Abstract
The reuse of waste materials as soil additives could be a welcome development in soil remediation. The mobility of Cd, Pb and As in a contaminated soil was investigated using natural and calcined poultry wastes (eggshell and chicken bone), CaCO3 and CaO at different application rates (0, 1, 3 and 5 %). The chemical composition accompanied with mineralogical composition indicated that CaCO3 and CaO were the major components in natural and calcined eggshells, respectively, while hydroxyapatite (HAP) dominated the natural and calcined chicken bones. The results showed that soil pH tended to increase in response to increasing application rates of all soil additives. The effectiveness of the additives in reducing Cd, Pb and As mobility was assessed by means of chemical extractions with 0.1 N HCl for Cd and Pb or 1 N HCl for As, according to Korean Standard Test (KST) method. Both calcined eggshell and chicken bone were equally effective with CaO or CaCO3 in reducing the concentration of 0.1 N HCl-extractable Cd from 6.17 mg kg−1 to below warning level of 1.5 mg kg−1, especially at the highest application rate. The application of calcined eggshell, CaO and CaCO3 also decreased the concentration of 0.1 N HCl-extractable Pb from 1,012 mg kg−1 to below warning level of 100 mg kg−1. The Pb concentration decreased significantly with an increasing application rate of chicken bone, but remained above warning level even at the highest application rate. On the contrary, natural and calcined chicken bones led to a significant increase in the mobility of As when compared with the control soil. These findings illustrate that calcined eggshell in particular is equally effective as pure chemical additives in stabilizing Cd and Pb in a contaminated agricultural soil. The presence of As in metal-contaminated soils should be taken into consideration when applying phosphate-containing materials as soil additives, because phosphate can compete with arsenate on adsorption sites and result in As mobilization.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Over the past several decades, industrial development throughout the world has resulted in the contamination of soil with heavy metals (Chen et al. 2000); in particular, mining and metallurgical industrial wastes which when dumped inevitably result in soil contamination (Reglero et al. 2008; Zhang et al. 2008). These metal-contaminated soils pose a risk to humans, animals and agricultural crops (Boularbah et al. 2006; Ok et al. 2007; Usman et al. 2012). In Korea, there are around 2,500 mines including 900 metallic mines (MOE 2005). More than 80 % of these mines are abandoned, with the ore reserves containing toxic metals such as Cd, Cu, Pb and Zn (Lee et al. 2011). Because of their acidic nature (pH < 4.0), the mine wastes are easily dispersed into the surrounding areas by flowing water and blowing wind. Therefore, nearby agricultural land is reported to be contaminated with heavy metals (Jung 2008; Ok et al. 2011a; Yang et al. 2008).
Various ex situ and in situ remediation technologies have been employed to reduce the risk arising from metal-contaminated soils (Ok et al. 2011b, c; Usman et al. 2004, 2005). Recently, the advantages of the in situ methods have overcome vulnerable points of the ex situ remediation technologies (De Kreuk 2005). In situ immobilization using chemical additives is a promising technology to remediate heavy metals in agricultural soils and to alleviate environmental and health risks (Ok et al. 2011a; Usman et al. 2006). In situ immobilization of metals can be achieved via the application of additives to the soil. The additives react with the contaminants and render them immobile or less available, eventually reducing plant uptake and groundwater contamination (Boisson et al. 1999a, b; Ok et al. 2011a). However, the formation of undesirable by-products should be avoided during the immobilization of heavy metals by soil additives. Therefore, the choice of the additive is a critical concern (Waterlot et al. 2010). Many additives, including natural or synthetic and organic or inorganic additives, have often been investigated for the in situ remediation of heavy metals in the contaminated soils (Geebelen et al. 2002; Kosobucki et al. 2008; Lee et al. 2008; O’Dell et al. 2007; Ok et al. 2010, 2011d; Singh et al. 2008; Usman et al. 2006). Naturally occurring waste materials were recently assessed for their immobilizing capacity. For example, waste eggshells (Ahmad et al. 2012a; Ok et al. 2011b), waste oyster shells (Ok et al. 2010), cow bones (Ahmad et al. 2012b), mussel shells (Ahmad et al. 2012c) and poultry waste materials (Hashimoto et al. 2008) have been successfully employed in immobilizing heavy metals in contaminated soils.
Calcination is the process by which a material can be upgraded by the transformation of one solid phase to another. The process takes place at or above the thermal decomposition temperature of the material (Ok et al. 2010). The main purpose of calcination is to eliminate water and organic matter, with the process generally taking place between 100 and 1,000 °C (Ozer et al. 2006). Some researchers used calcined materials such as calcined phosphate (Aklil et al. 2004), calcined eggshell (Ahmad et al. 2012a; Park et al. 2007) and calcined clay (Vieira et al. 2010) to remove heavy metals from the water and wastewater (Ahmad et al. 2012d). However, little information is available on the use of calcined materials for stabilizing heavy metals in contaminated soil. In a study by Ok et al. (2010), the authors showed that both natural and calcined oyster shells can be used as immobilizing substances for the remediation of metal-contaminated soil. The findings also showed that calcined oyster shells were more effective for immobilizing heavy metals in a contaminated soil than natural oyster shells. Other amendments such as phosphorus (P)-containing minerals are also known to decrease the availability of toxic metals to plants, animals and humans (Cui et al. 2010; Khan and Jones 2008; Park et al. 2001). In particular, the ability of bone meal, which is rich in P, to immobilize metals in soil through the formation of low-solubility metal phosphates has been evaluated by several authors (Hodson et al. 2000; Sneddon et al. 2006). To the author’s knowledge, however, no studies have been conducted to evaluate the use of waste chicken bone for the remediation of soils contaminated with heavy metals. In this study, the abilities of natural and calcined poultry wastes (eggshell and chicken bone) to reduce heavy metal mobility in a contaminated soil are compared.
