca2+ in the rock, the exchange is known as direct when the indices are positive. If the exchange is reverse, then the exchange is indirect and the indices are found to be negative. Positive CAI values noted in 1, 6, 7, 11, 13, 14, 15, 16, and 17 well areas suggest that sodium and potassium from water are exchanged with magnesium and calcium in rock following base exchange reactions (chloro-alkaline equilibrium), whereas negative CAI values noted at 2, 3, 4, 5, 8, 9, 10, 12, 18, and 19 wells suggest that magnesium and calcium from water are exchanged with sodium and potassium in rock favoring cation–anion exchange reactions (chloro-alkaline disequilibrium).
Hydrochemical facies
The term hydrochemical facies is used to describe the bodies of groundwater in an aquifer that differs in chemical composition (Aghazadeh and Mogaddam 2011). The facies are a function of the lithology, solution kinetics, and flow patterns of the aquifer (Raju et al. 2009). Large tables of analytical data are usually difficult to interpret regarding the variations in water quality. Graphs are useful for this purpose and several specialized types are in use. Piper’s diagram is one of them. Based on the dominant cations and anions, four water types were found among the water samples (Fig. 4):
Na + HCO3, Na + Cl, Ca + Mg + HCO3 + Cl, and Ca + Mg + HCO3 represent 5, 79, 11, and 5 % of the total number of water samples, respectively (Fig. 5).
Distribution of metal and other solutes
Concentration of metals in groundwater, including As, Cu, Se, Fe, and Mn are presented in Table 2. Most metal concentrations in water depend on the mineral solubility, and pH, Eh, and salinity of the solution (Caron et al. 2008). Concentration of Cu in groundwater is observed between 2.4 and 20.3 μg/l in the study area. Bioavailability of Cu in the water influenced by size of the initial Cu amendment, time-dependent changes in speciation, and soil humidity and the retention of Cu are enhanced under stable humid conditions (Halim et al. 2009). The contents of As, Fe, and Mn are 0.38–26, 7.5–100, and 1.87–7.26 μg/l, respectively. Concentrations of Fe and Mn in the studied groundwater samples show a distinct relationship to the concentration of As (Table 2). The groundwaters with high dissolved Fe relative to Mn have elevated levels of As and suggest that As is released into groundwater due to reductive dissolution of Fe-oxyhydroxide. The Mo, Se, V, and Pb concentrations range from 0.9 to 19.8, 1.1 to 24.6, 6 to 84.2, and 0.08 to 4.3 μg/l, respectively. The content of these metals is dependent on oxidation–reduction condition of groundwater. There are high positive correlations between V and Se (R = 0.82), Co (R = 0.78), between Ni and Co (R = 0.67), Cu (R = 0.70), between Se and Co (R = 0.68), between Cu and Co (R = 0.72), between Mo and As (R = 0.69), and positive correlations between Fe and As, Fe and Co, V and Cu, Ni and Se, Ni and V (Table 3). These robust correlations indicate strong geochemical and hydrogeochemical relationships between these metals. Contrary to many investigations which confirm negative correlation between arsenic and sulfate (e.g. Hasan et al. 2007; Halim et al. 2009), a positive correlation exists between arsenic and this anion in the study area that is due to mixing of ground waters from different sources. The low concentrations of SO4 2− in groundwater samples and negative correlation between SO4 2− and Cd (R = −0.26) and Pb (R = −0.07) indicate that Pb and Cd have not been directly mobilized from sulfide minerals. The negative correlation coefficient indicates that the dissolved Cu, Co, Fe, Mn, Ni, Se, V, and Pb concentration is likely not controlled by the amount of uncomplexed carbonate ions in the groundwater.
REE concentrations
The concentrations of individual REE and ΣREE in the studied groundwaters are presented in Table 4.
