Abstract
The interaction between heavy metals and river sediment is very important because river sediment is the sink for heavy metals introduced into a river and it can be a potential source of pollutants when environmental conditions change. The Kumho River, the main tributaries of the Nakdong River in Korea, can be one of the interesting research targets in this respect, because it runs through different geologic terrains with different land use characteristics in spite of its short length. Various approaches were used, including mineralogical, geochemical, and statistical analyses to investigate the distribution and behavior of heavy metals in the sediments and their sources. The effect of geological factor on the distribution of these metals was also studied. No noticeable changes in the species or relative amounts of minerals were observed by quantitative X-ray diffraction in the sediments at different stations along the river. Only illite showed a significant correlation with concentrations of heavy metals in the sediments. Based on an average heavy metal concentration (the average concentrations of Cd, Co, Cr, Cu, Ni, Pb, and Zn were 1.67, 20.9, 99.7, 125, 97.6, 149, 298 ppm, respectively), the sediments of the Kumho River were classified as heavily polluted according to EPA guidelines. The concentrations of heavy metals in the sediments were as follows: Zn > Pb > Cu > Ni > Cr > Co > Cd. In contrast, contamination levels based on the average I geo (index of geoaccumulation) values were as follows: Pb > Cd > Zn > Cu > Co = Cr > Ni. The concentrations of heavy metals increased downstream (with the exception of Cd and Pb) and were highest near the industrial area, indicating that industrial activity is the main factor in increasing the concentrations of most heavy metals at downstream stations. Sequential extraction results, which showed increased heavy metal fractions bound to Fe/Mn oxides at the downstream stations, confirmed anthropogenic pollution. The toxicity of heavy metals such as Ni, Cu, and Zn, represented by the exchangeable fraction and the fraction bound to carbonate, also increased at the downstream stations near the industrial complexes. Statistical analysis showed that Pb and Cd, the concentrations of which were relatively high at upstream stations, were not correlated with other heavy metals, indicating other possible sources such as mining activity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The elements produced by weathering of rocks and anthropogenic activities are transported by rivers and redistributed in geological environments. One of the most important processes affecting this migration of elements is interaction with geological materials (Kim et al. 2006, 2007). The accumulation and distribution of heavy metals depend mostly on the characteristics of the geological materials, such as mineral species and grain size (Hochella et al. 2005; Liu et al. 2006; Norra et al. 2006; Sakai et al. 1986; Taylor 2007). River sediments are usually composed of fine grains of minerals with high cation exchange capacity (CEC), such as clay minerals and iron oxides. As a result, heavy metals introduced into an aquatic environment can easily accumulate in the sediments. The interaction between heavy metals and river sediment is very important because river sediment is the sink for heavy metals introduced into a river and can be a potential source of pollutants when environmental conditions change (Demirak et al. 2006; Farkas et al. 2007).
In sediment, these metals are partitioned in different chemical forms depending on the geochemical conditions. Therefore, the bioavailability and toxicity of metals, and their potential for environmental pollution are largely dependent on the chemical forms rather than the total concentration (Singh et al. 2005). The sources of heavy metals in river sediments are diverse, ranging from lithogenic to anthropogenic sources such as discharge of urban and industrial waste water, combustion of fossil fuels, mining and smelting operations, processing and manufacturing industries, and waste disposal (Segura et al. 2006; Zhou et al. 2008). Therefore, information regarding both the chemical forms of heavy metals and their possible sources is essential for estimating potential toxicity and reducing environmental risk.
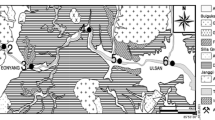
The Kumho River is the largest tributary of the Nakdong River, which is the largest river in South Korea, located in the southeastern part of the Korean Peninsula (Fig. 1). Although the Kumho River’s total length is relatively short, it runs through Daegu, the third largest city in Korea, just before it meets the Nakdong River; therefore, its contamination potential is high. In Daegu, many industrial complexes (including textile, machinery, and dyeing factories, which can be the important sources of pollutants to the river) have been developed near the Kumho River since 1967. The Kumho River is also used as an important source for the public water supply. However, the accumulation of heavy metals in the sediment of this river has not yet been systematically studied. In this paper, the chemical forms and accumulation patterns of heavy metals in the sediments of the Kumho River were investigated and statistical analysis was used to confirm the interpretation of data. These results provide more detailed insight into the distributions and fractionation patterns of heavy metals from various sources in the sediment. In addition, the effects of geological and mineral compositions on the distribution patterns of heavy metals are also discussed.
