Abstract
The concentrations and speciation of heavy metals (As, Cd, Cr, Cu, Hg, Ni, Pb and Zn) in the sediments of the nearshore area, river channel and coastal zones of the Yangtze estuary, China, were systematically investigated in this study. The concentrations of all heavy metals except Ni in the sediments of the nearshore area were higher than those of the river channel and coastal zones. In the nearshore area, the concentrations of most heavy metals except Hg in the sediments of the southern branch were higher than those of the northern branch because of the import of pollutants from the urban and industrial activities around. When compared with the threshold effect level (TEL) and geochemical background levels, Cr, Ni and As accumulated and posed potential adverse biological effects. The speciation analysis suggested that Cd, Pb and Zn in the sediments of the three zones showed higher bioavailability than the other heavy metals, and thus posed ecological risk. Significant correlations were observed among Cr, Cu, Ni and Zn (r > 0.77) in the nearshore area, Ni, Cu, Zn and Pb (r > 0.85) in the river channel and Ni, Cu, Cr, Pb and Zn (r > 0.75) in the coastal zone. Principal component analysis (PCA) indicated that the discharge of unban and industrial sewage, shipping pollution and the properties of the sediments (contents of Fe, Mn, Al, TOC, clay and silt) dominated the distribution of heavy metal in the nearshore area, river channel and coastal zones of the Yangtze estuary.
Similar content being viewed by others

Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Heavy metal pollution in natural environment has caused serious environmental problems around the world because of their toxicity, wide source, non-biodegradable properties, and accumulation behaviors (Wei et al. 2009; Yu et al. 2008). Therefore, the fate of heavy metals in sediment has recently been the subject of extensive discussion (Sundaray et al. 2011; Tang et al. 2010). With the rapid industrialization and economic development, heavy metals are continuing to be introduced to the water environment by several pathways, including industrial activities, agricultural activities, and atmospheric deposition (Karimi et al. 2011; Yang et al. 2011). These heavy metals from nearby urban zones and industrial areas could accumulate in sediment because of complex and dynamic environments (Li et al. 2007). When the environmental conditions change, adsorbed heavy metals could be desorbed from sediment into water and cause a secondary pollution, which may affect population health and ecological environment if the contents exceed the limit of bearing capacity (Bai et al. 2011; Kong et al. 2011).
The Yangtze River, the largest river in China and the third largest in the world, carries about 470 × 106 tons of sediment to the East China Sea annually (Chen et al. 2004; Song et al. 2011). The Yangtze estuary is one of the world’s largest estuaries, where half of Yangtze River-derived sediment is deposited (Lin et al. 2002). In addition, one of the most developed regions in China, including Shanghai city, is located on the southern branch of the Yangtze estuary, containing a dense population of 13 million and concentrated industry (Chen et al. 2004; Wang and Liu 2003). After the recent two decades of prosperous economy and booming industrial development, a tremendous amount of both natural and anthropogenic heavy metals is carried by the Yangtze River and settled in the estuary (Feng et al. 2004; Zhang et al. 2009, 2001). For example, every year, it is estimated that about 3 × 1010 tons of sewage is discharged along the Yangtze River into the estuary, consisting of 68 % industrial and 32 % domestic waste water discharge (Zhang 1999). The environment of the Yangtze estuary is dynamic and complex. Heavy metals here, which are affected by the special physicochemical conditions, such as salinity, pH and redox (Yue et al. 2003) could be transported and transformed easily. In addition, they can be absorbed by the plants and animals in the estuary, which are considered as an indispensable component of the food chain and are thus potentially harmful to human health (Buggy and Tobin 2008). Therefore, heavy metals in the Yangtze estuary should attract more attention and social concern.
Generally, it is regarded that the bioavailability of heavy metals is closely related to the chemical forms rather than the total concentrations in sediment (Clozel et al. 2006). The different geochemical forms of heavy metals in sediments have distinct mobility, biological toxicity and chemical behaviors, which offer a more realistic estimate of actual environmental impact (Cuong and Obbard 2006). Therefore, it is essential to distinguish and quantify the forms of heavy metals to obtain a better estimate of the potential and environmental impact of contaminated sediments.
In this paper, comparative studies of the contents, speciation and source of heavy metals in surface sediments from the nearshore, river channel and coastal areas in the Yangtze estuary were systematically conducted and statistically analyzed by correlation analysis, principal component analysis (PCA) and cluster analysis (CA). The results obtained here could provide foundation for the future remediation as well as the protection of water quality of the Yangtze estuary.
