Abstract
The degradation of groundwater quality, which has been noted in the recent years, is closely connected to the intensification of agriculture, the unreasonable use of chemical fertilizers and the excess consumption of large volumes of irrigation water. In the hilly region of central Thessaly in Greece, which suffers the consequences of intense agricultural use, a hydrogeological study is carried out, taking groundwater samples from springs and boreholes in the Neogene aquifers. The aim of this study is the investigation of irrigation management, water quality and suitability for various uses (water supply, irrigation), the degradation degree and the spatial distribution of pollutants using GIS. The following hydrochemical types prevail in the groundwater of the study area: Ca–Mg–HCO3, Mg–Ca–Na–HCO3 and Na–HCO3. In the above shallow aquifers, especially high values of NO3 − (31.7–299.0), NH4 + (0.12–1.11), NO2 − (0.018–0.109), PO4 3− (0.07–0.55), SO4 2− (47.5–146.5) and Cl− (24.8–146.5) are found, particularly near inhabited areas (values are in mg L−1). The water of shallow aquifers is considered unsuitable for human use due to their high polluting load, while the water of the deeper aquifers is suitable for human consumption. Regarding water suitability for irrigation, the evaluation of SAR (0.153–7.397) and EC (481–1,680 μS cm−1) resulted in classification category ‘C3S1’, indicating high salinity and low sodium water which can be used for irrigation in most soils and crops with little to medium danger of development of exchangeable sodium and salinity. The statistical data analysis, the factor analysis and the GIS application have brought out the vulnerable-problematic zones in chemical compounds of nitrogen and phosphates. The groundwater quality degradation is localized and related exclusively to human activities. Based on 2005 and 2008 estimates, the annual safe yield of the region’s aquifers were nearly 41.95 MCM. However, the existing situation is that 6.37 MCM of water is over extracted from these aquifers.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In central Greece, in the broader area of the Thessaly valley, the need for drinking water and, especially, irrigation water, is increasing continuously, as this area exhibits intense agricultural activity and the drought hitting the area in the recent years does not contribute to the full renewal of groundwater which is consumed to a great extent by the overexploitation of aquifers.
The increase in irrigation demands, which has occurred after the 1970s in the full extent of Thessaly, often results in significant deficit of water during the summer months (Filintas et al. 2008a). The size of the water deficit depends on the hydrological conditions. The most important problem of water scarcity appear in the areas of Larissa, where the discharge of Pinios river is significantly reduced and declining of the groundwater levels in dry seasons is occurred (Marinos et al. 1995; Lazaridis et al. 1996; Filintas 2005; Koutseris et al. 2010). Water scarcity in Thessaly can only be confronted with the construction of water-storing works and the necessary water reclamation works (Filintas et al. 2008a; Koutseris et al. 2010).
Intense agriculture as well as disposal of urban and livestock waste has negative environmental effects on soil and water. As a result, of the wasteful use of fertilizers and pesticides, as well as of the intense land cultivation (without fallow periods) soil’s organic matter is diminished and the soil is degraded. The loss of soils ability to support agriculture and the increase of their erodibility is the end result of the above (Filintas 2005).
Nitrogen is a nutriment substance of vital importance, necessary for the growth of plants (Delgado and Shaffer 2002; Follett and Delgado 2002; Filintas 2005; Filintas et al. 2006; Filintas et al. 2007a; Filintas 2008). Therefore it is often added to the soil to increase productivity. Nitrate (ΝΟ3 −) which is percolated in agricultural lands has been long recognized and remains a growing concern due to the application of nitrogen fertilizers (Tan et al. 2002; Filintas 2005; Filintas et al. 2007b; Filintas 2008; Filintas et al. 2008b) and the returns-waste of animals (Silva et al. 1999).
In Greece, in recent years, the increase in irrigation lands was very significant and was accompanied by modernization of technological equipment, the use of new crop varieties which are efficient and resilient to diseases, as well as the increased application of ‘new technology’ fertilizers which increase the degree of utilization by the plants. In total, 2,077,067 tn nitrogen and 4,832,354 tn phosphate fertilizers were consumed in the period 1980–1991 (Filintas 2005). Nitrates from fertilizers and other sources are characterized by their great ability to move towards the shallow and the groundwater as they are soluble and are not blocked by soil particles.
Surpluses of nitrates can cause pollution of water reservoirs and aquifers (Solley et al. 1990; Postma et al. 1991; Dioudis et al. 2009), thus creating health problems to humans and animals. The problem of pollution by nitrates is not only noticed in the Thessaly plain but also in many other areas in Greece (Lambrakis et al. 2001; Stamatis and Boudouris 2003; Dimopoulos et al. 2003; Stamatis et al. 2006; Filintas et al. 2008a; Daskalaki and Voudouris 2008); at the same time, eutrophication phenomena have started appearing in river estuaries (Bellos et al. 2004; Filintas 2005; Filintas et al. 2008b).
Irrigation water of the study area derives either from groundwater or from surface waters. Surface waters are, often, transported to the fields with draining trenches, from where they are pumped with portable pumping equipment and usually, are applied to the fields using sprinkler, furrow or drip irrigation. The central Thessaly area suffers intense agricultural exploitation and irrigation is applied empirically and is not based on scientific methods such as calculation of evapotranspiration so that soil water deficit is exactly replenished; nor is the rate of water percolation in the soil seriously taken into account. The result of this empirical application of irrigation water in the fields is the loss of significant quantities of water through runoff, where this is favored by terrain slopes as well as through deep percolation, especially in sandy soils. The surface runoff contributes to pollution by nitrates of the surface water bodies, while deep percolation contributes to aquifer pollution by nitrates. Intense precipitations, irrigation methods as well as likely floods influence the concentration of nitrates which are accumulated in the surface and groundwater.
Moreover, two main concerns arise from the presence of nitrate and phosphate in water: (a) the high loading levels in surface water systems can increase the growth of algae blooms, thus causing a deterioration in the aquatic biotope of fish and other aquatic organisms, as well as wild fauna and generally an environmental deterioration and (b) the high levels of nitrates in drinking water can have adverse effects in human health.
In the present study, the results of the hydrogeological investigation of the area based on the application of hydrochemical methods and the use of GIS are presented. The aim of the study is to bring out the problem and the extent of groundwater pollution by nitrates and ammonium, which is due to the intense agricultural activity in the broader area. The results constitute important evidence for taking environmental measures for the protection of groundwater and, generally, for controlling the further deterioration of the natural resources in the broader area.
Materials and methods
Study area
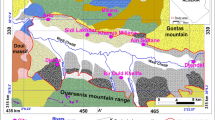
The study area occupies the south central part of Thessaly plain (Fig. 1a, b and c). It is restricted in the northeast by Larissa sub-basin (part of the eastern Thessaly basin) and southern by Farsala sub-basin (part of the western Thessaly basin). Pinios River, which drains Thessaly plain and falls into Aegean Sea, bounds to the North the study area. In north-western it is delimited by the marbles and gneisses of Titanos mountain as well as the carbonate mass of Fyllion mountain, while the carbonates and metamorphic formations of Halkodonio mountain bound to the southern–western the study area which is part of the intrabasinal high of central Thessaly that occurs among the grabens of eastern and western Thessaly. The study area of Nikaia constitutes generally a hilly until a semi-mountainous elongate zone with relatively smooth to intense relief, where the altitudes oscillate, in the flat-hilly region from 100 to 300 m, while in the northwestern and the southeastern mountainous masses it reaches 525 m. A relief differentiation is presented between the northeastern and the southwestern part of the area related to the tectonic deformation of the broader area (Fig. 1c).
The climate is characterized by a humid and cold period beginning in October followed by a dry and hot period beginning in May (Fig. 2a, b and c). As it results from meteorological data of the time period 1966–2008 (HNMS 2010; Filintas et al. 2008c; Dioudis et al. 2009; Filintas et al. 2010), from the two representative meteorological stations (Larissa station in the northern part and Farsala station in the southern part of the study area), the atmospheric temperature oscillates between 5.25°C in January and 27.08°C in July (Fig. 2b). From the climatic data of the study area, the ombrothermic diagram of a 43-year time period (1966–2008) was constructed and is presented in Fig. 2b. The mean rainfall value for the study area is 513.68 mm year−1. The average monthly temperature, of the last observed year (2008), is ranging from 5.1°C in January to 28.6°C in July (Fig. 2a). The study area has a temperate Mediterranean climate with warm dry summer and a mild winter, and is designated as Csa according to the Koeppen climatic classification (Filintas 2005), and also it is characterized as XERIC MOISTURE REGIME according to Soil Survey Staff (1975).