Study area
The Kumho River runs through about 116 km starting from Pohang until it meets the Nakdong River. Its catchment area is 2,110 km2. It passes through an agricultural area and past industrial complexes as it moves downstream. Annual precipitation in this area is 1,020.6 mm which is slightly lower than the Korean average. About 65% of the annual precipitation, 670 mm is concentrated in the summer season (June–September). The geology of the upstream area is composed of volcanic rock such as andesite, rhyolite, and tuff, but most areas are composed of sedimentary rocks (Fig. 1). The rock types of this area belong to Cretaceous Kyungsang Super Group which is divided into three groups: Sindong, Hayang, and Yuchon Groups (Chang 1975). Sindong and Hanyang Groups are mainly sedimentary rocks, and Yuchon Group is basically volcanic rocks. The sedimentary rocks of Sindong Group are composed of pebble and cobble conglomerates, pebbly sandstone, silty shale, mudstone, and marlstone. It is divided, primarily on the basis of variation in rock color, into the Nakdong, Hasandong, and Jinju Formations in ascending order. This group is characterized by lacking contemporaneous volcanic components and predominantly consisting of fluvial clastics, with rarely intercalated impure fresh-water limestone. The Hayang Group is characterized by bearing sparsely intercalated volcanic layers and is distinguished from the underlying Sindong Group which is entirely non-volcanic and from the overlying Yuchon Group which is dominantly composed of volcanic layers. The Hayang Gourp is composed of shale, siltstone, sandstone, conglomerate, marlstone, and volcanic layers of basic and intermediate compositions, and is subdivided as follows in ascending order: Chilgok Formation (variegated red beds), Sinla Conglomerate Formation (variegated red conglomerates and partly volcanic), Haman Formation (variegated red beds), Banyawol Formation (dark-gray shale and sandstone), and Songnaedong Formation (variegated red beds).
Methods
Sampling
Eleven sampling stations were selected considering accessibility to sampling points along the river at intervals of 5–10 km, and sampling was carried out in May 2003 (Fig. 1). At each station, the surface sediment samples from a depth of ~10 cm were collected with a plastic scoop and sieved to separate fractions less than 1 mm. The collected sediments were stored together with water in vinyl bags in the field and carried to the laboratory. The sediment samples smaller than silt size (<64 μm) were again separated in the laboratory using sieves, and the sediments were finally separated from water by centrifuge. The separated samples were dried at 60°C in an oven for 24 h and kept in a desiccator until further analysis.
Sample analysis and data treatment
X-ray diffraction (XRD) analysis was carried out by a Phillips X-ray diffractometer with Ni-filtered Cu–Kα radiation at 40 kV and 30 mA at the Daegu Branch of the Korea Basic Science Institute. The XRD data were collected from 5 to 50°2θ with steps of 0.02°2θ. The counting time was 1 s per step. For the quantitative analysis of minerals, Siroquant computer program by Sietronics was used for XRD data.
Heavy metal contents were analyzed using a 4:4:1 mixture of NHO3, HF, and HClO4 according to the total digestion method (modified EPA3052) suggested by the Environmental Protection Agency of the United States. The accuracy of the analysis was checked using standard samples such as an estuarine sediment (USGS SRM 1646a) and NIST Montana Soil (NIST SRM 2711). The measurement of the standard samples was performed in triplicate, and the average showed recovery errors within ±5%.