Rare earth element concentrations in the study area are low. The correlations of ΣREE with TDS and pH are also presented in Fig. 6. The ΣREE concentrations in groundwater exhibit a positive correlation with the TDS contents (R = 0.55) indicating that ΣREE concentrations are related to the salinity (TDS) of the water samples. Many studies have shown that pH can play an important role in the mobility of REEs and that higher REE concentrations are typically associated with lower pH values (Keasler and Loveland 1982; Guo et al. 2010). The pH affects carbonate complexations of the REEs, with higher pH leading to the predominance of REE decarbonate complexes. On the other hand, pH control REE read sorption onto oxide surfaces. However, our plot of REE concentrations versus pH (Fig. 6) shows no correlation between pH and ΣREE concentrations. The Ce anomaly is mostly negative with the Ce/Ce* values ranging from 0.019 to 1.057. The possible explanation for slightly negative Ce anomalies would be that more Ce has been fixed as CeO2 than that adsorbed on Fe oxy-hydroxides in an oxic environment. When conditions become more reducing (the actual situation), reduction of Fe oxy-hydroxides first releases the adsorbed Ce, while reduction of CeO2 is slower and only gradually mobilizes Ce into the waters. In contrast, the Eu anomaly is more variable than the Ce anomaly and was predominantly positive with the Eu/Eu* values narrowly ranging between 0.752 and 3.934. In the results from this study, Eu/Eu* ratios have a negative correlation with Eh values (Fig. 6). The ratios are systematically higher in the lower Eh (reducing) groundwaters than in the higher Eh (oxidizing-mildly reducing) groundwaters suggesting that reducing conditions are the dominant factors contributing to the positive Eu anomalies. Europium is the only lanthanide that, under reducing conditions, can be divalent, and is thus segregated from other REEs under such conditions (Lee et al. 2003). The poor correlation between dissolved Fe and Eu/Eu* suggests that the reductive dissolution of Fe oxy-hydroxides should not be the only source for the Eu anomalies (Fig. 6). The preferential desorption of Eu(II) should also play an important role (Guo et al. 2010).
Normalized patterns with respect to the estimated average composition of the North American Shale Composite (NASC) are displayed on Fig. 7 for groundwaters from the study area. The groundwaters exhibit enrichment in the HREEs relative to the LREEs (Fig. 7). This can be explained by the differences in geochemical mobility between LREEs and HREEs during water–rock interaction at depth.
Conclusions
The hydrogeochemical characteristics of groundwater are evaluated in Ferdows area, northeastern Iran. Groundwater is mostly Na–Cl type for the study area with Cl− as the dominant anion, although other types of water were observed. The results show that groundwater in the region with volcanic rocks are in chemical reaction and ion exchange and major cations Ca2+, Mg2+ and Na+ may release through the dissolution of carbonates, cation-exchange, and weathering of silicates. Concentration of the main metals, such as As, Cu, Fe, Mn, and Se, in groundwater, is low and is released into groundwaters from different sources. The TDS has an important role in the mobility of REEs in the study area. The REE concentrations and shale-normalized patterns show highly positive Eu anomalies and slightly negative Ce anomalies. There are differences in geochemical mobility between LREEs and HREEs during water–rock interaction at depth; therefore, groundwaters exhibit enrichment in the HREEs relative to the LREEs. The major contribution to positive Eu anomalies in groundwaters is expected to be preferential mobilization of Eu2+ relative to the trivalent REEs.