The higher mean monthly rainfall for the year 2008 was r w = 67.60 mm and it was observed in September, while the lowest one was r d = 3.60 mm at the month of May. Also, the effective rainfall Pe was calculated based on USDA-SCS (1970) method and is presented in Fig. 2a and b.
Based on the meteorological data of the wider region and by application of the Coutagne method (Coutagne 1954a, b), the annual evapotranspiration (E) (Filintas 2005; Filintas 2008) was calculated (Fig. 2c). The Coutagne (1954a, b) calculates the annual evapotranspiration (E) and is based on average annual values of precipitation (P) and air temperature (T) or the corrected air temperature (T c); the method has certain limits for T and P outside of which it is no longer valid. In the present study it was used the corrected air temperature T c (Soulios et al. 1991; Filintas 2005), the calculations were inside the method limitations and the mean annual evapotranspiration E was found to be 415.03 mm (80.7% of precipitation).
Two basic lithological types are developed in the study area, the Neogene sediments and the Holocene (quaternary) deposits which cover 65.36 and 34.64% of the region respectively, as the GIS analysis revealed. The groundwater studied emanates from water springs and wells supplied by these two lithological units.
From, studies that have been realized in the wider space of Thessalian basin and in other basins of Greece, it has been find out that the infiltration factor in the Holocene deposits and in the neogene sediments amounts about 14 and 7% of precipitation that fall in their surface respectively (Kallergis 1970; Soulios et al. 1991; Voudouris 1995; Soulios 1996).
Geology
The study area is structured from alpine and post-alpine formations and belongs geotectonically to the Eastern Greece unit. It covers the bigger part of the intrabasinal high of central Thessaly, which occurs among the grabens of Eastern and Western Thessaly. The area is formed from a NNE–SSW main fault system and a NNW–SSE fault system. Fault tectonics has contributed in the formation of the relief of the region (Fig. 3). The post-alpine formations of the study area consist of:
A simplified geological map of the study area (Bornovas et al. 1969, 1983; Katsikatsos et al. 1983; Plastiras and Karantasi 1985), (1 Holocene deposits, 2 Neogene sediments (carbonates, marls, conglomerates, clays, sands, etc.), 3 Flysch of Upper Cretaceous, 4 Carbonates of Upper Cretaceous, 5 Gneisses, Schists and Amphibolites, 6 Geological boundary, 7 Fault probable, 8 B1-B25: Groundwater sampling point of Borehole, 9 S1-S11: Groundwater sampling point of Spring)
-
(a)
Holocene recent deposits: they are alluvial sediments constituted from fluvial sand deposits covering the internal parts of the basins and talus cones as well as scree developed at the margins of the hills.
-
(b)
Neogene formations: they cover the most part of the study area and consist of clastic materials derived mainly from limestones, dolomites, flysh, schist and ophiolites that structure the surrounding mountains. The upper part of the Neogene formations including Pliocene–Pleistocene fluvial and continental deposits such as clays, loams, sand and conglomerates are developed at the northern and southern slopes of the hills. In the central part of the region Miocene lacustrine to marine marls were deposited. At certain places marly limestones with plant remains and thickness up to 15 m are occurred. The thickness of Neogene formations aquifers is appreciated in 300–400 m roughly (Bornovas et al. 1969; Katsikatsos et al. 1983; Katsikatsos 1992). The alpine substratum of the abovementioned sediments consist of upper Cretaceous flysh underlain by the upper Cretaceous karstified limestones outcropping at the eastern and the southwestern margins of the study area with a thickness of 100 m approximately. Metamorphic rocks, such as gneisses, marbles, chlorite and mica schists, occur in limited extent at the north-western part of the area. This metamorphic system belongs to Pelagonian geotectonic zone (Bornovas et al. 1969; Katsikatsos et al. 1983; Jacobshagen 1986).
Hydrogeology
Two different types of aquifers exist in the study area, the aquifer hosted in carbonate formations and the aquifer in neogene formations. In southeastern and northwestern section of Neogene formations different karst systems are developed in the carbonates. The intensively karstified limestones (upper Cretaceous) of Mavrovounio Mountain are thrusted at the impermeable flysh. At the northeastern margin of this karst system at the contact layer with the neogene formations, small karst springs drain the karst system. A significant amount of the karst water moves and contributes to the lateral feed of the surrounding neogene sediments. The Cretaceous limestones of Fillion Mountain form an important karst system, which undergoes an intense exploitation through boreholes for drinking and irrigation purposes (Kallergis et al. 1973; Mariolakos et al. 2001a, b). The neogene formations, which cover the most part of the study area and exhibit a thickness of 400 m, host locally unconfined and confined aquifers due to the alternation of permeable and impermeable layers. These aquifers undergo also intense exploitation through a large number of boreholes for irrigation purposes.
Unfortunately, there are no official data of the technical characteristics for the wells of the study region, so it is difficult to derive a precise description of groundwater horizons, which are developed inside the Neogenes’ formations. Also at the available geological mapping (Bornovas et al. 1969; Katsikatsos et al. 1983) was not performed in detail a stratigraphy description and thus the Neogene formations of the region in question are indivisible. The few available data elements of technical characteristics description for the area’s boreholes (wells), as depth of well (drilling), their water volume supply, the type of rock that they had drill etc., emanates from information of the householders. Based on this information, all the boreholes (wells) are supplied by Neogene formations. The discharge rates of the boreholes under favorable development conditions reach 20–30 m3 h−1. In some deeper boreholes, a characteristic rotten egg smell declares the presence of hydrogen sulfide. This could be attributed to the existence of organic matter in the neogene sediments, especially in the northeastern and southern part of the area, where the central and lower sediments are developed in fluvial-lacustrine and lacustrine-marine environments (Katsikatsos et al. 1983; Katsikatsos 1992). A small number of springs are occurred in the neogene sediments at different altitudes (Fig. 1c). The springs’ discharges range is between 0.01 L s−1 and 0.22 L s−1 (Table 1), while in dry periods most of them fall dry due to the presence of the large number of the boreholes.
In the broader area, in the Quaternary deposits of the northeastern Larissa basin and the southwestern Farsala basin, important unconfined aquifers are developed. These aquifers, suffers overexploitation mostly for irrigation usages, due to the intense agricultural activity in the area. The thickness of the unconsolidated deposits ranges between a few meters in the intrabasins and some ten meters in the grabens basins (Kallergis et al. 1973; Mariolakos et al. 2001a; Mariolakos et al. 2001b).
Hydrochemistry
Groundwater field sampling and laboratory analysis
In the frame of this study, water sampling was undertaken in May 2008 (May 5–10); according to the climatic characteristics of the broader area this time is the beginning of the dry period. Samples were collected at 11 springs’ outlets and 25 boreholes that were bored in neogene formations, in two different types of polyethylene bottles (100 mL and 1,000 mL volume). The specific boreholes have been chosen based on their depth and their spatial distribution, while the springs based on their discharges (Figs. 1c, 3). The first bottle type of 100 mL volume was filtered on site through 0.45 μm pore size Millipore filters. It was then acidified to a pH about 2 with 65% ultra pure HNO3 for heavy metals determination. The second non-acidified aliquot (1 L volume) was retained to determine major cation analyses and non-metal ions (Ca2+, Mg2+, Na+, K+, HCO3 −, Cl−, SO4 2−, NO3 −, ΝΟ2 −, ΝΗ4 +, PO4 3−, Br−, I−, F−) as well as SiO2, H2S and carbonate and total hardness. Uns
table parameters, temperature (T), electrical conductivity (EC), pH, dissolved oxygen (DO) were measured from untreated samples in situ using the portable devices WTW/LF-330 for electrical conductivity, WTW/330i for pH and WTW/OXI-96 for dissolved oxygen. Springs’ discharge rate and pumping rates in boreholes were also measured in situ.