For information on the chemical speciation of heavy metals, the sequential extraction method modified by Li et al. (1995) from the scheme proposed by Tessier et al. (1979) was used. This scheme is composed of five steps for each fraction: exchangeable metals (F1), metals bound to carbonates (F2), metals bound to Fe/Mn oxides (F3), metals bound to organic matter and sulfides (F4), and metals in the residual fraction (F5). Seven heavy metals, Pb, Cr, Co, Ni, Cu, Zn, and Cd, were analyzed for the sediment samples using Inductively Coupled Plasma–Atomic Emission Spectrometer (ICP–AES, Perkin Elmer optima 4300DV) and Inductively Coupled Plasma Mass Spectrometer (ICP–MS, VG Elemental Ltd. X-series) at the Korea Basic Science Institute. To support discussions, Spearman’s correlation analysis, two-way clustering analysis, and principal component analysis (PCA) were also performed using SPSS (v. 11.0).
Results and discussion
Mineral composition of sediments
Mineral composition in river sediment can sometimes be an indicator of the lithology and geology of the sediment’s origin (Churchman et al. 1988; Upadhyay et al. 2006). In addition, the information on mineral composition is essential for knowing the distribution and migration of heavy metals in the sediments because clay minerals, which are important components of sediments, play important roles in fixing heavy metals (Gier and Johns 2000; Gu and Evans 2007; Hochella et al. 2005; Liu et al. 2006). For the sediment samples collected along the river, no significant changes were observed in the mineral weight percentage (wt%) although some minor minerals, such as illite, showed slight changes from upstream to downstream stations (Table 1). The sediments were mostly composed of silicate minerals, with quartz comprising the greatest fraction (39.7–58.8 wt%), followed by feldspar, albite (21.6–34.7 wt%), and orthoclase (1.8–12 wt%). The sediments also included small amounts of clay minerals such as chlorite (3.3–6.4 wt%), illite (1.5–6.8 wt%), and kaolinite (0.4–2.7 wt%). At Stations 3 to 11, hornblende (2.4–6.7 wt%) was also observed as a minor component.
The catchment area of the Kumho River is composed of two distinct geologic areas, volcanic and sedimentary regions. The geologic difference, however, did not affect the mineral composition significantly; therefore, the effect of mineralogy on heavy metal composition in sediments should be limited, indicating that other factors, such as vicinity to the pollutant sources, are more important. Although clay minerals were identified in the sediments, smectite and vermiculite, which are known to have high cation exchange capacity, were not detected in studied samples. Among the identified clay minerals, illite and chlorite were the primary minerals with a smaller amount of kaolinite. Chlorite, however, is not a strong adsorbent for cations. Illite, whether by itself or layered with smectite, has a certain amount of cation exchange capacity (McBride 1984) and preferential adsorption of certain cations such as Cs (Kim et al. 1996a, b). Illite seemed to be the only mineral that increased its content from upstream to downstream stations. This trend, however, is not clear, as illite content drops from Stations 4 to 5 and from Stations 7 to 8.
Heavy metal concentrations in the sediments and possible contaminant sources
In the sediment of the Kumho River, heavy metal concentrations were generally high and showed high spatial variability (Table 2; Fig. 2). Among those metals, Zn had the highest average concentration (298 ppm); its concentration increased up to 653 ppm at Station 10, where a small confluent dominated by the effluent of an industrial wastewater treatment plant is discharged into the Kumho River. The average concentration of Pb (149 ppm) was the second highest in the sediment. The average concentrations of heavy metals were in the order of Zn > Pb > Cu > Cr > Ni > Co > Cd.
The concentrations of heavy metals generally increased downstream, except Cd and Pb (Table 2; Fig. 2). This trend was clearer for Cr, Co, Ni, and Zn. The concentrations of Cd and Pb at Stations 2–4 were higher than those at Stations 10 and 11, where most of the other heavy metals showed the highest concentrations. However, no noticeable industrial facilities were identified around those stations. Considering the presence of unreported small-scale mines in the upstream areas, the sources of Cd and Pb may be the abandoned mines. The concentration of Pb increased to 527 ppm at Station 6 and decreased again to 116 ppm at Station 11 where it meets the larger Nakdong River. At Station 6, other heavy metals did not show this abrupt increase, but rather decreased. Just 300 and 1,500 m before this station, there are two small confluents flowing into the Kumho River; the increase in Pb concentration was probably caused by other sources affecting these confluents, which cannot be clearly identified at this stage. With the exception of Cd and Pb, the increase in concentrations of most metals is well correlated with the increasing industrial area near Daegu. The highest levels were seen at Station 10, where the sediments were affected by discharge from the wastewater treatment plant. The industrial complexes are mainly composed of textile and metal factories. In particular, Ni, which is used mainly for metal plating, showed the most abrupt increase at this station. Pb and Cd, however, did not increase as much as compared with Cr, Ni, and Zn.