The hypothesis is that the reuse of waste materials in a beneficial way may lead to the development of an environment-friendly soil remediation technology. Therefore, this study was conducted to evaluate the effectiveness of natural and calcined poultry wastes (eggshell and chicken bone) on the mobility of Cd, Pb and As in contaminated agricultural soil near a mining site.
Materials and methods
Soil sampling and analysis
Soil was collected from an agricultural land near the closed Seoseong mine in Chungnam Province, South Korea. The soil samples were air dried and sieved (<2.0 mm). The soil texture was determined by the hydrometer method (Gee and Bauder 1986). The soil pH and electrical conductivity (EC) were measured using a glass electrode at a soil-to-water ratio of 1:5. The cation exchangeable capacity (CEC) was calculated from exchangeable cations (Ca, Mg, Na and K) extracted with 1 M ammonium acetate, following the Brown method (NIAST 2000). The soil organic matter was analyzed with the Walkley–Black method (Walkley and Black 1934). The total content of Cd, Pb and As was determined by an aqua-regia extraction (MOE 2009). Heavy metal concentrations were analyzed using an atomic-absorption spectrometry (AAS, AAnalyst 700, Perkin Elmer, USA).
Poultry waste as a soil additive
Waste eggshells and chicken bone were processed to produce powders for use as soil additives. Waste eggshells were collected from a local restaurant, while chicken bone were obtained from Wonju City in the southwest of Gangwon Province. Waste materials were washed several times with hot water, then subsequently dried at 100 °C for 72 h and ground to pass through a 1-mm sieve. Portions of the eggshell and chicken bone powders were calcined at 900 °C for 4 h in a furnace (Ok et al. 2010). The prepared soil additives were then stored in airtight containers for further study.
The thermal stability of the eggshell and chicken bone powders was evaluated by a thermo gravimetric analyzer (TGA, SDT Q600, TA Instruments, USA) to determine the weight loss during the calcination process. The elemental composition of the soil additives was determined by X-ray fluorescence (XRF-1700, Shimadzu, Japan) spectroscopy. X-ray diffraction (XRD) patterns were obtained using an XRD spectrometer (X’pert PRO MPD, PANalytical, Netherlands) to evaluate the mineralogical composition of the soil additives. Surface structure analyses of the soil additives were then performed using a scanning electron microscopy (SEM, S-4300, Hitachi, Japan).
Incubation experiment
The contaminated agricultural soil was treated with the prepared soil additives in an incubation experiment. Natural and calcined poultry wastes (eggshell and chicken bone), CaCO3 and CaO were mixed with 100 g of the air-dried contaminated soil in a sealed plastic container at 0 % (control), 1, 3 and 5 % by weight. Deionized water was then added based on the 70 % water holding capacity of the soil, with the moisture content being maintained by periodically measuring the weight loss. The soil was incubated for 30 days, after which the air-dried treated soil samples were analyzed for pH and Cd, Pb and As concentrations. The soil pH was measured using a 1:5 soil/water ratio. The Korean Standard Test (KST) method was employed to assess the mobility of Cd, Pb and As in the treated soil. The KST method is proposed for soils contaminated with Cd, Cu, Pb and As (Lim et al. 2009). In order to detect the contaminant concentrations, 10 g of each soil sample was reacted with 50 mL of 0.1 N HCl (for Cd and Pb) by shaking at 100 rpm for 1 h, and 1 N HCl (for As) by shaking at 100 rpm for 30 min. The suspension was then centrifuged at 3,200 rpm for 20 min and the supernatant was filtered through Whatman No. 42 filter paper. The filtered solution was finally analyzed for Cd and Pb concentrations using an atomic-absorption spectrometer.
Statistical analysis
All of the treatments were undertaken in triplicate with the Statistical Analysis System (SAS 9.1 TS Level 1M3) software being used for the statistical analysis. One-way ANOVA was applied using Tukey’s honestly significant difference (HSD) studentized range test to determine any significant differences among different means of the treatments at p < 0.05. Pearson’s correlation coefficients between the different variables were also calculated.
Results and discussion
Soil characterization
The selected physico-chemical properties of the contaminated soil are shown in Table 1. The texture of the soil samples used in the experiment was loam, with a slightly acidic soil pH of 6.21. The organic matter content and CEC of the soil sample were 38.69 g kg−1 and 15.11 cmol(+) kg−1, respectively. The total content of heavy metals digested by the aqua-regia extraction was 15.27 mg kg−1 for Cd, 1,233 mg kg−1 for Pb and 119.82 mg kg−1 for As, which are 3.8, 6.2 and 4.8 times higher than the Korean warning levels of 4, 200 and 25 mg kg−1 respectively.