Study area and collection of samples
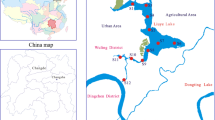
The Yangtze estuary is the largest estuary in China (Zhang et al. 2001). Industrial and domestic sewage from coastal cities like Shanghai and tributaries mainly runs through this long channel to the East China Sea.
According to the spatial distribution (Fig. 1), 33 samples from the nearshore area of the Yangtze estuary (N1–N33), 10 samples from the river channel (R1–R10) and 20 samples from the coastal area (C1–C10) were collected in May and August 2010. Figure 1 showed the details of the sampling points. The surface sediments were sampled to a depth of 2–5 cm using a Van Veen Grab. The samples were stored in a polyethylene bag, placed in a cooler, and then shipped immediately to the laboratory.
Data and methods
Analytical methods
The concentrations and speciation of heavy metals were measured at Institute of Geophysical and Geochemical Exploration (IGGE), Chinese Academy of Geological Sciences, which is certificated by the China National Accreditation Board for Laboratories. Detailed description of the method is showed here.
In the laboratory, sediments were defrosted and air-dried at room temperature. The samples were then ground with agate pestles and mortars, and sieved with 200-mesh nylon sieve (pore diameter: 0.074 mm). For digestion, the dried sediment (0.25 g) was put into digestion solution (5 mL HNO3, 10 mL HF, 2 ml HClO4) at 200 °C in a closed system for 12 h. After digestion and cooling, the residue was digested again in the same way and then mixed with 8 mL aqua regia and heated until 2–3 mL solution was left. Next, the solution was rinsed with 25 mL ultrapure water. This diluted solution was recorded as solution (A). Solution (A) (1 mL) was taken and diluted with ultrapure water to 10 mL for analysis of Cr, Cu, Ni, Zn and Mn using Inductively Coupled Plasma Mass Spectrometer (ICP-MS, Thermo) and Fe2O3, Al2O3 using Inductively Coupled Plasma Optical Emission Spectrometer (ICP-OES, Thermo). Solution (A) (1 mL) was taken and diluted with 2 % nitric acid to 10 mL for analysis of Cd and Pb using Inductively Coupled Plasma Mass Spectrometer (ICP-MS, Thermo). The dried sediment (0.25 g) was digested with 8 mL aqua regia and heated until 2–3 mL solution was left. Next, the solution was rinsed with 25 mL ultrapure water. The diluted solution was deal with 1 % KMnO4 and 1 % oxalic acid for analysis of As and Hg by Hydride Generation-Atomic Fluorescence Spectrometry (HG-AFS, IGGE).
Particle size analysis was performed using a Microtrac S3500 Particle Size Analyzer (0.25–1,400 μm) (Microtrac, USA). Total organic carbon (TOC) content of the sediment samples was determined using a Liqui TOC analyzer (Elementar, Germany).
Various extraction methods, such as Tessier procedure (Tessier et al. 1979), BCR procedure (Ure et al. 1993), and GSC procedure (Hall et al. 1996) are widely used for heavy metals speciation. When considering the nature of the studied sediment (neutral pH, medium organic matter and extractable Fe2O3 content), Tessier procedure was used in our study. The procedure is illustrated as follows (Tables 1, 2, 3):
-
1.
Exchangeable fraction (EXC) Two grams soil was extracted with 20 mL of 1.0 mol/L MgCl2 in a Teflon centrifuge tube for 2 h at 25 °C and pH = 7 with continuous agitation, then centrifuged for 20 min at 4,000 rpm/min. The wash water was added to the supernatant. The mixture was filtered through a 0.45 μm membrane and deionized water was added to make a volume of 25 mL for measurement. The residue was retained for the next steps. The same centrifugation–decantation–washing procedure was also used after each of the following steps.
-
2.
Carbonate bound fraction (CARB) Residue from exchangeable fraction was extracted with 20 mL of 1.0 mol/L NaOAC (pH = 5) for 5 h at 25 °C with continuous agitation, then centrifuged for 20 min at 4,000 rpm/min.
-
3.
Fe–Mn oxide fraction (Fe–Mn) Residue from the carbonate fraction was extracted with 20 mL of 0.04 mol/L NH2OH/HCl in 25 % acetic acid for 5 h at 25 °C with continuous agitation, and then centrifuged for 20 min at 4,000 rpm/min.
-
4.