The rest of the analyses were conducted at the laboratory of Mineralogy-Geology, Agriculture University of Athens. The parameters Mg2+, Fetot, Mn, Cu and Cr were determined by atomic absorption spectroscopy (GBC/908AA), while the alkali-cations Na+ and K+ by flame photometry (Flamephotometer ΙΝTECH/420). Total Hardness (Tot-H), calcium hardness (Per-H) and temporal hardness as well as Cl− were determined using titration kits (Total Hardness: ManVer Buret Titration Method 8226 of HACH, Calcium Hardness: Buret Titration Method 8222 of HACH, Temporal Hardness: Buret Titration Method, HCl 0,1 N Titrant, Methyl-red indicator and Cl−: Buret Titration Method AgNO3 0,1 N Titrant and KCrO2 indicator (Höll 1979). The parameters SO4 2−, NO3 −, ΝΟ2 −, ΝΗ4 +, PO4 3−, Br−, I−, F−, H2S and SiO2 were determined by spectral photometry (HACΗ DR/3000) using the suitable HACH kits.
Data analysis
Graphical methods as the expanded Durov diagram (Lambrakis 1991), scatter plot diagrams, eigenvalue diagram (Filintas 2005), statistical methods such as descriptive statistics, factor analysis (Davis 1986) and R-mode factor analysis as well as geostatistical methods (Filintas et al. 2007b; Filintas 2008; Hatzopoulos 2008; Filintas et al. 2009; 2010) and Trend surface analysis (Filintas 2008) were used for the data processing of chemical analyses but also for the presentation of the results. The software PHREEQC was used for the simulations of chemical analyses.
The statistical, geostatistical, hydrogeological GIS analysis, GIS delineation of catchments and aquifers, GIS parametric mapping, precipitation and evapotranspiration calculations, groundwater-wastewater and irrigation environmental management analysis, water Balance (recharge-discharge) and the water suitability for irrigation analysis were conducted at the laboratory of Remote Sensing and GIS, Department of Environment, University of the Aegean. For the statistical analysis, it was used the statistical software SPSS 13. A computer digital geo-database in GIS was also designed and developed (Filintas 2005; Hatzopoulos 2008; Filintas 2008; Filintas et al. 2009; 2010) for the study area, which contained five matrixes with the spatial and attribute data of the studied parameters and of the spatial data of the water samples and the results of the laboratory analyses as attribute data. For the use of various maps, the georeferencing of the GIS and geological maps, for the development of digital databases, the digital elevation model and the output parametric GIS maps were used the software: (a) ArcView 3.2 and (b) ArcGIS 9.1. So, by use of methods of laboratory chemical analyses of water samples, GPS verification, GIS, geostatistical and statistical methods and computer data processing, the spatial variability of the NO3, NH4, PO4 and EC was modeled and mapped in digital form in the Greek Geodetic System of Reference called EGSA87, (Projection Type: Transverse Mercator, Spheroid name: GRS 1980 and datum: EGSA87).
Results and discussion
Hydrogeological GIS analysis results, types and boundaries of aquifers, delineation of catchments and climatic data of the region
From the hydrogeological GIS analysis of the study area, the definite boundaries of surface area catchments (Fig. 1b, c) and their amount were derived. There are four (4) catchments in Nikaia region with an amount of 866.56 km2 (Table 2). Also, the GIS analysis revealed that there are two different types of continental aquifers in Nikaia region, the carbonate formations type of aquifer (with an area found to be 300.18 km2), and the neogene formations type of aquifer (with an area found to be 566.38 km2). The definite boundaries of the two continental aquifers of the study area are presented in Fig. 4.
Unluckily, there aren’t any available official data records for long term water extraction from the study area aquifers, so it is not possible to present the water extraction history of the area.
By applying the moving average technique (3 years averages for 43 years data series) to the annual average values of air temperature data from 1966 to 2008 (HNMS 2010; Filintas et al. 2010; Filintas et al. 2008c; Filintas 2005), an increasing air temperature value of 0.64°C from 1966 to 2008 was observed. Unfortunately, the climate change, that causes the increase of air temperature has also reduced rainfall intensity over the wider area of Thessaly plain in Central Greece and the statistical analysis of rainfall indicates that, there is 10.7% rainfall reduction nowadays from the beginning of this century. The mean monthly average rainfall values of the time period 1966–2008 are ranging between 15.87 mm month−1 in August and 66.91 mm month−1 in October. The higher values were observed mostly during the period October to March (Fig. 2b, c).
Available water resources, precipitation, evapotranspiration, groundwater, wastewater, irrigation environmental management analysis and water balance (recharge-discharge)
The main aquatic source which replenishes the water resources of Nikaia study area is the rainfall and also a small source portion is contributed by the Pinios River streams, Nempegleriotis and Ennipeas River.
Available water resources, precipitation, evapotranspiration
The average rainfall value for the study region is 513.68 mm year−1 and the amount of average precipitation water volume over the four catchments surface (Table 2) was found 445.13 MCM (106 m3) in annual basis. The Zappion catchment receives the highest precipitation water volume that is 213.92 MCM, while the Mosxoxori catchment has the lowest one, which is 69.39 MCM (Table 2).
Based on 43 years rainfall data series (HNMS 2010; Filintas et al. 2008c; Filintas et al. 2010), and by applying of the moving average technique to the annual average values of precipitation, from 1966 to 2008 (Fig. 2c), a decreasing trend of 1.39 mm per annum was observed. Also, the analysis revealed that in the last 10 years, there is 10.3% reduction in rainfall measurements, whereas, 10.6 and 11.6% reduction is reached when only the last 5 and 3 years values of precipitation are used respectively (Fig. 2c). From the aforementioned, it seems that water scarcity is at our doors.
Water scarcity inevitably tantalizes peoples, regions and nations in a struggle for existence and moreover when rivers cross borders within or between nations, water scarcity is a harbinger of trouble which leads to water stress and possibly to water wars between nations. In such times, construction of big dams is a necessity (Filintas et al. 2008a). Although, there have been some proposals for dam constructions in the area, till now none was constructed, due to lack of funding, society opposition from NGOs and environmental agencies, etc.
The society opposition stands because, despite the reception of measures, the improvements in the programming, planning, construction, operation and management of dams and water reservoirs, nowadays remain a widely widespread environmental concern that the big dams lead in important negative environmental impacts in a wide spectrum of functions of natural ecosystems and of human societies (Filintas et al. 2008a).
Moreover, various studies of rainfall (precipitation) analysis and evapotranspiration calculations (Soulios et al. 1991; Voudouris 1995; Soulios 1996; Filintas 2005; Filintas 2008; Hatzopoulos and Filintas 2009), for Greece and central Greece catchments (basins) with similar conditions as those of the present study region, revealed that, the variability of evapotranspiration was 70.1–82.8% of the average annual amount of precipitation. So, only a 17.2–29.9% of the precipitation is really contributing to the water budget of the area.
Based on the meteorological data and by application of the Coutagne method (Coutagne 1954a, b), the total annual evapotranspiration E (Fig. 2c) and also the annual evapotranspiration E of each catchment (Filintas 2005; Filintas 2008) were calculated (Table 2). For the Coutagne method (1954a, b) application, it was used the corrected air temperature T c (Soulios et al. 1991; Filintas 2005), the calculations were found to be inside the method limitations and the mean annual evapotranspiration E was found to be 415.03 mm (80.7% of precipitation). The utilization of corrected air temperature gives more satisfactory results equally to this method in Central Greece prevailing conditions according to various researchers (Soulios et al. 1991; Voudouris 1995; Soulios 1996; Filintas 2005) and that was the reason of applying the corrected air temperature T c .
The average annual precipitation water volume of the neogene sediments surface (Neogene formations type of Aquifer which is 65.36% of the study region) for the four catchments, amounts in 290.93 MCM. From this volume of water the 80.79% is evaporated, that is to say 235.07 MCM, the 7.0% (20.36 MCM) infiltrates to the groundwater storage, the 0.12% (0.359 MCM) is used for municipal water consumption (Drinking water, Houses, Hotels, etc.) only in Ennipeas River catchment and the rest 12.08% (35.15 MCM) is storm runoff.
For the Holocene deposits surface [Carbonate formations type of Aquifer] (34.64% of the study region), it is appreciated that annually accepts an average precipitation water volume of 154.19 MCM, from which 124.58 MCM are evaporated, 21.58 MCM infiltrate and enrich the groundwater storage, and the remaining 8.02 MCM is storm runoff.