Effects of calcination on poultry waste characteristics
Waste eggshell and chicken bone powders were incinerated at high temperature to convert them from the less reactive CaCO3 to the more reactive CaO form (Ok et al. 2010). The calcination process can be explained by the following reaction:
Transitional changes during calcination, which were associated with the weight loss of eggshell and chicken bone, were measured using TGA and are shown in Fig. 1. Normal and derivative weight loss curves were drawn to identify the decomposition of the organic and inorganic components in the materials (Fig. 1). The TGA curve for eggshell showed a transitional change at about 650–850 °C, corresponding to the decomposition temperature of CaCO3. A weight loss of 45.6 % was observed at 789 °C due to the removal of the CO2 gas from CaCO3. Above 800 °C, no change in weight loss was observed, indicating that the calcination of eggshell had been completed at this temperature according to the above reaction. The results are consistent with that of Park et al. (2007) who reported the complete calcination of eggshell at 770 °C.
In the case of chicken bone, however, the thermal calcination process was more complex and had various phase changes. At around 50–250 °C, there was a decrease in weight due to the loss of water and from 250 to 600 °C the weight loss was associated with the thermal decomposition of organic matter. Above 600 °C, decomposition of the carbonate resulted in a weight loss of chicken bone. Almost similar phase changes were observed by the TGA analysis in other studies using pig bone (Raja et al. 2009), human femur bone (Lim 1975) and chicken bone (Park et al. 2001; Phiraphinyo et al. 2005).
Variations in the calcination processes of eggshell and chicken bone can be explained by their different compositions. The eggshell mainly comprised CaCO3 (Ok et al. 2011b), which can be deduced from the single phase change in terms of carbonate decomposition. The chicken bone, however, containing organic matter and calcium phosphate showed three phase changes due to water loss, organic matter and carbonate decomposition (Phiraphinyo et al. 2005). These results are supported by the XRF elemental analyses (Table 2) presenting CaO as the major component (93.23 %) in calcined eggshell, whereas besides CaO (80.21 %) and P2O5 (13.76 %) were also observed in the calcined chicken bone.
It was found that the loss on ignition (LOI) for calcined chicken bone (0.89 %) was high when compared with calcined eggshell (0.62 %), which indicates the presence of higher water content and organic matter composition resulting in different phase changes during the calcination process (Ok et al. 2010). The mineralogical composition of natural and calcined poultry waste (eggshell and chicken bone) is shown in Fig. 2 as XRD spectra. The primary minerals predicted in the natural and calcined eggshells were calcite (CaCO3) and calcium oxide (CaO), as indicated by the main peaks observed at 29.5 and 37.3 two θ degrees, respectively. However, the primary mineral predicted in the poultry waste of the chicken bone was hydroxyapatite (HAP), as indicated by the peak observed at 31.7 two θ degrees. The chemical composition, accompanied by the mineralogical composition revealed that CaO was the major compound found in calcined eggshell, while HAP dominated calcined chicken bone.
Soil pH as affected by the natural and calcined soil additives
It is well known that the soil pH is the main factor that affects soil chemical processes (Jung et al. 2011). At low soil pH, the solubility of heavy metals is increased and thus can have adverse effects on plant growth. The results showed that the soil additives affected the soil pH after an incubation period of 30 days (Table 3). It was noted that the soil pH increased in response to increasing application rates of all of the soil additives. In general, the largest increase in the soil pH was found for the soil receiving the highest application rate (5 %). The soil pH increased significantly from 6.52 in the control soil to 8.47, 12.54, 8.04, 12.51, 7.90 and 7.99 in the soil treated with the highest application rate of CaCO3, CaO, natural eggshell, calcined eggshell, natural chicken bone and calcined chicken bone, respectively. In general, the soil pH of the liming additives (natural and calcined eggshells) was higher than that of the control and chicken bone additives.
The largest increase in soil pH was observed for the CaO and calcined eggshell treatments with pH values of 12.54 and 12.51, respectively, at 5 % application rate. These increases in the soil pH are mainly due to the release of two hydroxyl (OH−) ions for each oxide of Ca in CaO and calcined eggshell according to the following chemical reaction (Ok et al. 2010):
The increase in soil pH caused by the calcined eggshell additive can be explained by the presence of calcium oxide as the dominant compound in calcined eggshells. In a previous study, similar effects on soil pH were also observed in the soil treated with calcined oyster shell powder (Ok et al. 2010). The increase in the soil pH resulting from the natural eggshell treatment could be ascribed to the dissolution of CaCO3 in water, leading to the production of hydroxyl ions (OH−) according to the following chemical reaction (Ok et al. 2010):
Ca2+ displaces H+ and Al3+ on exchange sites where OH− neutralizes the H+ in the soil solution. Natural and calcined chicken bones increased the soil pH to a maximum value of 7.90 and 7.99 at 5 % application rate, respectively. The liming action of the chicken bone containing HAP may be due to the presence of free CaCO3 as an impurity and to the fact that HAP consumes H+ in the dissolution process, reducing soil acidity (Shellis et al. 2010). The lower increase in soil pH noted for chicken bone was attributed to the association of Ca with phosphate as HAP [Ca5(PO4)3OH] rather than to CaO.