Organic matter bound fraction (OM) Residue from the Fe–Mn oxide fraction was extracted with 3 mL of 30 % H2O2 and 2 mL of 0.02 mol/L HNO3 for 2 h at 85 °C and pH = 2 with occasional agitation; additional 3 mL H2O2 was added again with occasional agitation for 3 h; and 10 mL of 3.2 mol/L NH4OAC in 20 % HNO3 was added for 30 min at 25 °C with continuous agitation, then centrifuged for 20 min at 4,000 rpm/min.
-
5.
Residual fraction (RES) Residues from the organic fraction were together digested with 3 mL HNO3, 1 mL HF and 1 mL HClO4 for 5 h, and then added with another 1 mL HNO3. Digestion was conducted in a water bath at 80 °C for 16 h.
Quality assurance and quality control
Quality assurance and quality control were assessed using duplicates, method blanks, and standard reference materials (GBW07436, GSS22, GSS23 and GSS24, obtained from Chinese Academy of Measurement Sciences) with each batch of samples (1 blank and 1 standard for each 5 samples). The qualified rates of primary reference material and duplicate sample were 100 %. The percentage of recovery ranged from 96 to 110 %. The detection limits were 0.5 μg/g for Cr, Pb and Zn, 0.3 μg/g for Ni and Cu, 0.1 μg/g for As, 0.02 μg/g for Cd, 0.002 μg/g for Hg, 3, 5, 10 μg/g for Fe2O3, Mn and Al2O3.
Statistical analysis
All statistical calculations, including Pearson’s correlation coefficients, principal component analysis (PCA) and cluster analysis (CA) were conducted using SPSS13.0.
Results and discussion
Distribution of heavy metals in sediments
The concentration range, median, mean, standard deviation (SD), coefficient of variation (CV) and the geochemical background levels of As, Cd, Cr, Cu, Hg, Ni, Pb and Zn in the sediments from the Yangtze estuary, as well as the threshold effect level (TEL) and the probable effect level (PEL) which were established by the Canadian Council of Ministers of the Environment, are given in Table 1. The adverse biological effects are expected to occur when the concentrations of heavy metals exceed the TEL (MacDonald et al. 1996). The ratios of heavy metal concentrations in the sediments from the nearshore, river channel and coastal areas of the Yangtze estuary and TEL are shown in Fig. 2.
When compared with the geochemical background levels, the mean concentrations of As, Cr, Ni, Pb and Zn in the sediments from the Yangtze estuary were higher. These result suggested that there was the accumulation of As, Cr, Ni, Pb and Zn due to anthropogenic factors. The mean levels of Cd, Cu and Hg were lower than the geochemical background levels, which indicated that no obvious contamination of these heavy metals occurred in the Yangtze estuary.
In the nearshore zone, the mean concentrations of all heavy metals expect Cd were higher than the geochemical background levels, which might be due to that anthropogenic factors were obvious in the nearshore area. In the coastal areas, the mean concentrations of As, Cr, Ni, Pb and Zn were higher than the geochemical background levels because pollutants from runoff accumulated there. In the river channel, the mean concentration of Pb was higher than the geochemical background level, which was also observed in the other two zones. This indicated there was the accumulation of Pb in all zones of the Yangtze estuary.
The mean concentrations of Ni, Cr and As in the nearshore, river channel and coastal areas were higher than the TEL, but lower than the PEL (Fig. 2a). The mean concentrations in the three zones were about 1.71–2.23 times as high as the TEL for Ni and Cr and 1.11–1.46 times for As. These suggested that Ni, Cr and As in the sediments from the three zones posed potential biological adverse effects. The mean levels of the other studied heavy metals in the three zones were lower than the TEL, which indicated that no adverse effects of these heavy metals occurred in the Yangtze estuary. In addition, the concentrations of all heavy metals except Ni in the sediments from the nearshore area were the highest among the three zones, which may be due to anthropogenic point and non-point input, such as the discharge of urban runoff, industrial and domestic sewage. The concentrations of all heavy metals except Cd and Hg in the sediments from the river channel were lower than that from the nearshore and coastal areas because of high flow rate. What’ more, the concentration of all heavy metals except Hg in the sediments from the south branch (N1–N11, N18–N23) of the nearshore area were higher than that from the north branch (N12–N17) (Fig. 2a). This distribution pattern might be related to the import of a number of tributaries into the south branch, such as the Liuhe mouth, Shidong mouth, and Huangpu River. These tributaries flow through Shanghai city, which is one of the largest cities of China, and bring a large number of pollutants into south branch. Additionally, industrial discharge from the factories which located at the south bank was another cause.