Contamination levels of metals
The concentration levels of heavy metals are generally much higher than the values reported for other river sediments and the world averages with the exception of Cr (Olivares-Rieumont et al. 2005; Singh et al. 2005). Although the concentrations of Cd were low relative to other metals, they were still 3.5–17 times higher than the world average. For Cu, Pb, and Zn, these values were also 1.5–7.25 times higher than the world average, indicating that the anthropogenic effects are much higher in the sediments of the Kumho River. Based on the average concentrations, most of the sediments were considered polluted according to EPA guidelines. Of 11 sampling points, the EPA guidelines for heavily polluted sediments were exceeded by the concentration of Cr for 9 stations (>75 ppm), Cu 10 stations (>50 ppm), Ni 6 stations (>50 ppm), Pb 9 stations (>60 ppm), and Zn for 7 stations (>200 ppm).
Igeo, the index of geoaccumulation introduced by Müller (1979), was also used to calculate the heavy metal contamination levels for the sediment samples (Table 3). The index is calculated as Igeo = log2[Cn/1.5Bn], where Cn is the concentration of the heavy metal n in sediments, and Bn is the background or pristine value of the element. The factor 1.5 was used to minimize the effect of possible variations in the background values due to lithogenic effects (Müller 1979). Here, the geochemical background value in average shale from Turekian and Wedepohl (1961) was used as Bn. Therefore, Igeo can provide insight into contamination intensity by anthropogenic activity. Depending on the values, the pollution levels are classified as follows:
-
Igeo ≤ 0: unpolluted (Class 0)
-
0 < I geo ≤ 1: unpolluted to moderately polluted (Class 1)
-
1 < I geo ≤ 2: moderately polluted (Class 2)
-
2 < I geo ≤ 3: moderately to strongly polluted (Class 3)
-
3 < I geo ≤ 4: strongly polluted (Class 4)
-
4 < I geo ≤ 5: strongly to extremely polluted (Class 5)
-
5 < I geo ≤ 6: extremely polluted (Class 6)
Based on the average I geo values, the contamination levels of sediments in the Kumho River were in the order of Pb > Cd > Zn > Cu > Co = Cr > Ni. The average sediments of the Kumho River can be classified as unpolluted for Co, Cr, Ni, unpolluted to moderately polluted for Cu and Zn, and moderately polluted for Cd and Pb. Although the contamination level of Ni was the lowest in the study area, the sediment collected from Station 10 indicated moderate to strong pollution with respect to Ni (I geo = 2.20). At this station, indices of all studied heavy metals were higher than 0, indicating that the impact of the wastewater treatment plant was very significant compared with other sources. Based on I geo values, the contamination intensity generally increased downstream due to the increasing influence of industrial activities. However, for Cd and Pb, the sediments from the upstream stations (2, 3, and 4) were classified as moderately to strongly polluted, which also reflects the influence of the abandoned mines around those areas. At Station 6, the sediment was classified as strongly to extremely polluted for Pb due to contaminated water from other confluents.
Chemical fractionation of heavy metals
Although total heavy metal concentrations provide useful information regarding the behavior of heavy metals (Hochella et al. 2005; Marengo et al. 2006), the metals in different chemical forms have different potential effects on the aquatic ecosystem; therefore, sequential extraction techniques have been developed and applied to the aquatic sediment samples (Filgueiras et al. 2004; Olivares-Rieumont et al. 2005; Relić et al. 2005; Singh 2001; Singh et al. 2005).