It is suggested that caution be exercised while using calcined eggshells as a soil additive as the high pH induced by the calcined eggshell may cause negative impacts on soil nutrient availability to agricultural crops. However, it is thought that the soil pH may decrease under field conditions with continuous cropping due to various factors, including crop removal, leaching of basic cations, application of ammonia-based nitrogen fertilizers and organic matter decomposition (Tang et al. 1999; Yang et al. 2009).
Metal mobility as affected by soil additives
Lime and phosphate minerals are well-known immobilizers that decrease the availability of toxic metals to plants, animals and humans. However, little information is available on the use of calcined waste materials to immobilize heavy metals in contaminated soils. In the current study, the effectiveness of natural and calcined poultry wastes in reducing Cd, Pb and As mobility was assessed by means of chemical extractions with 0.1 N HCl for Cd and Pb and 1 N HCl for As. Many investigators reported that diluted HCl extraction could be employed to determine the mobility and potential availability of heavy metals to plants (Lu and Bai 2009; Ok et al. 2010, 2011b).
Cd mobility
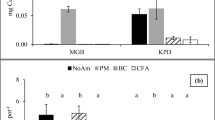
The mobile Cd concentrations extracted by 0.1 N HCl in response to soil additives are shown in Table 3. The concentration of 0.1 N HCl-extractable Cd in untreated soil (6.17 mg kg−1) was four times greater than the Korean warning standard level of 1.5 mg kg−1 (Ok et al. 2011b). Thus, these findings suggest that Cd can be easily mobilized and poses a potential threat to the environment. The results showed that all soil additives effectively reduced the concentration of 0.1 N HCl-extractable Cd below the warning level, with increasing effectiveness by increasing application rate. The concentration of 0.1 N HCl-extractable Cd was reduced by 8.25, 9.80, 7.93, 12.07, 4.24 and 7.86 % in soil treated with a 1 % application rate of CaCO3, CaO, natural eggshell, calcined eggshell, natural chicken bone and calcined chicken bone, respectively (Fig. 3a). However, a 5 % application rate of these treatments led to significant reductions in the 0.1 N HCl-extractable Cd, with reductions of 90.72, 99.90, 16.11, 99.95, 11.69 and 98.15 %, respectively, when compared with the control. These reductions in Cd mobility are assumed to be related to the chemical immobilization action of the soil additives. Cadmium may be adsorbed by soil colloids and may be precipitated as Cd-carbonate, phosphate or hydroxide, leading to its reduced mobility (McLean and Bledsoe 1992). Cadmium sorption depends on factors such as the pH, CEC, organic matter and the total soil Cd. In the current study, the increased soil pH induced by the soil additives is thought to be the main cause of the decreasing extractability of Cd as a result of its precipitation as carbonate or hydroxide. Additionally, the presence of phosphate in chicken bone additives could result in the precipitation of Cd as phosphate. A large number of studies have been conducted on the interaction of phosphate and Cd (Middelburg and Comans 1991; Yu and Zhou 2009). With bone meal treatments, Sneddon et al. (2006) found a significant decrease in Cd release from the soil. Fayiga et al. (2006) also observed that the application of phosphate rock was effective in decreasing the Cd uptake by Pteris vittata in a soil spiked with heavy metals. According to Yu and Zhou (2009), phosphate-induced Cd immobilization can be explained by several mechanisms: (1) an increase in the negative charge; (2) co-sorption of H2PO4 − and Cd as an ion pair; (3) formation of Cd3(PO4)2; (4) surface complex formation of Cd on the P compounds. Chen et al. (1997) suggested that Cd removal from aqueous solutions in the presence of apatite was due to surface complexation and ion exchange rather than precipitation of metal phosphates.
Pb mobility
The data in Table 3 show a significant decrease in the extractable Pb concentrations in soil following treatment with CaCO3, CaO and poultry waste (eggshell and chicken bone). The concentration of 0.1 N HCl-extractable Pb in the untreated soil (1,012 mg kg−1) was ten times greater than the Korean warning standard (100 mg kg−1) (Ok et al. 2011b). Among all of the soil additives tested, only 3 and 5 % application rates of calcined eggshell, CaCO3 and CaO decreased the Pb concentration to below warning level. Chicken bone and calcined chicken bone were unable to decrease the Pb concentration below the warning level even at a higher application rate. Nevertheless, they decreased the availability of Pb to 59.5 and 60.8 %, respectively, at a 5 % application rate as compared to the control (Fig. 3). In the current study, the primary mineral predicted to be found in the chicken bone was HAP. Indeed, the application of HAP to aqueous Pb or Pb-contaminated soils has been suggested by several authors (Boisson et al. 1999b; Ma et al. 1993, 1994). In situ Pb immobilization in contaminated soil via phosphate addition is widely accepted (Cui et al. 2010; Khan and Jones 2008). The addition of P amendments to Pb-contaminated soils reduces Pb mobility via ionic exchange and precipitation of the pyromorphite-type products [Pb5 (PO4)3X; X = F, Cl, Br or OH], reducing the risk of Pb toxicity in the environment (Cao et al. 2004; Udeigwe et al. 2011). Chen et al. (2010) quantitatively compared the efficiency of aqueous Pb removal by bone char meal and phosphate rock. They concluded that the Pb removal efficiency by bone char meal and phosphate rock was mainly controlled by the dissolution of phosphatic components associated with HAP, followed by a subsequent precipitation of the geochemically stable pyromorphite [Pb10(PO4)6(OH, Cl)2].