The concentrations of Ni and Cr in all sediments from the three zones were higher than the TEL (Fig. 2b). The mean concentrations of Ni and Cr in the nearshore and coastal areas were higher than that in the river channel. These results indicated that potential adverse effects of Ni and Cr occurred in the nearshore and coastal areas. The higher concentrations of Ni in the three zones occurred at N5, R5, C9 and C20. The level of TOC in the sediment from N5 site (about 1.31 %) was significantly higher than that in other sites, so more Ni could be attached to organic matter by adsorbing, ion exchanging or chelate effect (Zhang et al. 2009). Ni is usually associated with Ni ore and the production of alloy and chemicals (Yang et al. 2009). Therefore, the increasing industrial discharge from the steel industry, ore–Ni mining and refining is responsible for the introduction quantity of Ni (such as at N5, R5). The suspended particulate matter, which could adsorb heavy metals and settle into sediment together, is another reason for the high levels of Ni in sediments, such as at site C4, which is in the greatest turbidity zone. In addition, the high Ni concentration at C20 might be due to low flow rate.
The higher concentrations of Cr in the three zones occurred at N2, N5, N31, R5 and C9. Obviously, the higher concentrations of Cr occurred at the same sites as Ni, such as N5, R5 and C9. Therefore, the distribution pattern of Cr might also be related to the influences of the increasing discharges of contaminants from local industrial, such as the mining, smelting, electroplating, paint manufacturing, urban activities (Yu et al. 2008).
The concentrations of As in 90.9 % of samples from the nearshore area, 35.3 % from the river channel, and 85.0 % from the coastal areas of the Yangtze estuary were higher than the TEL. Maximum levels of As from the three zones occurred in the sediments from N31, R10 and C6, which were 2.55, 1.83 and 2.28 times as high as the TEL, respectively. Besides the discharging of urban sewage and industrial wastewater (such as N31), agricultural non-point pollution discharged into the Yangtze estuary might explain the high As levels in the sediment from some site such as R10.
The distribution patterns of Cu, Pb and Zn in the nearshore area were obviously different from those in the river channel and coastal areas (Fig. 2c). The concentrations of Cu, Pb and Zn in all sites from the nearshore area were fluctuating around the TEL, while they were less than the TEL in the river channel and coastal areas. This suggested that Cu, Pb and Zn in the nearshore area posed potential adverse effects. Higher concentrations of Cu, Pb and Zn, ranged from 1.15 to 1.51 times as high as the TEL, occurred at the sampling sites N18, N19, N27 and N26. The sites N18 was located at BaoYang dock. Therefore higher Cu, Pb and Zn contents there might be due to the leak through transportation, such as mineral slag, crude oil and sewage. In addition, a large amount of urban wastewater, industrial sewage and import of tributaries was responsible for high levels of Cu, Pb and Zn at some site (such as N26 and N27) (Liu et al. 2003).
The concentrations of Cd and Hg in the sediments from all sampling sites of the Yangtze estuary were lower than the TEL except Hg for N16 and R4 (Fig. 2d). Accordingly, there were no adverse effects of Cd and Hg in the sediments from the Yangtze estuary.
Speciation of heavy metals
The bioavailability of heavy metals in sediments is closely associated with their chemical forms rather than the total concentrations. The partitioning of the speciation including EXC fraction, CARB fraction, Fe–Mn oxide fraction, OM fraction and RES fraction of heavy metals in the sediments from the nearshore, river channel and coastal areas of the Yangtze estuary was showed in Fig. 3.
The chemical speciation partitioning of Cd was similar in the samples from the three zones. Higher EXC and CARB fractions in all areas were observed. The EXC-Cd and CARB-Cd in the three zones accounted for as high as 25–31 and 30–34 %, respectively. As is known, the EXC and CRAB fractions have the greatest tendency toward remobilization from sediment phase to the more bioavailable pore water phase (Kim et al. 2010). Therefore, when the environmental conditions changed, Cd would pose a higher ecological risk at the Yangtze estuary.