Each form of heavy metals was plotted in Fig. 3 as the percentage of the sum of all fractions. In general, the residual fraction has the highest percentage, and the fraction bound to Fe/Mn oxide phase is the second highest. For Cr, Cd, Ni, and Co, the residual metal fractions were dominant. For Ni, Zn, and Co, the residual metal fractions became less important downstream, while the fractions of the Fe/Mn oxide phase increased. In contrast, the residual fractions of Pb and Cd decreased downstream with the fractions bound to Fe/Mn oxide not showing any distinct trend.
Based on I geo, the sediments were most polluted with Pb as compared with other metals. The highest fraction of Pb in the sediments was in the Fe/Mn oxide phase (30.8–57.0%), followed by those bound to organic matter (12.5–39.6%) and in residual metals (10.3–32.6%). The exchangeable fraction of metals and the fraction bound to carbonate, which can be potentially toxic, were negligible or very small. The concentration of Pb increased abruptly from 140 to 527 ppm at Station 6. The sequential extraction result showed that at this station, the fraction of Pb bound to Fe/Mn oxides also increased compared with other fractions. In general, the high proportion of metals with the exchangeable and Fe/Mn oxide phases is indicative of anthropogenic pollution (Farkas et al. 2007; Filgueiras et al. 2004; Klavins et al. 2000; Relić et al. 2005). Therefore, the main source for the abrupt increase of Pb can be attributed to the anthropogenic pollutants coming from the small confluent located upstream of Station 6, as discussed before. Although the total concentration of Pb was lower at Station 10 than Station 6, the fraction of Pb bound to iron oxide also increased at this point. At Station 10, one possible reason for the increase in the concentrations of most metals is the discharge from the wastewater treatment plant. This result shows that Fe/Mn oxides play important roles in fixing Pb at those stations.
The sediments of this river were generally unpolluted with Cr, Ni, and Co based on I geo. However, the concentrations of those metals typically increased downstream, especially for Ni at Station 10. At this station, the sediments became unpolluted to moderately polluted with Cr and Co, but moderately to strongly polluted with Ni. The fractions of metals bound to Fe/Mn oxide increased noticeably at this station, especially for Ni, which increased from 11.7 to 66.0%, and the fractions of exchangeable and carbonate-bound fractions increased from 0.8 and 1.6% to 3.0 and 7.0%, respectively. This result indicates that the anthropogenic effects were the most important sources of metals here, mainly due to the pollutants from the wastewater treatment plant. The significant proportions of Ni were exchangeable or carbonate-bound, can easily be released into the water and are potentially toxic (Farkas et al. 2007).
Most Cr existed as residual fractions (more than 50%). However, this proportion became lower downstream, especially at Stations 9–11. For all samples, the fractions bound to organic matter were generally slightly larger than those in Fe/Mn oxides. At Station 10, like Ni and Pb, the fraction bound to Fe/Mn iron oxides increased markedly. The Cr fractions of exchangeable and carbonate-bound forms were negligible here. Co showed a similar trend to Cr except that its fractions bound to Fe/Mn oxides were higher than those of Cr and became higher than the residual fractions at Stations 7, 9, 10, and 11.
For Cu, the fraction bound to organic matter (34.0–58.0%) was highest due to the high affinity of Cu for organic matter (McBride 1994). However, no significant changes were observed in fractions bound to organic matter. Compared with other heavy metals, the exchangeable fraction of Cu was high at the downstream stations, with 6.8% at Station 10. At this station, the fraction bound to Fe/Mn oxides also increased from 12.8 to 24.2%, which also emphasizes the importance of Fe/Mn oxides fixing Cu. The high Cu fractions of exchangeable and Fe/Mn oxide-bound forms also indicate an anthropogenic origin. The same trend was also observed in Zn, and the fractions bound to Fe/Mn oxides were high at several downstream stations. One noticeable feature was that the fraction of Zn bound to carbonates was relatively high compared with other metals, increasing the possibility of this metal being released due to a change in the saturation state with respect to carbonate minerals.
For Cd, which was the second most polluted heavy metal based on the I geo value, the residual fraction did not show a noticeable decrease at the downstream stations, which is similar to Pb. The fraction bound to Fe/Mn oxides was the second highest yet did not show a significant increase at the upstream stations, although the sediments were moderately to strongly polluted with Cd at these stations. This result indicates that the sources of Cd and Pb were probably different from other metals, possibly from small-scale abandoned mines in the upstream regions.