Globally, in the study area, the amount of average annual precipitation is 445.13 MCM of water volume, 359.66 MCM are evaporated, 41.95 MCM infiltrate to the groundwater storage and the rest 43.53 MCM is storm runoff (Table 2).
Groundwater, wastewater
Groundwater constitutes the major part of the water resources of Nikaia region. However, over extraction of water from the continental aquifers of the study area, nitrate pollution due to the intense agricultural activity and the middle recharge capacities resulted in the depletion of available freshwater within the aquifers. At three catchments of the Nikaia region (Nempegleriotis R.-Nikaia, Zappion and Mosxoxori catchments) a domestic total water volume of 1.19 MCM is supplied by water imports (transported with pipelines) from the neighboring Larissa-Pinios catchment (Municipality of Larissa) for municipal consumption (Drinking water, etc.). In the fourth catchment (Ennipeas River catchment) a freshwater volume of 0.359 MCM is used for municipal water consumption (drinking water, houses, hotels, etc.) (Table 2).
Irrigation water is supplied from: boreholes (wells), available perennial springs, streams and rivers, irrigation pipelines and channels (in some areas).
For the time being, the effluent water (wastewater) of domestic uses (houses, hotels, etc.) and livestock breeding are not used for irrigation (Kalavrouziotis et al. 2011), due to traditional negative opposition among the local farmers’ society (Hatzopoulos and Filintas 2009).
The aquifers of the area characterized into two groups within four hydrologic regions (catchments) (Fig. 4), which are having variable capacities (Tables 2, 3). Although, in the region, 41.95 MCM infiltrate and enrich the groundwater storage, the aquifers are depleting due to high irrigation demands in the last three decades, and the average water table level is reaching 30–45 m below surface level in the east and south farm land, while in the rest of the farm land is reaching 80–180 m below surface (Filintas 2005). Based on 2005 and 2008 estimates, the annual safe yield of the region’s aquifers were nearly 41,95 MCM however, the existing situation is that 6,37 MCM of water (deficit) is over extracted from these aquifers, as it is presented in Table 3, (Filintas 2005).
Irrigation environmental management analysis and water balance (recharge-discharge)
Agriculture, contributes a lot to the economy of the four catchments. The current crop patterns were grouped into 12 groups of crops (corn, cotton, sugarbeet, alfalfa, medic, tomato, vegetables, vineyards, fruit-bearing trees, olive trees, watermelon, melon, etc.), exhibiting similar characteristics with respect to water consumption (NSSG 2009; Mpliatsos 2009; Filintas 2008; Filintas 2005).
The ratio of population working in agricultural (farm and livestock) sector in the region is 56.10% based on census 2001 (Filintas 2005). Irrigation water is supplied from: boreholes (wells), available perennial springs, streams and rivers, irrigation pipelines and channels (in some areas). The total area used for irrigation in all the catchments is 193.07 km2 (Table 2). Moreover, the percentages of irrigated land of each catchment and the percentages of irrigated land of the agricultural catchment land are respectively: at Nempegleriotis R.-Nikaia catchment 24.94 and 26.77%, at Zappion catchment 24.74 and 32.27%, at Mosxoxori catchment 19.80 and 25.69%, and finally at Ennipeas River catchment 24.11 and 30.08%.
Until the 1980s and 1990s, old flood irrigation techniques were commonly applied, when water shortage, new plantations water demands and environmental water management forced the local farmers to install water-saving irrigation equipment and apply new water-saving irrigation techniques such as drip irrigation, etc. The government and the European Union supported these changes by subsidizing the investment equipment costs (Filintas 2005). Water outputs from the water balance (recharge-discharge) budget are agricultural irrigation, municipal water consumption (only at Ennipeas River catchment), industrial water consumption, stock farming, hydro network system and transportation pipelines losses, unused effluent water (wastewater) and some uncontrolled springs and streams flows that flow to neighboring catchments and finally are discharged to the Aegean sea.
The complete data on agricultural use (net irrigation), domestic use (residences, hotels, livestock, etc.), available water resources (infiltration recharge, surface waters, water imports) and water withdrawals, as well as monthly balance of water resources vs. water consumption are summarized in Table 4. Generally the study area is a region with relatively low hydrodynamics that is related straightforward with its lithology.
Groundwater quality
Groundwater temperature presents great differentiation (Table 1), between springs (12.8–17.2°C) and deep boreholes (14.6–23.1°C). The highest groundwater temperature exhibits in boreholes at the southern part of the study area (Β18: 22.1°C, Β20: 23.1°C and Β22: 23.2°C, see Figs. 1c and 3). Generally, the study area but especially the southern part, which is formed by fault tectonics and is characterised by the presence of higher groundwater temperature in deep boreholes, is part of the broader area of East Sterea Hellas, where an increase in geothermal fluid is observed coinciding with the Pliocene-Quaternary volcanic activity resulted in the creation of the volcanic centers of Lichades in Evoikos Golf and of Mikrothives in northeastern Orthis mountain (Fytikas and Kolios 1979). In Farsala basin, which extends southern of the study area, geothermal reservoirs have been found in Krini and Ampelia regions with temperature up to 26.2 and 39.1°C respectively (Stamatis et al. 2007).
Groundwater presents an alkaline character with pH values ranging among 6.9 and 8.1 (Table 1). The spring waters exhibit high content of dissolved oxygen (5.8–9.3 mg L−1 corresponding to 58–92% saturation degree). On the contrary the boreholes present a great range of the DO content between 1.6 and 9.1 mg L−1. The deeper boreholes Β5, Β14, Β16, Β17, Β18, Β19, Β20, Β21 and Β22 reveal the lowest values of d DO (1.6–5.9 mg L−1) due to the presence of organic matter in the neogene marl formations and the prevailing reduction conditions in deeper layers. In the abovementioned boreholes the relatively high concentration of hydrogen sulfide is characteristic. The concentration of hydrogen sulfide in boreholes varies between 0.0 and 2.6 mg L−1. In springs S3 and S8 low concentration of hydrogen sulfide (0.5 mg L−1) is detected (Table 1).
Spring waters present high values of electrical conductivity and TDS (total dissolved solids) ranging from 950 to 1680 μS cm−1, as well as 714–1,356 mg L−1 respectively. On the contrary the groundwater of boreholes exhibits lower values of EC and TDS ranging among 481 and 970 μS cm−1, as well as 429 and 985 mg L−1 respectively, with an exception of the wells Β20 (1,050 μS cm−1) and Β21 (1,146 μS cm−1), which are also characterised by high temperature values.
The most important characteristic of the water springs is the pollution by nitrates (NO3 − : 31.7–299.0 mg L−1) due to the agricultural activity of the broader area. Moreover, the high values of NH4 + (0.12–1.11 mg L−1), NO2 − (0.018–0.109 mg L−1) and PO4 3− (0.07–0.55 mg L−1) indicate groundwater pollution caused by anthropogenic activities. The concentrations, which are shown in Table 1, in most groundwater samples from spring and shallow boreholes, exceed the upper limits given by the directive concerning the water quality for human use (EEC98/83 1998; WHO 1984). Relatively similar groundwater quality deterioration reveals the boreholes in depths from 80 to 120 m. High nitrate concentration in groundwater present the boreholes Β23 in 100 m depth (NO3 −:83.6 mg L−1) and Β25 in 120 m depth (NO3 −:77.4 mg L−1). The highest NH4 + concentration shows the borehole Β20 in 80 m depth (NH4 +: 7.43 mg L−1), while Β15 in 120 m depth present high PO4 3− concentration (PO4 3−: 0.53 mg L−1). On the contrary the boreholes in greater depths present lower groundwater quality deterioration with NO3 − values ranging from 11.4 to 62.1 mg L−1, NH4 + between 0.02 and 1.11 mg L−1, NO2 − from 0.002 to 0.059 mg L−1 and PO4 3− among 0.08 and 0.49 mg L−1.
Groundwater from both sources is characterized by great variety of concentrations of sodium (15.9–144.0 mg L−1), chloride (14.2–127.7 mg L−1) and sulfate (1.3–146.5 mg L−1). The higher concentrations of sodium are located in groundwater of deep boreholes, while chloride and sulfate are presented with higher concentrations in spring waters and shallow boreholes. Their presence is related on geogenic factors, such as the existence of residual evaporates within the neogene sediments but also with anthropogenic influences. The F−, Br− and I– reveal low concentrations (Table 1). The higher halogens concentrations were detected in groundwater of the deep boreholes at the southern part of the study area. The concentrations of SiO2 in spring waters oscillate between 19.2 and 29.5 mg L−1 and in the boreholes between 11.1 and 33.2 mg L−1, with the higher concentrations found in the boreholes with the higher water temperature.