Pb, Zn and Cu exhibited similar chemical partitioning in the sediments from the three zones. The predominant fraction for Pb, Zn and Cu in the three zones was RES fraction (about 45–62 % for Pb, 57–74 % for Zn and 46–72 % for Cu). In addition, their EXC fraction was all lower than 1.3 %. For Pb, Fe/Mn-Pb, CARB-Pb and OM-Pb fraction were 20–31, 7.8–14 and 9.4–16.0 %, respectively. The Fe/Mn-Pb was related with Fe and Mn dioxide. When the surrounding redox potential changed, this fraction can be easily released from sediment into water (Liu et al. 2003). In addition, Pb, as a kind of environmental hormone, could affect the procreation ability of the biological system (Yang et al. 2009). Therefore, Pb in the sediments from the three zones of the Yangtze estuary posed ecological risk. Furthermore, Pb in the nearshore and coastal areas possessed relative higher risk than that in the channel because of higher fractions of CARB-Pb and Fe/Mn-Pb. The sum of CARB-Zn and Fe/Mn-Zn fraction reached 31 % for the nearshore area, 25 % for the channel and 17 % for the coastal areas, respectively. These suggested that Zn at the Yangtze estuary also posed ecological risk. The sum of CARB-Cu and Fe/Mn-Cu fraction in the nearshore sediments was higher than the other two zones. These indicated that Cu had more mobility in the nearshore zones of the Yangtze estuary.
Hg exhibited different chemical partitioning in the sediments from the three zones, but the main fractions were the RES-Hg and OM-Hg. The percentage of OM-Hg from the coastal areas (about 51 %) was far more than that from the other two zones (about 25 % for the nearshore area and 33 % for the river channel). The high data for OM-Hg in the coastal areas may be connected with the high contents of organic matter and sulfides in the sediments from the coastal zones, where Hg could be bound to active sites of molecules, such as carboxyl and phenolic OH− groups or precipitated matter as sulfides (Fernandes 1997). In addition, in the river channel, the sum percentage of EXC-Hg, CARB-Hg and Fe/Mn-Hg (about 28 %), which might be released from sediments to water easily, was much higher than that in the nearshore and coastal areas (for only 5.6 and 6.2 %). These suggested that higher bioavailability of Hg occurred in the river channel than the other two zones.
The predominant partitioning of As, Cr and Ni in sediment from three zones was RES fraction. The percentages were as high as 85–92, 79–93 and 73–82 %, respectively, while the other fractions were all less than 8 %.The results were consistent with the distribution of Cr and Ni in the sediments of Loure River (Filgueiras et al. 2004). Overall, As, Cr and Ni posed lower bioavailability and less ecological risk compared to the other metals in the Yangtze estuary.
Multivariate analyses
Pearson’s correlation coefficient among heavy metals, TOC and grain sizes in the surface sediments from three zones of the Yangtze estuary were presented in Table 2. In the nearshore area, the data showed a significantly positive correlation among Cr, Cu, Ni and Zn (r > 0.77) (the main metals commonly produced in industrial activities), which suggested the same or similar source input. Pb and Cd also correlated to most heavy metals positively except for Cd–Hg, Pb–As and Pb–Hg pairs, suggesting that Pb and Cd may be impacted by some industrial activities, such as smelting, mining activities (Wei et al. 2009). In the river channel, the results showed intensive correlations among Ni, Cu, Zn and Pb (r > 0.85). As and Ni correlated to most heavy metals positively except for As–Cr, As–Hg, Ni–Cd and Ni–Hg pairs, and Cr showed significant correlations(r > 0.67) with Cu, Ni, Pb and Zn. In the coastal areas, there were significant correlations among Ni, Cu, Cr, Pb and Zn (r > 0.75), the main metals used in the industrial activities. The results indicated that these metals might originate from the discharging of industrial, such as pollutants from mining, smelting, printing and so on (Yang et al. 2009). However, in the nearshore area of the Yangtze estuary, there were no significant correlations between As, Hg and the most of other heavy metals except for As–Cu, As–Ni, Hg–Ni and Hg–Zn pairs. In the river channel, Hg did not showed significant correlations with all of other heavy metals. In the coastal zones, except for As–Pb and Cd–Hg, As and Cd had no significant correlations with others. The results suggested that these heavy metals were not associated with others, and they had different behavior during transport in most areas of the Yangtze estuary; except Cd, Cu and Zn in the nearshore area, As, Cd and Hg in the river channel and As and Cd in the coastal areas, Mn, TFe2O3 and Al2O3 showed statistically significant positive correlation with other heavy metals. These might be due to that heavy metals could be adsorbed with Mn, TFe2O3 and Al2O3 by bounding to surface active sites.
Significant correlations occurred between TOC and most heavy metals in the Yangtze estuary except Hg, As and Cd in the sediments from some zones. This indicated that they might have a common resource, or the heavy metals introduced to environment were attached to organic matters by adsorbing, ion exchanging or chelate effect (Yu et al. 2008). In the nearshore area, there were no significant correlations between heavy metals and grain size except the pairs of Cr–silt, Cu–silt and Ni–silt. This suggested that grain size might be unimportant in heavy metal distribution as compared to TOC in the sediments from the nearshore area. In the river channel and coastal areas, clay and silt contents correlated with most heavy metals positively except Cd, Hg–clay and As, Cd–silt pairs. This implied that clay and silt here could adsorb heavy metals easily and move together (Fukue et al. 2006).