Statistical analysis and sources of heavy metals
Statistical analysis was used to confirm the sources and correlations of heavy metals in the sediment. First, nonparametric correlation analysis was conducted on heavy metals and mineral components because the concentrations of heavy metals did not show normal distributions (Table 4). Although the mineralogy did not show significant changes as the river flows downstream, some significant correlations were observed among minerals. However, these were not the typical type of correlations that can be observed in the normal sediments, in which the distance of migration is the main factor controlling the distribution of minerals (Kim et al. 2006, 2007). Generally, clay minerals, such as kaolinite, show a negative correlation with primary minerals, such as quartz, feldspars, and hornblende. The sediment samples in this study area did not show this kind of relationship because this study was performed using sediment from a relatively short segment of the river. The heavy metals showed no significant correlations with minerals except with illite (Table 4), indicating that the enrichment of heavy metals here was not influenced by the mineral components. However, illite, which has a large surface area and great absorption capacity, may have played a certain role in the absorption of Co, Cu, and Zn. The statistical analysis results also showed significant correlations between heavy metals, such as between Ni and Cr, the main metals used in the plating industry, suggesting that they are anthropogenic. However, Pb and Cd did not show significant correlations with other metals, implying that their sources are different from other metals.
The hierarchical clustering analysis (CA) based on Ward’s method was also used to obtain additional information on the behavior of heavy metals. The result showed that Stations 10 and 11 can be grouped separately from other stations, indicating different sources of heavy metals for these two stations (Fig. 4a). The large industrial complexes were responsible for the abrupt increase in the heavy metal concentrations at these two stations, which was reflected in the statistical result. The upstream Stations 2–4 can also be grouped together, possibly due to the influence of abandoned mines around those areas.
The CA results for the heavy metals show that Cd and Pb are grouped separately from other heavy metals, indicating that their origins were basically different from those of Ni, Zn, Cr, Co, and Cu (Fig. 4b). This is also well reflected in the results of PCA (Table 5). Two principal components (PC1, PC2) with eigenvalues higher than 1.0 were extracted from the PCA. PC1 explains 59.9% of the total variance and is characterized by the strong positive loading in Co, Cr, Cu, Ni, and Zn (Table 5). Figure 5 shows higher PC1 scores at downstream reaches, reflecting the influence of industrial activities on Co, Cr, Cu, Ni, and Zn. The weak loadings of PC1 on Cd and Pb indicate that industrial activities did not play significant roles in accumulation of Cd and Pb in river sediments. PC2 showed a strong positive loading in Cd and a moderate negative loading in Pb. The different behaviors between those two metals were partly due to the addition of Pb from different sources. The significant supply of Pb from other sources, which cannot be clearly identified at this stage, makes the PC2 score much lower at Station 6 in Fig. 5. At headwater area, however, these two metals were likely originated from the scattered abandoned mines because no significant industrial facilities were found upstream and their concentrations were generally high at upstream stations despite some exceptions.
Conclusions
The sediments of the Kumho River were studied to obtain information on mineralogical compositions, distribution, speciation, and sources of heavy metals. No noticeable changes in mineralogical components were observed by qualitative XRD study, although the river flows through areas with differing geologies. Illite was the only mineral that showed significant correlations with several heavy metals due to its high surface area and sorption capacity. Based on average heavy metal concentrations, the sediments of the Kumho River were classified as heavily polluted according to EPA guidelines. The average concentrations of heavy metals in the sediments were in the order of Zn > Pb > Cu > Ni > Cr > Co > Cd. However, the contamination levels based on average I geo values were in the order of Pb > Cd > Zn > Cu > Co = Cr > Ni. The concentrations of heavy metals generally increased downstream as the river passed through the urban area. The increases in heavy metals were closely related to the input of contaminated tributary waters, especially at Stations 10 and 11. The sediments at those stations were highly contaminated by the tributary containing discharge from the treatment plant for industrial wastewater. This indicates that the anthropogenic effects were dominant in the increase of heavy metal contents in the Kumho River. The increase of heavy metals was also correlated with the increased fraction of metals bound to Fe/Mn oxides, indicating the importance of Fe/Mn oxides in the sorption of those metals. The potential toxicity of heavy metals for Ni, Cu, and Zn, represented by the exchangeable and the carbonate-bound fractions, also increased near industrial complexes. Metals such as Pb and Cd were relatively high at the upstream stations and statistically well correlated with each other, suggesting that the small-scale abandoned mines were the main sources of these two metals in the sediments.