From the results obtained for Cd and Pb mobility, it is suggested that the rise in soil pH induced by the soil additives was responsible for decreasing their availability in the soil. These results are consistent with those of Kim et al. (2010) and Ok et al. (2010) who found that at high pH, metals became immobile and their uptake by plants was significantly reduced. In general, soil pH is reported to be the most important factor in controlling the availability of metals (Chen et al. 2000). Many studies on the adsorption and immobilization of heavy metals by the use of soil additives have shown that the pH is the master variable in the soil (Appel and Ma 2002; Barrow and Whelan 1998; Msaky and Calvet 1990). Increasing the soil pH increases the cationic heavy metal retention to soil surfaces via adsorption, inner sphere surface complexation and/or precipitation and multinuclear type of reactions (Appel and Ma 2002). Many adsorption sites in soils are pH dependent, i.e., Fe and Mn oxides, organic matter, carbonate and clay minerals (McLean and Bledsoe 1992). Metal hydroxides formed at a higher soil pH are generally immobile. The results of this study indicate that calcined eggshell is as effective as CaO in stabilizing Pb and Cd in a contaminated agriculture soil due to similar effects on the soil pH, but at different application rates. It was found that calcined eggshell was more effective than other treatments, which induced less effect on the soil pH. Precipitation as metal hydroxides or carbonates may be one of the possible mechanisms for the immobilization of Cd and Pb in soil treated with liming materials (Bolan et al. 2003; Ok et al. 2010, 2011b, c). This process can play a major role in the chemical stabilization of heavy metals, especially in alkaline soils which contain high metal concentrations.
As mobility
The effectiveness of natural and calcined poultry wastes in reducing As mobility was assessed by chemical extraction with 1 N HCl. The concentration of 1 N HCl-extractable As (22.38 mg kg−1) in the control soil was 3.7 times greater than the Korean warning standard level of 6 mg kg−1 (Moon et al. 2011). The concentration of 1 N HCl-extractable As decreased slightly after the addition of lime-based treatments (CaCO3, CaO and natural or calcined eggshell) (Table 3). It was found that the addition of natural and calcined chicken bone resulted in a significant increase in the 1 N HCl-extractable As compared to the control soil. The As concentrations were increased by 20.45, 47.96 and 67.20 % in soil treated with 1, 3 and 5 % of natural chicken bone additive, respectively (Fig. 3). At the same application rates, calcined chicken bone treatment increased the 1 N HCl-extractable As to a greater extent with an increase of 72.84, 97.60 and 110 %, respectively, when compared with the control. The highest As mobilization was observed in soil treated with calcined chicken bone at the highest application rate. The mobilization of As in the soil with chicken bone additives could be due to the competition of phosphate with As for surface binding sites on the solids (Boisson et al. 1999a). Many studies on immobilization/mobilization of As in soil have shown that phosphate may effectively compete with As for surface binding sites, resulting in As desorption/dissolution (Boisson et al. 1999a, b; Impellitteri 2005). For example, with the treatments of steel shots, beringite and hydroxyapatite, Boisson et al. (1999a) found that the addition of HAP led to higher As mobility due to phosphate–arsenate competition for the sorption complex of the solid soil phase. The findings presented in this study illustrate that the presence of arsenic in metal-contaminated soils should be taken into consideration, as phosphate competes with arsenate for adsorption sites and can cause As mobilization.
Conclusions
In the current study, natural and calcined poultry wastes, including eggshell and chicken bone, were applied as soil additives to study their effects on immobilization of Cd, Pb and As in a contaminated agricultural soil. The additives significantly reduced Cd and Pb mobility in the treated soil when compared with the control. The reduction was highly correlated with the soil pH attributing to the formation of insoluble metal hydroxides. On the contrary, chicken bone led to a significant increase in the mobility of As in the soil when compared with the control. These findings suggest that natural and calcined eggshells were equally effective with CaCO3 and CaO in stabilizing Cd and Pb in a contaminated agriculture soil. Future studies are needed to investigate the effect of natural and calcined poultry wastes on heavy metal availability to plants, as well as to predict the exact mechanism of metal transformation from available to unavailable forms in metal-contaminated soils.