The concentrations of heavy metals Fetot, Mn, Cu and Cr in groundwater of springs and boreholes oscillate in relatively low levels. Their mean values (in mg L−1) amount in 0.032, 0.031, 0.018 and 0.014 respectively and they do not exceed the maximum acceptable levels, as these are determined by the Directives concerning waters of human consumption (EEC98/83 1998; WHO 1984).
Classification of groundwater
As is shown in Piper diagram (Fig. 5a), groundwater samples are classified in three groups. In first group belong all the spring waters and 32% of the groundwater samples of boreholes. In percentage 55% on the total of the spring waters are classified into the groundwater types Ca–HCO3 and Ca–Mg–HCO3, while in the rest 45% the Ca–Mg–HCO3–Cl–SO4 type is the dominant type. In this group, Ca2+ (21–72%) and Mg2+ (18–63%) present the dominant cations, while HCO3 − (41–74%) and SO4 2− (8–26%) prevail the dominant anions. The water samples belonging in this group could be considered as fresh water, younger than the groundwater of the second and third group (Lloyd and Heathcote 1985; Appelo and Postma 1994). In the second group, belongs the higher percentage of boreholes, approximately 52% of their total number. The samples are classified into a variety of water types including Mg–Ca–Na–HCO3, Mg–Na–HCO3, Na–Mg–Ca–HCO3 and Na–Ca–Mg–HCO3–Cl types, with no dominant type. These boreholes are located in the southern and western part of the study area. Their spatial distribution represents a wide transitional zone from fresh recharge waters in mixed type waters (Lloyd and Heathcote 1985; Appelo and Postma 1994). Obviously the water–rock interactions are still in progress. In this groundwater type, Na+ (20–55%) and Mg2+ (12–55%) are the dominant cations, while HCO3 − (50–76%) presents the dominant anion. In the third group belongs a small number of boreholes (Β2, Β3, Β9 and Β11), roughly 16% of their total number, located mainly in the northern and eastern part of the study area. The hydrochemical type Na–HCO3 prevails in this group. Na+ (68–81%) and Ca2+ (13–19%) are the dominant cations, while HCO3 − (71–77%) presents the dominant anion. The low calcium concentration could be attributed to cation exchange processes, which took place in the neogene aquifers of the area due to the abundance of clay minerals in neogene sediments (Lloyd and Heathcote 1985; Appelo and Postma 1994). According to the geochemical simulation of the groundwater Sylvite (KCl), Halite (NaCl), Anhydrite (CaSO4), Quartz (SiO2), Feldspar (NaAlSi3O8), Dolomite (CaMg[HCO3]2) and in certain cases Carbonate (CaCO3) are the main minerals that participate in the rocks of the study area and are dissolved in groundwater.
Figure 5b and c presents an expanded Durov diagram with the classification of the groundwater. According to this diagram, the following hydrochemical types prevail in the groundwater of the study area: Ca–HCO3, Mg–HCO3 and Na–HCO3. In Ca–HCO3 water type belong fresh water samples, which are younger than the groundwater of the other groups. Generally, they are fresh recharge waters, which are enriched in elements in relation to their lithological environment and individually to the human activities. In the Mg–HCO3 Water type, belongs the group of the transitional zone from fresh in mixed waters. They are considered as modified by cation exchange processes and obviously by hydrolysis of magnesium minerals in alkalis (Na+ and K+) and in Mg2+ infiltrated fresh water into the neogene sediments. Obviously the water–rock interactions are in progress and consequently no dominant water type is represented. In Na–HCO3 water type, belongs the recharge water, which undergoes the cation exchange processes.
Origin of the chemical elements of groundwater
Most groundwater samples of the study area are characterized from high concentrations of dissolved salts of geogenic origin, mainly in Ca2+, Mg2+, Na+, SO4 2− and Cl−. Based on the lithological environment and the various geochemical processes took place during the infiltration in deeper layers, groundwater obtain its chemical character. The presence of residual soluble evaporates in the neogene sediments, which have been deposited in lacustrine–marine environment, is a primary factor for the high salt concentrations in the groundwater of the study area. All the sampling points are located in places with positive hydraulic gradient and in distance of enough kilometers from the sea, thus seawater intrusion due to over pumping is excluded. Table 5 presents the ion ratio of Na/Cl and Cl/Br. The Na/Cl ratio has a value of 0.858 for seawater. Lower values obtain seawater intrusion. The mean value of Na/Cl in the groundwater of the study area is 1.162 indicating that seawater intrusion does not occur in the study area. The ratio Cl/Br for seawater is 648. Lower values denote seawater intrusion, while higher values imply evaporate dilution in groundwater. In the study area the Cl/Br ratio exhibit values ranging between 1,397–4,395 (mean value 3,215) in spring waters and 852–19,180 (mean value 4,359) in boreholes. These values denote the presence of evaporates in neogene formations of the study area and the enrichment of groundwater in salts due to their dilution.
Figure 6a–d, depicts representative cross-plots, where Na, Ca and SO4 are plotted in relation to Cl− concentration as well as Ca is plotted in relation to SO4. The lines join the end members in all diagrams (groundwater from the borehole Β15 with the lowest TDS (429 mg L−1) and groundwater from spring S5 with the highest TDS (1,356 mg L−1)). In the case of Na and Cl−, a small percentage of samples approximately 30%, seems to originate from mixed waters with similar chemical composition with that of the end members. The majority of water samples present a sodium excess. In this group belong the deep boreholes located in the northeastern and the southern part of the study area. In the presence of the clay minerals in the deeper layers of the neogene sediments could be attributed this sodium excess due to the cation exchange phenomena. In diagram b of Fig. 6 is shown that all spring waters present a calcium excess caused by the carbonate mineral dilution, while the groundwater in boreholes depict calcium deficit indicating that cation exchange phenomena took place. In diagram d of Fig. 6 sulfate excess in relation to chloride is realized in spring waters, while in groundwater from boreholes the opposite is found. This sulfate excess could be attributed in the dilution of anhydrite as is shown in diagram b. Anhydrite dilution could be also related to the excess of calcium due to high relation coefficient presenting the calcium–sulfate relation.
Factor analysis
In multivariate statistical analysis the Factor analysis is a useful explanatory tool and it can be applied to discover and interpret relations among variables or to test hypotheses (Davis 1986). Factor analysis attempts to find a way to identify underlying variables that explain the pattern of correlations within a set of original (observed) variables. This modeling procedure is often used to condense the information contained in the original variables and reduce the number of variables in a data set with a minimum loss of information, but can also be used to explore the latent structure of the variables in a data file (Filintas 2005). In the present study modeling, it was applied primary the factor analysis and finally the R-mode factor analysis for extracting factors governing the water chemistry and its physical characteristics of the study area Basins. The data matrix of 30 variables (T, pH, EC, SAR, TDS, DO2, DO2 (sat.%), H2S, Tot-H (Total Hardness), Temp-H (Temporal Hardness), Per-H (Calcium Hardness), Ca, Mg, Na, K, NH4, HCO3, Cl, SO4, NO3, PO4, NO2, Br, I, F, Fe, Mn, Cu, Crtot, SiO2) and 36 observations has been used in the present factor analysis.
The resulted eigenvalue diagram (Fig. 6e), of the factor’s analysis initial solution helped in the determination of the optimal number of components (factors), which was seven (Table 6).
-
Factor-1 has high loadings of carbonate Hardness (Temporal Hardness), HCO3, TDS, EC, Mg and NH4. Factor-1 may be treated as a “bicarbonate–carbonate hardness” factor and explains the dissolution of Dolomite (CaMg [HCO3]2), Anhydrite (CaSO4), Mg silicate, Sylvite (KCl) and part of Halite (NaCl) as the dominant chemical processes in the Pinios river basin. Factor-I accounts for 21.54% of the variance in the data matrix. The presence of ammonium is related with the intense agricultural activity that takes place in the region (Filintas 2005).