References
Chang KH (1975) Cretaceous stratigraphy of Southeast Korea. J Geol Soc Korea 11:1–23
Churchman GJ, Hunt JL, Glasby GP, Renner RM, Griffiths GA (1988) Input of river-derived sediment to the New Zealand continental shelf: II. Mineralogy and composition. Estuar Coast Shelf Sci 27:397–411. doi:10.1016/0272-7714(88)90096-0
Demirak A, Yilmaz F, Tuna AL, Ozdemir N (2006) Heavy metals in water, sediment and tissues of Leuciscuc cephalus from a stream in southwestern Turkey. Chemosphere 63:1451–1458. doi:10.1016/j.chemosphere.2005.09.033
Farkas A, Erratico C, Viganò L (2007) Assessment of the environmental significance of heavy metal pollution in surficial sediments of the River Po. Chemosphere 68:761–768. doi:10.1016/j.chemosphere.2006.12.099
Filgueiras AV, Lavilla I, Bendicho C (2004) Evaluation of distribution, mobility and binding behaviour of heavy metals in surficial sediments of Louro River (Galicia, Spain) using chemimetric analysis: a case study. Sci Total Environ 330:115–129. doi:10.1016/j.scitotenv.2004.03.038
Gier S, Johns WD (2000) Heavy metal-adsorption on micas and clay minerals studied by X-ray photoelectron spectroscopy. Appl Clay Sci 16:289–299. doi:10.1016/S0169-1317(00)00004-1
Gu X, Evans LJ (2007) Modelling the adsorption of Cd(II), Cu(II), Ni(II), Pb(II), and Zn(II) onto Fithian illite. J Colloid Interface Sci 307:317–325. doi:10.1016/j.jcis.2006.11.022
Hochella M Jr, Moore JN, Putnis CV, Putnis A, Kasama T, Eberl DD (2005) Direct observarion of heavy metal-mineral association from the Clark Fork River Superfund Complex: implications for metal transport and bioavailability. Geochim Cosmochim Acta 69:1651–1663. doi:10.1016/j.gca.2004.07.038
Kim Y, Cygan RT, Kirkpatrick RJ (1996a) 133Cs NMR and XPS investigation of Cs adsorbed on clay minerals and related phases. Geochim Cosmochim Acta 60:1041–1052. doi:10.1016/0016-7037(95)00452-1
Kim Y, Kirkpatrick RJ, Cygan RT (1996b) 133Cs NMR study of Cs on the surfaces of kaolinite and illite. Geochim Cosmochim Acta 60:4059–4074. doi:10.1016/S0016-7037(96)00257-8
Kim Y, Cho S, Kang HD, Kim W, Lee HR, Doh SH, Kim K, Yun SG, Kim DS, Jeong GY (2006) Radocesium reaction with illite and organic matter in marine sediment. Mar Poll Bull 52:659–665. doi:10.1016/j.marpolbul.2005.10.017
Kim Y, Kim K, Kang HD, Kim W, Doh SH, Kim DS, Kim BK (2007) The accumulation of radiocesium in coarse marine sediment: effects of mineralogy and organic matter. Mar Poll Bull 54:1341–1350. doi:10.1016/j.marpolbul.2007.06.003
Klavins M, Briede A, Rodinov V, Kokorite I, Parele E, Klavina I (2000) Heavy metals in rivers of Latvia. Sci Total Environ 262:175–184. doi:10.1016/S0048-9697(00)00597-0
Li XD, Cloes BJ, Ramsey MH, Thornton I (1995) Sequential extraction of soils for multi-element analysis by ICP-AES. Chem Geol 124:109–123. doi:10.1016/0009-2541(95)00029-L
Liu L, Li F, Xiong D, Song C (2006) Heavy metal contamination and their distribution in different size fractions of the surficial sediment of Haihe River, China. Environ Geol 50:431–438. doi:10.1007/s00254-006-0226-0
Marengo E, Gennaro MC, Robotti E, Rossanigo P, Rinaudo C, Roz-Gastaldi M (2006) Investigation of anthropic effects connected with metal ions concentration, organic matter and grain size in Bormida river sediments. Anal Chim Acta 560:172–183. doi:10.1016/j.aca.2005.11.086