References
Ahmad M, Hashimoto Y, Moon DH, Lee SS, Ok YS (2012a) Immobilization of lead in a Korean military shooting range soil using eggshell waste: an integrated mechanistic approach. J Hazard Mater 209–210:392–401
Ahmad M, Lee SS, Yang JE, Ro HM, Lee YH, Ok YS (2012b) Effects of soil dilution and amendments (mussel shell, cow bone, and biochar) on Pb availability and phytotoxicity in military shooting range soil. Ecotoxicol Environ Saf 79:225–231
Ahmad M, Moon DH, Lim KJ, Shope CL, Lee SS, Usman ARA, Kim KR, Park JH, Hur SO, Yang JE, Ok YS (2012c) An assessment of the utilization of waste resources for the immobilization of Pb and Cu in the soil from a Korean military shooting range. Environ Earth Sci. doi:10.1007/s12665-012-1550-1
Ahmad M, Usman ARA, Lee SS, Kim SC, Joo JH, Yang JE, Ok YS (2012d) Eggshell and coral wastes as low cost sorbents for the removal of Pb2+, Cd2+ and Cu2+ from aqueous solutions. J Ind Eng Chem 18:198–204
Aklil A, Mouflih M, Sebti S (2004) Removal of heavy metal ions from water by using calcined phosphate as a new adsorbent. J Hazard Mater A112:183–190
Appel C, Ma L (2002) Concentration, pH, and surface charge effects on cadmium and lead adsorption in three tropical soils. J Environ Qual 31:581–589
Barrow NJ, Whelan BR (1998) Comparing the effects of pH on the sorption of metals by soil and by goethite, and on uptake by plants. Eur J Soil Sci 49:683–692
Boisson J, Mench M, Vangronsveld J, Ruttens A, Kopponen P, De Koe T (1999a) Immobilization of trace metals and arsenic by different soil additives: evaluation by means of chemical extractions. Commun Soil Sci Plant Anal 30:365–387
Boisson J, Ruttens A, Mench M, Vangronsveld J (1999b) Evaluation of hydroxyapatite as a metal immobilizing soil additive for the remediation of polluted soils. Part 1. Influence of hydroxyapatite on metal exchangeability in soil, plant growth and plant metal accumulation. Environ Pollut 104:225–233
Bolan NS, Adriano DC, Mani PA, Duraisamy A (2003) Immobilization and phytoavailability of cadmium in variable charge soils. Plant Soil 251:187–198
Boularbah A, Achwartz C, Bitton G, Morel JL (2006) Heavy metal contamination from mining sites in South Morocco: 1. Use of a biotest to assess metal toxicity of tailings and soils. Chemosphere 63:802–810
Cao X, Ma LQ, Rhue DR, Appel CS (2004) Mechanisms of lead, copper, and zinc retention by phosphate rock. Environ Pollut 131:435–444
Chen XB, Wright JV, Conca JL, Peurrung LM (1997) Effects of pH on heavy metal sorption on mineral apatite. Environ Sci Technol 31:624–631
Chen HM, Zheng CR, Tu C, Shen ZG (2000) Chemical methods and phytoremediation of soil contaminated with heavy metals. Chemosphere 41:229–234
Chen S, Ma Y, Chen L, Wang L, Guo H (2010) Comparison of Pb(II) immobilized by bone char meal and phosphate rock: Characterization and kinetic study. Arch Environ Contam Toxicol 58:24–32
Cui Y, Du X, Weng L, van Riemsdijk WH (2010) Assessment of in situ immobilization of lead (Pb) and arsenic (As) in contaminated soils with phosphate and iron: solubility and bioaccessibility. Water Air Soil Pollut 213:95–104
De Kreuk JF (2005) Advantages of in situ remediation of polluted soil and practical problems encountered during its performance. In: Perminova IV et al (eds) Use of humic substances to remediate polluted environments: from theory to practice. Springer, Netherlands, pp 257–265
Fayiga AO, Lena Q, Ma LQ (2006) Using phosphate rock to immobilize metals in soil and increase arsenic uptake by hyperaccumulator Pteris vittata. Sci Total Environ 359:17–25
Gee GW, Bauder JW (1986) Particle-size analysis. In: methods of soil analysis. Part I. Physical and mineralogical methods-agronomy Monograph No.9, 2nd edn. Madison, pp 383–411
Geebelen W, Vangronsveld J, Adriano DC, Carleer R, Clijsters H (2002) Amendment-induced immobilization of lead in a lead-spiked soil: evidence from phytotoxicity studies. Water Air Soil Pollut 140:261–277
Hashimoto Y, Matsufuru H, Sato T (2008) Attenuation of lead leachability in shooting range soils using poultry waste amendments in combination with indigenous plant species. Chemosphere 73:643–649
Hodson ME, Valsami-Jones E, Cotter-Howells JD, Dubbin WE, Kemp AJ, Thornton I (2000) Effect of bone meal (calcium phosphate) amendments on metal release from contaminated soils—a leaching column study. Environ Pollut 112:233–243
Impellitteri CA (2005) Effects of pH and phosphate on metal distribution with emphasis on As speciation and mobilization in soils from a lead smelting site. Sci Total Environ 345:175–190