-
Factor-2 accounts for 18.47% variance in the data matrix and consists of variables Ca, NO3, Calcium and Total Hardness, Na and SAR. Factor-2 may be treated as a “nitrate-carbonate-calcium hardness” factor and explains the dissolution of Carbonate (CaCO3), Na silicate, Feldspar (NaAlSi3O8), Halite (NaCl), SiO2 as Quartz and part of Anhydrite (CaSO4). This factor is mainly represented by NO3 and Ca and shows negative loading of SAR and Na. The nitrate ion has no significant lithological source in the area and it may be associated with surface runoff of nitrate fertilizer from the catchments, nitrates leaching into deeper soil layers from the intense agricultural utilization (EEC91/676 1991; Filintas 2005) and from atmospheric inputs.
-
Factor-3 may be treated as a “dissolved oxygen-hydrogen sulfide” factor and shows negative loading of H2S and temperature in relation with dissolved oxygen as it was expected. The third Factor accounts for 13.25% of the variance in the data matrix. The high loadings of dissolved oxygen are mostly attributed to springs samples as laboratory and field analysis revealed high values (6.0–9.3 mg L−1) that correspond to 58–92% saturation and secondary to a limited number of the boreholes.
-
Factor-4 accounts for 9.62% variance in the data matrix and is mainly represented by pH. The groundwater of the study area shows an alkaline character with pH values between 6.9 και 8.1.
-
Factor-5 may be treated as a “halogen” factor and shows high loadings of iodine and bromium. This factor accounts for 8.66% variance in the data matrix and is mainly attributed to the presence of organic matter (lignite).
-
Factor-6 has high loadings of iron, copper and manganese and accounts for 8.49% variance in the data matrix. Factor-6 may be treated as a “heavy metal” factor. However the actual measured values do not exceed the maximum acceptable levels, as these are thus determined by the Directives that concern waters of human consumption (EEC98/83 1998; WHO 1984).
-
The factor-7 is less significant and accounts for only 5.00% of the total variance. This factor may be treated as a “phosphor-potassium” factor and may be associated with the intense agricultural utilization of the study area.
Factors 1, 2, and 3 account for 53.25% of the variance in the data matrix and dominate over the other factors in controlling the water chemistry. The rest four factors account for 31.77% of the variance and all the seven factors taken together explain about 85.02% of the total variance in the data matrix, indicating that the determined variables, which control the water chemistry are rock weathering with significant contribution of NO3, NH4 and PO4 from anthropogenic (agricultural) sources.
Trend surface and geostatistical analysis
In general, what makes GIS different from other kinds of computer mapping systems is that the attribute data and spatial information are always linked and processed jointly in GIS (Filintas 2005; Hatzopoulos 2008; Filintas 2008; Filintas et al. 2010). In the study, whenever it was judged necessary, it was applied the appropriate transformation to the data sets. A trend surface analysis was performed for each one of the input datasets of the NO3, NH4, PO4 and EC in a three-dimensional data space in order to identify trends in the input dataset and then the results used in the geostatistical analysis. Trend was resulted in the cases of NO3 and NH4, which was attributed to the geology, elevation and slope patterns in conjunction with the agricultural utilization patterns of the study area.
Then, by use of methods of laboratory chemical analysis of water samples, GPS verification, GIS, geostatistical and statistical methods and computer data processing, the spatial variability of the NO3, NH4, PO4 and EC was modeled and mapped in digital form in the Greek Geodetic System of Reference (EGSA87) (Filintas 2005; Hatzopoulos 2008; Filintas et al. 2008c). In order to identify the appropriate geostatistical method of spatial interpolation the below mentioned criteria were applied: (a) Mean Standardized Prediction Error (MSPE), as close as possible to 0 and (b) Root Mean Square Standardized prediction error (RMSS), as close as possible to 1 (Filintas 2005; Hatzopoulos 2008; Filintas et al. 2008c). An extent examination was carried out in all the results obtained applying different geostatistical methods of spatial interpolation with non-independent control points. It was revealed that the most efficient and prominent method for measured data was the ordinary Κriging (οr Κrig). Table 7 shows the best final results that were obtained regarding MSPE and RMSS of: NO3, NH4, PO4 and EC, applying non-independent control sets of spatial interpolation. These result values are acceptable and are in accordance with the above-mentioned criteria, proving the suitability of the methods applied.
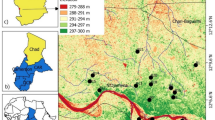
The GIS mapping results have interpreted the spatial variability of the NO3 − values, and as a consequence showed the nitrate pollution dispersion in the study area (Fig. 7a). After the creation of the first map and the classification by Defined Interval method (Filintas 2005; Filintas et al. 2008c), the boundaries of nitrate classes concentrations in the waters were determined. In the GIS map produced (after spatial interpolation), the problematic and vulnerable zones subjected to nitrate pollution have been located (Filintas 2005), and two main zones (with three classes) of nitrate pollution risk were identified (Fig. 7b). These zones are described below: The first zone of nitrate pollution covers almost the half of the study area (50.76%) shown in Table 8, consist of two subclasses 0–25 and 25–50 mg L−1 NO3 − respectively. The 25 mg L−1 class actually is the suggestive European Commission (EC) limit while the 50 mg L−1 class refers to the maximum limit (EEC91/676 1991; EEC98/83 1998; Filintas 2005; Filintas et al. 2008c), for drinking water. In certain parts of this zone, the concentrations of nitrates are exceeded the proposed European and Greek limit of acceptance for safety (25 mg L−1) reaching values up to the maximum upper limit of 50 mg L−1 and are subjected from small to medium risk of nitrate pollution. For this reason the monitoring of these waters, springs and boreholes is of great importance in order to early detect any increasing trends of nitrate concentrations. The second vulnerable zone of nitrate pollution covers the rest of the study area (Fig. 7b). The nitrate concentrations which varied from 50.1 to 298.9 mg L−1 are well above the European maximum limit of 50 mg L−1 for drinking water (EEC98/83 1998). This zone refers to the northeast, northwest, central-west, southeast and southwest part of the study area. The above mentioned areas are of great nitrate pollution risk, and apart from the continuous monitoring of the nitrate concentrations in the springs and boreholes, other actions are of significant importance. Thus, priority must be given in the political decisions that should be made, the management rules for environmental protection that should be applied and the decrease of nitrate human impact from agricultural activity that should be achieved. The top priority must be given to the degraded springs and in a second run to the boreholes belonging to this zone.
Regarding the ammonium content, the acceptance limits for drinking water are between 0.05 and 0.50 mg L−1 (EEC98/83 1998). The values of NH4+ determined in the laboratory and the parametric GIS mapping interpretation of the data in the area (Fig. 7c), varied from 0.02 to 7.43 mg L−1. The initial ammonium prediction map (Fig. 7c), was created after classification using Defined Interval method. After bordering the ammonium water concentrations in the study area, another GIS map of spatial interpolation included the problematic and vulnerable zones subjected to ammonium pollution have been located (Filintas 2005), which consisted of two main zones (classes) of ammonium pollution risk (Fig. 7d). The first zone of ammonium concentration (0–0.50 mg L−1) covers the greater part of the study area (78.11%) as shown in Table 8. The levels of concentrations observed, were not exceeded the maximum acceptance limits concerning waters for human consumption (EEC98/83 1998; Filintas 2005). In this zone a low ammonium pollution risk should be attributed. The second zone covering the 21.89% of the study area presents ammonium concentrations from 0.50 to 7.43 mg L−1. These values are exceeded the acceptance levels for drinking waters (EEC98/83 1998) defining this zone vulnerable and of high pollution risk (Fig. 7d).
The phosphate results from GIS mapping showed that its concentration varied from 70 to 740 μg L−1 (Fig. 8a). All values covering the 100% of the study area (Table 8) are considered as acceptable because they lay well below the normal levels of PO4 for drinking water, while the maximum acceptance limit is 5,000 μg L−1 (EEC98/83 1998).
The GIS mapping of EC values showed that they vary from 481 to 1,680 μS cm−1 (Fig. 8b). It is deduced with the aid of GIS techniques, that 100% of the values in the study area are considered as acceptable values (Table 8) because they lie between the normal acceptable levels with an upper level of 2,500 μS cm−1 for the drinkable waters (EEC98/83 1998). Regarding irrigation use of water, the GIS mapping and classification showed that groundwater of the study area in spatial percentage 100% (Table 8) are classified in medium (250–750 μS cm−1) to high (750–2,250 μS cm−1) salinity zone (Richards 1954), indicating medium το high salinity and can be used for irrigation in most soils and crops with little to medium danger of development of exchangeable sodium and salinity.