McBride MB (1994) Environmental chemistry of soils. Oxford University Press, New York, p 332
Müller G (1979) Schwermetalle in den sedimenten des Rheins–Veränderungen seit 1971. Umschau 79:778–783
Norra S, Lanka-Panditha M, Kramar U, Stüben D (2006) Mineralogical and geochemical patterns of urban surface soils, the example of Pforzheim, Germany. Appl Geochem 21:2064–2081. doi:10.1016/j.apgeochem.2006.06.014
Olivares-Rieumont S, de la Rosa D, Lima L, Graham DW, Alessandro KD, Borroto J, Martínez F, Sánchez J (2005) Assessment of heavy metal levels in Almendares River sediments—Havana City, Cuba. Water Res 39:3945–3953. doi:10.1016/j.watres.2005.07.011
Relić D, Đorđević D, Popović A, Blagojević T (2005) Speciations of trace metals in the Danube alluvial sediments within an oil refinery. Environ Inter 31:661–669. doi:10.1016/j.envint.2004.11.003
Sakai H, Kojima Y, Saito K (1986) Distribution of metals in water and sieved sediments in the Toyohira river. Water Res 20:559–567. doi:10.1016/0043-1354(86)90019-9
Segura R, Aranchibia V, Zúñiga MC, Pastén P (2006) Distribution of copper, zinc, lead and cadmium concentrations in stream sediments from the Mapocho River in Santiago, Chile. J Geochem Expl 91:71–80. doi:10.1016/j.gexplo.2006.03.003
Singh M (2001) Heavy metal pollution in freshly deposited sediments of the Yamuna River (the Ganga river tributary): a case study from Delhi and Agra urban centres India. Environ Geol 40:664–671. doi:10.1007/s002549900091
Singh KP, Mohan D, Singh VK, Amrita Malik (2005) Studies on distribution and fractionation of heavy metals in Gomti river sediments—a tributary of the Ganes, India. J Hydrol 312:14–27. doi:10.1016/j.jhydrol.2005.01.021
Taylor MP (2007) Distribution and storage of sediment-associated heavy metals downstream of the remediated Rum Jungle Mine on the east branch of the Finniss River, Northern Territory, Australia. J Geochem Expl 92:55–72. doi:10.1016/j.gexplo.2006.07.005
Tessier A, Campbell PGC, Bisson M (1979) Sequential extraction procedure for the speciation of particulate trace metals. Anal Chem 1:851–888
Turekian KK, Wedepohl KH (1961) Distribution of the elements in some major units of the Earth’s crust. Bull Geol Soc Am 72:175–192. doi:10.1130/0016-7606(1961)72[175:DOTEIS]2.0.CO;2
Upadhyay AK, Gupta KK, Sircar JK, Deb MK, Mundhara GL (2006) Heavy metals in freshly deposited sediments of the river Subernarekha, India: an example of lithogenic and anthropogenic effects. Environ Geol 50:397–403. doi:10.1007/s00254-006-0218-0
Zhou J, Ma D, Pan J, Nie W, Wu K (2008) Application of multivariate statistical approach to identify heavy metal sources in sediment and waters: a case study in Yangzhong, China. Environ Geol 54:373–380. doi:10.1007/s00254-007-0824-5
Acknowledgements
This work was supported by a Korean Research Foundation Grant (KRF-2004-015-C00571).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, Y., Kim, BK. & Kim, K. Distribution and speciation of heavy metals and their sources in Kumho River sediment, Korea. Environ Earth Sci 60, 943–952 (2010). https://doi.org/10.1007/s12665-009-0230-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12665-009-0230-2