Jung MC (2008) Contamination by Cd, Cu, Pb, and Zn in mine wastes from abandoned metal mines classified as mineralization types in Korea. Environ Geochem Health 30:205–217
Jung K, Ok YS, Chang SX (2011) Sulfate adsorption properties of acid-sensitive soils in the Athabasca oil sands region in Alberta, Canada. Chemosphere 84:457–463
Khan MJ, Jones DL (2008) Chemical and organic immobilization treatments for reducing phytoavailability of heavy metals in copper-mine tailings. J Plant Nutr Soil Sci 171:908–916
Kim RY, Sung JW, Kim SC, Jang BC, Ok YS (2010) Effect of calcined eggshell on fractional distribution and plant uptake of Cd, Pb and Zn in contaminated soils near mine. 19th World Congress of Soil Science, Soil Solutions for a Changing World, 1–6 August 2010, Brisbane, Australia
Kosobucki P, Kruk M, Buszewski B (2008) Immobilization of selected heavy metals in sewage sludge by natural zeolites. Bioresour Technol 99:5972–5976
Lee YB, Ha HS, Lee CH, Kim PJ (2008) Coal fly ash and phospho-gypsum mixture as an amendment to improve rice paddy soil fertility. Commun Soil Sci Plant Anal 39:1041–1055
Lee SH, Kim EY, Park H, Yun J, Kim JG (2011) In situ stabilization of arsenic and metal-contaminated agricultural soil using industrial by-products. Geoderma 161:1–7
Lim JJ (1975) Thermogravimetric analysis of human femur bone. J Biol Phys 3:111–129
Lim M, Han GC, Ahn JW, You KS, Kim SH (2009) Leachability of arsenic and heavy metals from mine tailings of abandoned metal mines. Int J Environ Res Public Health 6:2865–2879
Lu SG, Bai SQ (2009) Contamination and potential mobility assessment of heavy metals in urban soils of Hangzhou, China: relationship with different land uses. Environ Earth Sci 60:1481–1490
Ma QY, Tralna SJ, Logan TJ, Ryan JA (1993) In situ lead immobilization by apatite. Environ Sci Technol 27:1803–1810
Ma QY, Tralna SJ, Logan TJ, Ryan JA (1994) Effects of aqueous Al, Cd, Cu, Fe(II), Ni, and Zn on Pb immobilization by hydroxyapatite. Environ Sci Technol 28:1219–1228
McLean JE, Bledsoe BE (1992) Behaviour of metals in soils. EPA Ground Water Issue, EPA 540-S-92-018. Environmental Protection Agency, Washington, p 25
Middelburg JJ, Comans RNJ (1991) Sorption of cadmium on hydroxyapatite. Chem Geol 90:45–53
MOE (2005) Investigation into the actual condition of soil contamination of abandoned mines (in Korean). Korean Ministry of Environment, Gwacheon
MOE (2009) The Korean Standard Test (KST) Methods for soils (in Korean). Korean Ministry of Environment, Gwacheon
Moon DH, Kim KW, Yoon IH, Grubb DG, Shin DY, Cheong KH, Choi HI, Ok YS, Park JH (2011) Stabilization of arsenic-contaminated mine tailings using natural and calcined oyster shells. Environ Earth Sci 64:597–605
Msaky JJ, Calvet R (1990) Adsorption behaviour of copper and zinc in soils: influence of pH on adsorption characteristics. Soil Sci 150:513–522
NIAST (2000) Method of soil and plant analysis (in Korean). National Institute of Agricultural Science and Technology, Suwon
O’Dell R, Silk W, Green P, Claassen V (2007) Compost amendment of Cu–Zn minespoil reduces toxic bioavailable heavy metal concentrations and promotes establishment and biomass production of Bromus carinatus (Hook and Arn.). Environ Pollut 148:115–124
Ok YS, Yang JE, Zhang YS, Kim SJ, Chung DY (2007) Heavy metal adsorption by a formulated zeolite–Portland cement mixture. J Hazard Mater 147:91–96
Ok YS, Oh SE, Ahmed M, Hyun S, Kim KR, Moon DH, Lee SS, Lim KJ, Jeon WT, Yang JE (2010) Effect of natural and calcined oyster shells on Cd and Pb immobilization in contaminated soils. Environ Earth Sci 61:1301–1308
Ok YS, Kim SC, Kim DK, Skousen JG, Lee JS, Cheong YW, Kim SJ, Yang JE (2011a) Ameliorants to immobilize Cd in rice paddy soils contaminated by abandoned metal mines in Korea. Environ Geochem Health 33:23–30
Ok YS, Lee SS, Jeon WT, Oh SE, Usman ARA, Moon DH (2011b) Application of eggshell waste for the immobilization of cadmium and lead in a contaminated soil. Environ Geochem Health 33:31–39
Ok YS, Lim JE, Moon DH (2011c) Stabilization of Pb and Cd contaminated soils and soil quality improvements using waste oyster shells. Environ Geochem Health 33:83–91
Ok YS, Usman ARA, Lee SS, Abd El-Azeem SAM, Choi BS, Hashimoto Y, Yang JE (2011d) Effect of rapeseed residue on cadmium and lead availability and uptake by rice plants in heavy metal contaminated paddy soil. Chemosphere 85:677–682
Ozer AK, Gulaboglu MS, Bayrakceken S, Weisweiler W (2006) Changes in physical structure and chemical composition of phosphate rock during calcination in fluidized and fixed beds. Adv Powder Technol 17:481–494