Principal component analysis (PCA), a multivariate statistical tool, is generally employed for pattern recognition, classification, modeling and the dimensionality of a data reducing (Filgueiras et al. 2004; Glasby et al. 2004; Tang et al. 2010). In our study, three principal components (PC) in sediments from the three zones were extracted respectively. They accounted for about 83.67, 93.86 and 92.67 % of total variance (Table 3), and played a critical role in explaining metal contamination.
In the nearshore zones, PC1 was dominated by Cr, Zn and Cu. These three heavy metals had significant correlations with each other (Table 2) and showed the same distributions (Fig. 2); especially, their levels were all higher at N5 and N7 site which were seriously polluted by urban and industrial sewage. Therefore, PC1 was related to the discharge of urban and industrial sewage. PC2 was the highest loading of As and Fe, Mn, Al and clay contents. As loading (0.851) was higher than the other heavy metals, and correlation analysis showed that As had no obvious relationship with most other heavy metals here (Table 2). All of these implied that As had an independent behavior (such as mining). Owing to high loading of Fe, Mn, Al and clay contents and their significant correlations with other metals, PC2 was controlled by the mining industry (which was related to As), releasing heavy metals binding with Fe, Mn, Al compounds and clay. PC3 was dominated by silt. Overall, the discharge of urban and industrial sewage, releasing of heavy metals binding with clay and Fe, Mn, Al compounds, and grain sizes dominated the heavy metal distribution in the sediments from the nearshore of the Yangtze estuary.
In the river channel and coastal zones, PC1 was both dominated by Cr, Ni and Cu. Moreover, significant correlations were observed among these three metals (Table 2), indicating same source such as shipping pollution and wastewater from the nearshore area. At the same time, the properties of sediment (Fe, Mn, Al, TOC, Clay and Silt) loading were obviously high. In general, PC1 was related to the discharge of wastewater, shipping pollution and the properties of the sediments from river channel and coastal zones. In the river channel zones, PC2 was dominated by As and Cd, while PC3 by Hg only. In the coastal zones, PC2 and PC3 were dominated by Cd and As, respectively. Cd and Hg in the river channel and Cd and As in the coastal areas had no obvious relationship with most other heavy metals (Table 2). These might be due to their different behavior. In all, discharge of pollutants from nearshore zone, shipping pollution, and the properties of sediments impacted the heavy metals distribution in the river channel and coastal zones of the Yangtze estuary.
Cluster analysis as an amalgamation rule, the squared Euclidean distance and a measure of the proximity between samples was investigated to quantitatively identify some areas of contamination (Fang et al. 2010; Zhang et al. 2009). Three dendrograms showed samples from the nearshore, river channel and coastal zones at Fig. 4. There were two major clusters noted at every zone. Clusters Na1, Rb (R5), Ca2 represent highly contaminated sites in the nearshore, river channel and coastal zones, respectively, where high ecological risk might be occurring. Accordingly, these sites should be paid more attention in remediation process. The other clusters noted moderate (Na2, Ra1 and Ca1) and low [Nb, Ra2 (R4), Cb (C4)] heavy metal contaminate sites in the sediments.
Conclusions
The assessment of heavy metals (As, Cd, Cr, Cu, Hg, Ni, Pb and Zn) in the sediments from the nearshore, river channel and coastal zones of the Yangtze estuary indicated that the concentrations of all heavy metals except Ni in the sediments from the nearshore area were higher than that from the river channel and coastal zones. Higher contents of most heavy metals except Hg in the sediments from the southern branch than the northern branch occurred because of the import of pollutants from urban and industrial activities around. The contents of Cr, Ni and As were higher than the TEL and geochemical background levels, which indicated they accumulated and posed potential adverse biological effects. The mean levels of Cd, Cu and Hg in the sediments were lower than the TEL and geochemical background levels. The mean concentrations of Pb and Zn were lower than the TEL, but higher than geochemical background levels. The speciation analysis suggested that Cd, Pb and Zn showed higher bioavailability than other heavy metals, and posed some ecological risk in the sediments from the three zones. Low ecological risk was observed for As, Cr, and Ni in the three zones because of high residual fraction. The result of correlation analysis demonstrated that significant correlations occurred among Cr, Cu, Ni and Zn (r > 0.77) in the nearshore, Ni, Cu, Zn and Pb (r > 0.85) in the river channel and Ni, Cu, Cr, Pb and Zn (r > 0.75) in the coastal zone. PCA analysis showed that the discharge of unban and industrial sewage, shipping pollution and the properties of sediments (contents of Fe, Mn, Al, TOC, Clay and Silt) dominated the heavy metal distribution in the nearshore, river channel and coastal zones of the Yangtze estuary.