References
Aghazadeh N, Mogaddam AA (2011) Investigation of hydrochemical characteristics of groundwater in the Harzandat aquifer, Northwest of Iran. Environ Monit Assess 176:183–195
Azaza FH, Ketata M, Bouhlila R, Gueddari M, Riberio L (2010) Hydrogeochemical characteristics and assessment of drinking water quality in Zeuss–Koutine aquifer, southeastern Tunisia. Environ Monit Assess. doi:10.1007/s10661-010-1457-9
Baghvand A, Nasrabadi T, Bidhendi NG, Vosoogh A, Karbassi A, Mehrdadi N (2010) Groundwater quality degradation of an aquifer in Iran central desert. Desalination 260:264–275
Caron ME, Grasby SE, Ryan MC (2008) Spring water trace element geochemistry: a tool for resource assessment and reconnaissance mineral exploration. Appl Geochem 23:3561–3578
Carrilo-Rivera JJ, Varsanyi I, Kovacs LO, Cardona A (2007) Tracing groundwater flow systems with hydrogeochemistry in contrasting geological environments. Water Air Soil Pollut 184:77–103
Eftekhar Nezhad J, Valeh N (1977) Geology map of Ferdows 1:100000 sheets. Geological survey of Iran
Guo H, Zhang B, Wang G, Shen Z (2010) Geochemical controls on arsenic and rare earth elements approximately along a groundwater flow path in the shallow aquifer of the Hetao Basin, Inner Mongolia. Chem Geol 270:117–125
Halim MA, Majumder RK, Nessa SA, Hiroshiro Y, Uddin MJ, Shimada J, Jinno K (2009) Hydrogeochemistry and arsenic contamination of groundwater in the Ganges Delta Plain, Bangladesh. J Hazard Mater 164:1335–1345
Hasan MA, Ahmed KM, Sracek O, Bhattacharya P, VonBromssen M, Broms S, Fogelstrom J, Mazumder ML, Jacks G (2007) Arsenic in shallow groundwater in Bangladesh: investigations from three different physiographic settings. Hydrol J 15:1507–1522
Jalali M (2006) Chemical characteristics of groundwater in parts of mountainous region, Alvand, Hamadan, Iran. Environ Geol 51:433–446
Keasler KM, Loveland WD (1982) Rare earth elemental concentrations in some Pacific Northwest rivers. Earth Planet Sci Lett 61:68–72
Lee SG, Lee DH, Chae BG, Kim Y, Kim WY, Woo NC (2003) Rare earth elements as indicators of groundwater environment changes in a fractured rock system: evidence from fracture-filling calcite. Appl Geochem 18:135–143
Meyback M (1987) Global chemical weathering of surficial rocks estimated from river dissolved loads. Am J Sci 287:401–428
Pazand K, Hezarkhani A, Ghanbari Y, Aghavali N (2011) Groundwater geochemistry in the Meshkinshahr Basin of Ardabil Province in Iran. Environ Earth Sci 65:871–879
Pazand K, Hezarkhani A, Ghanbari Y, Aghavali N (2012) Geochemical and quality assessment of groundwater of Marand Basin, East Azarbaijan Province, northwestern Iran. Environ Earth Sci 67:1131–1143
Pourlatifi A (1995) Geology map of Ferdows 1:100000 sheets. Geological Survey of Iran
Raju NJ, Ram P, Dey S (2009) Groundwater quality in the lower Varuna river basin, Varanasi district, Uttar Pradesh. J Geol Soc India 73:178–192
Schoeller H (1977) Geochemistry of groundwater. In: Groundwater studies—an international guide for research and practice, vol 15. UNESCO, Paris, pp 1–18
Sharif MU, Davis RK, Steele KF, Kim B, Kresse TM, Fazio JA (2008) Inverse geochemical modeling of groundwater evolution with emphasis on arsenic in the Mississippi River Valley alluvial aquifer, Arkansas (USA). J Hydrol 350:41–55
Tang J, Johannesson KH (2006) Controls on the geochemistry of rare earth elements along a groundwater flow path in the Carrizo Sand aquifer, Texas, USA. Chem Geol 225:156–171
Wood SA (1990) The aqueous geochemistry of the rare-earth elements and yttrium: 1. Review of the available low-temperature data for inorganic complexes and inorganic REE speciation in natural waters. Chem Geol 82:159–186
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pazand, K. Geochemical and hydrogeochemical evolution of groundwater in Ferdows area, northeast of Iran. Environ Earth Sci 71, 685–695 (2014). https://doi.org/10.1007/s12665-013-2470-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12665-013-2470-4