Park HJ, Lee BH, Lee BH (2001) Calcination characteristics of waste cow bone and eggshell (in English abstract). J Korean Solid Wastes Eng Soc 18:731–736
Park HJ, Jeong SW, Yang JK, Kim BG, Lee SM (2007) Removal of heavy metals using waste eggshell. J Environ Sci 19:1436–1441
Phiraphinyo P, Taepakpurenat S, Lakkanatinaporn P, Suntornsuk W, Suntornsuk L (2005) Physical and chemical properties of fish and chicken bones as calcium source for mineral supplements. Songklanakarin J Sci Technol 28:327–335
Raja S, Thomas PS, Stuart BH, Guerbois JP, O’Brien C (2009) The estimation of pig bone age for forensic application using thermogravimetric analysis. J Therm Anal Calorim 98:173–176
Reglero MM, Gonzalez LM, Taggart MA, Mateo R (2008) Transfer of metals to plants and red deer in an old lead mining area in Spain. Sci Total Environ 406:287–297
Shellis RP, Barbour ME, Jones SB, Addy M (2010) Effects of pH and acid concentration on erosive dissolution of enamel, dentine, and compressed hydroxyapatite. Eur J Oral Sci 118:475–482
Singh A, Sharma RK, Agrawal SB (2008) Effects of fly ash incorporation on heavy metal accumulation, growth and yield responses of Beta vulgaris plants. Bioresour Technol 99:7200–7207
Sneddon IR, Orueetxebarria M, Hodson ME, Schofield PF, Valsami-Jones E (2006) Use of bone meal amendments to immobilise Pb, Zn and Cd in soil: a leaching column study. Environ Pollut 144:816–825
Tang C, Unkovich MJ, Bowden JW (1999) Factors affecting soil acidification under legumes. III. Acid production by N2-fixing legumes as influenced by nitrate supply. New Phytol 143:513–521
Udeigwe TK, Eze PN, Teboh JM, Stietiya MH (2011) Application, chemistry, and environmental implications of contaminant-immobilization amendments on agricultural soil and water quality. Environ Int 37:258–267
Usman ARA, Kuzyakov Y, Stahr K (2004) Effect of clay minerals on extractability of heavy metals and sewage sludge mineralization in soil. Chem Ecol 20:123–135
Usman ARA, Kuzyakov Y, Stahr K (2005) Effect of clay minerals on immobilization of heavy metals and microbial activity in a contaminated soil. J Soil Sediment 5:245–252
Usman ARA, Kuzyakov Y, Lorenz K, Stahr K (2006) Remediation of a soil contaminated with heavy metals by immobilizing compounds. J Plant Nutr Soil Sci 169:205–212
Usman ARA, Lee SS, Awad YM, Lim KJ, Yang JE, Ok YS (2012) Soil pollution assessment and identification of hyperaccumulating plants in chromated copper arsenate (CCA) contaminated sites, Korea. Chemosphere 87:872–878
Vieira MGA, Neto AFA, Gimenes ML, da Silva MGC (2010) Removal of nickel on Bofe bentonite calcined clay in porous bed. J Hazard Mater 176:109–118
Walkley A, Black IA (1934) An examination of the Degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci 37:29–38
Waterlot C, Pruvot C, Ciesielski H, Douay F (2010) Effects of a phosphorus amendment and the pH of water used for watering on the mobility and phytoavailability of Cd, Pb and Zn in highly contaminated kitchen garden soils. Ecol Eng 37:1081–1093
Yang JE, Ok YS, Kim WI, Lee JS (2008) Heavy metal pollution, risk assessment and remediation in paddy soil environment: research and experiences in Korea. In: Sanchez ML (ed) Ecological research progress. Nova Science Publishers, New York, pp 341–369
Yang JE, Lee WY, Ok YS, Skousen J (2009) Soil nutrient bioavailability and nutrient content of pine trees (Pinus thunbergii) in areas impacted by acid deposition in Korea. Environ Monit Assess 157:43–50
Yu Z, Zhou Q (2009) Growth responses and cadmium accumulation of Mirabilis jalapa L. under interaction between cadmium and phosphorus. J Hazard Mater 167:38–43
Zhang J, Provis JL, Feng D, van Deventer JSJ (2008) Geopolymers for immobilization of Cr6+, Cd2+, and Pb2+. J Hazard Mater 157:587–598
Acknowledgments
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Education, Science and Technology (2012R1A1B3001409) and by Korea Ministry of Environment as “Geo-Advanced Innovative Action Project (No.172-112-011)”. The instrumental analysis was partly supported by the Korea Basic Science Institute, the Central Laboratory and Environment Research Institute of Kangwon National University in Korea.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lim, J.E., Ahmad, M., Usman, A.R.A. et al. Effects of natural and calcined poultry waste on Cd, Pb and As mobility in contaminated soil. Environ Earth Sci 69, 11–20 (2013). https://doi.org/10.1007/s12665-012-1929-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12665-012-1929-z