References
Bai JH, Cui BS, Chen B, Zhang KJ, Deng W, Gao HF, Xiao R (2011) Spatial distribution and ecological risk assessment of heavy metals in surface sediments from a typical plateau lake wetland, China. Ecol Model 222(2):301–306
Buggy CJ, Tobin JM (2008) Seasonal and spatial distribution of metals in surface sediment of an urban estuary. Environ Pollut 155(2):308–319
Chen ZY, Saito Y, Kanai Y, Wei TY, Li LQ, Yao HS, Wang ZH (2004) Low concentration of heavy metals in the Yangtze estuarine sediments, China: a diluting setting. Estuar Coast Shelf Sci 60(1):91–100
Clozel B, Ruban V, Durand C, Conil P (2006) Origin and mobility of heavy metals in contaminated sediments from retention and infiltration ponds. Appl Geochem 21(10):1781–1798
Cuong DT, Obbard JP (2006) Metal speciation in coastal marine sediments from Singapore using a modified BCR-sequential extraction procedure. Appl Geochem 21(8):1335–1346
Fang SB, Xu C, Jia XB, Wang BZ, An SQ (2010) Using heavy metals to detect the human disturbances spatial scale on Chinese Yellow Sea coasts with an integrated analysis. J Hazard Mater 184(1–3):375–385
Feng H, Han XF, Zhang LZ, Yu WG (2004) A preliminary study of heavy metal contamination in Yangtze River intertidal zone due to urbanization. Mar Pollut Bull 49(11–12):910–915
Fernandes HM (1997) Heavy metal distribution in sediments and ecological risk assessment: the role of diagenetic processes in reducing metal toxicity in bottom sediments. Environ Pollut 97(3):317–325
Filgueiras AV, Lavilla I, Bendicho C (2004) Evaluation of distribution, mobility and binding behaviour of heavy metals in surficial sediments of Louro River (Galicia, Spain) using chemometric analysis: a case study. Sci Tot Environ 330(1–3):115–129
Fukue M, Yanai M, Sato Y, Fujikawa T, Furukawa Y, Tani S (2006) Background values for evaluation of heavy metal contamination in sediments. J Hazard Mater 136(1):111–119
Glasby GP, Szefer P, Geldon J, Warzocha J (2004) Heavy-metal pollution of sediments from Szczecin Lagoon and the Gdansk Basin, Poland. Sci Tot Environ 330(1–3):249–269
Hall GEM, Vaive JE, Beer R, Hoashi M (1996) Selective leaches revisited with emphasis on the amorphous Fe oxyhydroxide phase extraction. J Geochem Explor 56(1):59–78
Karimi R, Ayoubi S, Jalalian A, Sheikh-Hosseini AR, Afyuni M (2011) Relationships between magnetic susceptibility and heavy metals in urban topsoils in the arid region of Isfahan, central Iran. J Appl Geophys 74(1):1–7
Kim Y, Kim BK, Kim K (2010) Distribution and speciation of heavy metals and their sources in Kumho River sediment, Korea. Environ Earth Sci 60(5):943–952
Kong SF, Lu B, Ji YQ, Zhao XY, Chen L, Li ZY, Han B, Bai ZP (2011) Levels, risk assessment and sources of PM10 fraction heavy metals in four types dust from a coal-based city. Microchem J 98(2):280–290
Li QS, Wu ZF, Chu B, Zhang N, Cai SS, Fang JH (2007) Heavy metals in coastal wetland sediments of the Pearl River Estuary, China. Environ Pollut 149(2):158–164
Lin S, Hsieh IJ, Kuo MH, Wang CH (2002) Influence of the Yangtze River and grain size on the spatial variations of heavy metals and organic carbon in the East China Sea continental shelf sediments. Chem Geol 182(2–4):377–394
Liu WX, Li XD, Shen ZG, Wang DC, Wai OWH, Li YS (2003) Multivariate statistical study of heavy metal enrichment in sediments of the Pearl River Estuary. Environ Pollut 121(3):377–388
MacDonald DD, Carr SR, Calder FD, Long ER, Ingersol CG (1996) Development and evaluation of sediment quality guidelines for Florida coastal waters. Ecotoxicology 5(4):253–278
Sheng JJ, Fan DJ, Yang DF, Qi HY, Xu L (2008) Distribution patterns of heavy metals in surface sediments of the Yangtze estuary and its adjacent areas and environmental quality assessment. J Environ Sci China 29(9):2405–2412