Water suitability for human consumption
All small communities in the study area are supplied with drinking water from local boreholes except the city of Nikaia which is connected with the water supply network of Larissa city. The main water quality problems appear to be the groundwater hardness, the pollution by nitrates and the presence in certain places of hydrogen sulfide and obviously of other gases. Considering the results obtained from chemical analyses, groundwater is characterized by high calcium and magnesium concentrations contributing to the increase in total hardness of spring waters, which varies from 25.8 to 40.4odH (German grade of hardness). Thus, spring waters could be characterized as very hard waters. The groundwater in boreholes presents a variety in hardness with values being between 4.0 and 28.1odH. The lowest hardness values (4.0–6.7odH) are observed in the waters of Na–HCO3 type, while the highest hardness values (11.8–28.1odH) have presented the waters of Mg–HCO3 type. Many from these waters can be used for covering human needs, unless they also contain H2S and/or other gas concentrations. The main problem of the water seems to be their high content in nitrogen compounds. The mean value of nitrates exceeds the 50 mg L−1 limit (EEC 98/83 1998; WHO 1984) and is observed in almost all springs. Therefore, groundwater from springs is unsuitable for water supply. In addition, unsuitable seems to be the groundwater from shallow boreholes (80–120 m depth), which are also characterized by high concentrations of the abovementioned pollutants exceeding the upper acceptable limits. On the contrary, boreholes of great depth (180–250 m) do not appear any groundwater degradation, since all chemical parameters lie bellow the acceptable limits and therefore their groundwater is considered suitable for human consumption.
Water suitability for irrigation uses
In order to evaluate groundwater quality and its suitability for irrigation, weightiness was given in evaluating and identifying potential problems (factors) related to irrigation water quality. The chemical parameters (Table 1) were compared with guideline values from literature (Richards 1954; Rhoades 1977; Ayers and Westcot 1985; Filintas 2005) in order to identify a potential problem water based on possible restrictions in use related to the following factors: (1) salinity, (2) rate of water infiltration into the soil, (3) specific ion toxicity and (4) some other miscellaneous effects. The water quality factors results of the present study are:
Salinity
Regarding salinity, EC and TDS were evaluated together. Salts in soil or water reduce water availability to the crop to such an extent that yield is affected (Ayers and Westcot 1985). The EC is an important and reliable indicator of the total dissolved solids (salts) content of the irrigation water (Filintas 2005; Filintas et al. 2008b, Filintas et al. 2008c) and the laboratory results showed that the mean EC and TDS of the boreholes (0.76 dS m−1; 663.68 mg L−1) and of the springs (1.68 dS m−1; 956.09 mg L−1) have a mean degree of restriction on use ‘slight to moderate’ (Ayers and Westcot 1985), and they are both acceptable for most crops (Ayers and Westcot 1985; Filintas 2005).
Infiltration
The infiltration factor affects infiltration rate of water into the soil. The evaluation of infiltration factor was performed using EC and SAR together. The Sodium Adsorption Ratio (SAR) is an indicator of the sodium hazard of water (Filintas 2005; Filintas et al. 2008b). The mean degree of restriction on use for the infiltration factor of the irrigation water of boreholes is ‘none’ (SAR = 2.266 < 3 and EC [dS m−1] = 0.76 > 0.70), according to irrigation water quality limits (Ayers and Westcot 1985). A plot of analytical data on the Rhoades (1977) diagram of relative rate of water infiltration relating salinity (EC) and SAR (Fig. 9a), shows that the waters of the boreholes B16, B17, B18, B19, B20, B23, B25 and of the whole dataset of springs are classified in ‘no reduction in rate of infiltration’, while the rest fall into second category ‘slight to moderate reduction in rate of infiltration’. Both water sources can be used for irrigation purposes according to Rhoades (1977).
EC and sodium concentration are very important in classifying irrigation water. While a high salt concentration (high EC) in water leads to formation of saline soil, a high sodium concentration leads to development of an alkaline soil (Filintas 2005). The calculated values of SAR range from 0.153 to 7.397 for boreholes and from 0.371 to 0.933 for springs. The plot of the data on the US salinity diagram (Fig. 9b) according to Richards (1954), in which the EC is taken as salinity hazard and SAR as sodic (alkalinity) hazard, shows that the mean of the water samples, the boreholes B8, B10, B16, B17, B18, B19, B20, B22, B23, B25 and the total set of springs (S1-S11) fall in the category ‘C3S1’, indicating high salinity and low sodium water which can be used for irrigation in most soils and crops with little to medium danger of development of exchangeable sodium and salinity. The boreholes B2, B3 and B9 fall in the category ‘C3S2’, indicating high salinity and medium sodium hazard and can be used for irrigation in certain soils and crops with caution and in association with soil analysis. The rest of the boreholes fall in the category ‘C2S1’, indicating medium salinity and low sodium water and can be used for irrigation in most soils and crops with little danger of development of exchangeable sodium and salinity.
Specific ion toxicity
The toxicity problems occur if certain constituents (ions) in the water are taken up by the plant and accumulate to concentrations high enough to cause crop damage or reduced crop yields. The ions of primary concern are chloride and sodium. Chloride (Cl−) is usually the source for the most common toxicity in the irrigation water. The mean value of chloride is 1.49 meq L−1 for the boreholes and 1.66 meq L−1 for the springs. The degree of restriction on use (Ayers and Westcot 1985) for the chloride is ‘none’ for surface irrigation, micro irrigation and sprinkler irrigation for the boreholes and the springs, except for springs S5 and S11 that have degree ‘none’ for surface irrigation but ‘Slight to Moderate’ degree for sprinkler irrigation. Chloride concentrations are considered acceptable for surface, micro irrigation and sprinkler irrigation in the study area (Filintas 2005). Sodium (Na+) is toxic and its toxicity is not as easily diagnosed as chloride’s (Filintas 2005). The mean value of Sodium is 3.09 meq L−1 for boreholes and 1.53 meq L−1 for the springs with a total mean (boreholes + springs) of 2.62 meq L−1. The degree of restriction on use (Ayers and Westcot 1985) for the Sodium is ‘Slight to Moderate’ for sprinkler irrigation with boreholes irrigation water, except in the cases of the water of boreholes B1, B4, B5, B12, B13, B14, B15, B16, B17, B18, B24, B25 and all the springs, which are classified to a degree of restriction on use ‘none’ and can be used without restrictions for surface, micro irrigation and sprinkler irrigation in the study area.
Other miscellaneous effects
The pH mean of the boreholes is 7.39 and of the springs is 7.54 and both are classified as ‘Normal range’. The Nitrate-Nitrogen (means 7.02 and 21.63 mg L−1) and the bicarbonate (means 5.97 and 7.30 meq L−1) for boreholes and springs respectively are both classified as ‘Slight to Moderate’ degree of restriction on use. The means (in mg L−1) of Ammonia–Nitrogen (0.362 and 0.251), the orthophosphate (0.307 and 0.176) and the iron (0.020 and 0.029) for boreholes and springs respectively are all classified as ‘none’ degree of restriction on irrigation use.
Conclusions
The study area is part from the Eastern Hellas unit and is structured from crystalline rocks of the substratum (schists, marbles and ophiolites), Neogene clastic sediments (marly limestones, marls, clays, sandstones and conglomerates) and Quaternary deposits. It is formed by NNE–SSW and NNW–SSE trending fault systems. In marls and marly limestones the presence of organic material (lignite) is characteristic. In granular formations unconfined and in certain places confined aquifers are developed. From the hydrogeological GIS analysis, were derived the definite boundaries of four (4) catchments with an amount of 866.56 km2. Also, the GIS analysis revealed two different types of continental aquifers in Nikaia region, the carbonate formations type of aquifer (with an area found to be 300.18 km2), and the neogene formations type of aquifer (with an area found to be 566.38 km2). Globally, in the study area, the amount of average annual precipitation is 445.13 MCM of water volume; 359.66 MCM are evaporated, 41.95 MCM infiltrate and enrich the groundwater storage and the rest 43.53 MCM is storm runoff. Irrigation water is supplied from: boreholes (wells), available perennial springs, streams and rivers, irrigation pipelines and channels (in some areas). For the time been, the effluent water (wastewater) of domestic uses (houses, hotels, etc.) and livestock breeding are not used for irrigation, due to traditional negative opposition among the local farmers’ society.