Song YX, Ji JF, Yang ZF, Yuan XY, Mao CP, Frost RL, Ayoko GA (2011) Geochemical behavior assessment and apportionment of heavy metal contaminants in the bottom sediments of lower reach of Changjiang River. Catena 85(1):73–81
Sundaray SK, Nayak BB, Lin S, Bhatta D (2011) Geochemical speciation and risk assessment of heavy metals in the river estuarine sediments—a case study: Mahanadi basin, India. J Hazard Mater 186(2–3):1837–1846
Tang WZ, Shan BQ, Zhang H, Mao ZP (2010) Heavy metal sources and associated risk in response to agricultural intensification in the estuarine sediments of Chaohu Lake Valley, East China. J Hazard Mater 176(1–3):945–951
Tessier A, Campbell PGC, Bisson M (1979) Sequential extraction procedure for the speciation of particulate trace metals. Anal Chem 51(7):844–851
Ure AM, Quevauviller P, Muntau H, Griepink B (1993) Speciation of heavy metal in soils and sediments e an account of the improvement and harmonization of extraction techniques undertaken under the auspices of the BCR of the Commission of the European Communities. INT J Environ Anal Chem 51(1–4):135–151
Wang ZL, Liu CQ (2003) Distribution and partition behavior of heavy metals between dissolved and acid-soluble fractions along a salinity gradient in the Changjiang Estuary, eastern China. Chem Geol 202(3–4):383–396
Wei CY, Wang C, Yang LS (2009) Characterizing spatial distribution and sources of heavy metals in the soils from mining-smelting activities in Shuikoushan, Hunan Province, China. J Environ Sci 21(9):1230–1236
Yang ZF, Wang Y, Shen ZY, Niu JF, Tang ZW (2009) Distribution and speciation of heavy metals in sediments from the mainstream, tributaries, and lakes of the Yangtze River catchment of Wuhan, China. J Hazard Mater 166(2–3):1186–1194
Yang ZP, Lu WX, Long YQ, Bao XH, Yang QC (2011) Assessment of heavy metals contamination in urban topsoil from Changchun City, China. J Geochem Explor 108(1):27–38
Yu RL, Yuan X, Zhao YH, Hu GR, Tu XL (2008) Heavy metal pollution in intertidal sediments from Quanzhou Bay, China. J Environ Sci 20(6):664–669
Yue C, Qing H, Wei QL (2003) The distributions of particulate heavy metals and its indication to the transfer of sediments in the Changjiang Estuary and Hangzhou Bay, China. Mar Pollut Bull 46(1):123–131
Zhang J (1999) Heavy metal compositions of suspended sediments in the Changjiang (Yangtze River) estuary: significance of riverine transport to the ocean. Cont Shelf Res 19(12):1521–1543
Zhang WG, Feng H, Chang JN, Qu JG, Xie HX, Yu LZ (2009) Heavy metal contamination in surface sediments of Yangtze River intertidal zone: an assessment from different indexes. Environ Pollut 157(5):1533–1543
Zhang W, Yu L, Hutchinson SM, Xu S, Chen Z, Gao X (2001) China’s Yangtze Estuary: I. Geomorphic influence on heavy metal accumulation in intertidal sediments. Geomorphology 41(2–3):195–205
Zhao YY, Yan MC (1992) The comparison of abundance of chemical elements in sediment of the Yellow River, the Yangtze River and China shallow sea. Chin Sci Bull 13:1202–1204
Acknowledgments
This work was supported by the National Basic Research Program of China (973 Project, Grant No. 2010CB429003), National Natural Science Foundation of China (Grant No. 21177013), the fundamental Research Funds for the Central Universities and Special Fund of State Key Laboratory of Water Environment Simulation (Grant No. 11K04ESPCN).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, X., Wang, Y., Li, B. et al. Distribution and speciation of heavy metals in surface sediments from the Yangtze estuary and coastal areas. Environ Earth Sci 69, 1537–1547 (2013). https://doi.org/10.1007/s12665-012-1988-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12665-012-1988-1