Although, in the region, 41.95 MCM recharge the groundwater storage, the aquifers are depleting due to high irrigation demands in the last 3 decades, and the average water table level is reaching 30–45 m below surface level in the east and south farm land, while in the rest of the farm land is reaching 80 to 180 m below surface. Based on 2005 and 2008 estimates, the annual safe yield of the region’s aquifers were nearly 41.95 MCM, however, the existing situation is that 6.37 MCM of water (deficit) is over extracted from these aquifers (Filintas 2005). The hydrochemical analyses, that were realized in 36 globally selectively sampling points (11 springs and 25 boreholes) distributed in the whole extent of the study area, after statistical treatment, hydrochemical diagrams development and GIS mapping (graphical and statistical methods, scatter plot and eighenvalue diagrams, factor analysis and R-mode factor analysis as well as geostatistical and Trend surface analysis) resulted in the following conclusions:
-
Groundwater samples are classified in three major groups (types): (a) Fresh water with low TDS value of Ca–HCO3 and Ca–Mg–HCO3 types (b) Transitional waters from fresh recharge in mixed waters including Mg–Ca–Na–HCO3, Mg–Na–HCO3, Na–Mg–Ca–HCO3 and Na–Ca–Mg–HCO3–Cl types, which reveal that the water–rock interactions are still in progress and (c) alkaline waters with Na–HCO3 type, which have undergone the cation exchange phenomena.
-
The high salt concentrations are due to the mineral dilution and the cation exchange processes. According to the geochemical simulation of the groundwater, Sylvite (KCl), Halite (NaCl), Anhydrite (CaSO4), Quartz (SiO2), Feldspar (NaAlSi3O8), Dolomite (CaMg[HCO3]2) and in certain cases Carbonate (CaCO3) are the main minerals contain in the rocks of the study area and are dissolved in groundwater.
-
The Cl–Na, Cl–Ca cross–plots reveal that in a small percentage of the water samples these elements have a common origin, while in the majority of the samples sodium excess indicates that cation exchange phenomena took place.
-
Chemical analyses confirm the existence of lacustrine-marine phase in neogene formations of the study area, which is responsible for the presence of residual, evaporates.
-
The factor analysis resulted in a significant data reduction and it helped to extract and identify the factors/sources responsible for variations in basins water quality. Factor-1 (21.54% of the variance) may be treated as a “bicarbonate-carbonate hardness” factor. The presence of ammonium is related with the intense agricultural activity that takes place in the region. Factor-2 accounts for 18.47% variance and may be treated as a “nitrate-carbonate-calcium hardness” factor. The nitrate ion has no significant lithological source in the area and it could be associated with surface runoff of nitrate fertilizer from the catchments, nitrates leaching into deeper soil layers from the intense agricultural utilization and from atmospheric inputs. Factors 1, 2, and 3 account for 53.25% of the variance and dominate over the other factors in controlling the water chemistry. All the seven factors taken together explain about 85.02% of the total variance in the data matrix, indicating that the determined variables, which control the water chemistry are rock weathering with significant contribution of NO3, NH4 and PO4 from anthropogenic (agricultural) sources.
-
The trend surface analysis that was performed for each one of the input datasets of the NO3, NH4, PO4 and EC in a three-dimensional data space resulted in trends for the cases of NO3 and NH4, which was attributed to the geology, elevation and slope patterns in conjunction with the agricultural utilization patterns of the study area.
The results of GIS mapping regarding nitrate and ammonium concentrations revealed two mainly pollution risk zones for each parameter. The first zone of nitrate pollution (0–50 mg L−1) covers almost the half of the study area (50.76%) and is subjected from small to medium risk of nitrate pollution, while the second nitrate pollution zone (nitrate 50.1–298.9 mg L−1) covers the rest of the study area (49.24%) and the nitrate concentrations are well above the European maximum limit of 50 mg L−1 for drinking water. Regarding NH4 +, the first zone covers the greater part of the study area (78.11%) and is characterized as low ammonium pollution risk zone. The second zone covering 21.89% of the study area presents ammonium concentrations from 0.50 to 7.43 mg L−1 and is defined as vulnerable and of high pollution risk.
-
Regarding the suitability of the water for human use, the groundwater from springs and shallow boreholes are unsuitable for water supply due to the high concentrations in nitrogen compounds exceeding the upper acceptable limits. On the contrary, boreholes of great depth (180–250 m) do not appear any groundwater degradation, since all chemical parameters lie bellow the acceptable limits and therefore, is considered suitable for human consumption.
-
Regarding water suitability for irrigation, the evaluation of SAR (0.153–7.397) and EC (481–1,680 μS cm−1) resulted in classification category ‘C3S1’, indicating high salinity and low sodium water which can be used for irrigation in most soils and crops with little to medium danger of development of exchangeable sodium and salinity. Also, the results showed that both water sources can be used for irrigation purposes with some limitations.
References
Appelo C, Postma D (1994) Geochemistry. Groundwater and pollution, Balkema
Ayers SR, Westcot DW (1985) Water quality for agriculture, FAO Irrigation and Drainage Paper 29-Rev.1. FAO Publications Food and Agriculture Organization of the United Nations, Rome
Bellos D, Sawidis T, Tsekos I (2004) Nutrient chemistry of River Pinios (Thessalia, Greece). Environ Int 30:105–115
Bornovas I, Filippakis N, Bizon JJG (1969) Geological map of Greece 1:50.000, Farsala Sheet. Publication IGME Athens
Bornovas I, Rontogianni-Tsiambaou TH (1983) Geological map of Greece 1:500.000, Publication IGME Athens
Coutagne A (1954a) Quelques considérations sur le pouvoir évaporant de l’atmosphère, le déficit d’écoulement effectif et le déficit d’écoulement maximum. La Houille Blanche 6:360–369
Coutagne A (1954b) Étude de quelques correlations hydrométéorologiques régionales et leur interpretation algebrique: La Houille blanche, 3 journées de l’Hydraulique de la Societé. Hydrotech. de France, Paris, pp 220–226
Daskalaki P, Voudouris K (2008) Groundwater quality of porous aquifers in Greece: a synoptic review. Env Geol 54(3):505–513
Davis JC (1986) Statistics and data analysis in geology. Wiley, New York
Delgado JA, Shaffer MJ (2002) Essentials of a national nitrate leaching index assessment tool. J Soil Water Conserv 57:327–335
Dimopoulos M, Chalkiadaki M, Dassenakis M, Scoullos M (2003) Quality of groundwater in Western Thessaly, the problem of nitrate pollution. Glob Nest Int J 5(3):185–191
Dioudis P, Filintas A, Koutseris Ε (2009) GPS and GIS based N-mapping of agricultural fields’ spatial variability as a tool for non-polluting fertilization by drip irrigation. Int J Sus Dev Plann 4(3):210–225
EΕC 91/676 (1991) Directive 91/676/EEC on nitrates from agricultural sources, European Commission Report COM(97) 473
EΕC 98/83 (1998) Council Directive 98/83/EC (Drinking Water Directive), European Commission, Brussels
Filintas A (2005) Land use systems with emphasis on agricultural machinery, irrigation and nitrates pollution, with the use of satellite remote sensing, geographic information systems and models, in watershed level in Central Greece. M.Sc. thesis, Dept. of Environment, University of Aegean, Mitilini, Greece
Filintas TΑG (2008) Study and mapping of biomass yield with the use of spatial statistics and geoinformation. M.Sc. thesis, Dept. of Natural Resources and Agriculture Engineering, Agricultural University of Athens, Athens, Greece
Filintas TΑG, Dioudis PI, Pateras DT, Hatzopoulos JN, Toulios LG (2006) Drip irrigation effects in movement, concentration and allocation of nitrates and mapping of nitrates with gis in an experimental agricultural field. In: Proc. of 3rd international conference haicta 2006 on: information systems in sustainable agriculture, agroenvironment and food technology, HAICTA, Volos, Greece, 20–23 September. ISBN 960-8029-43-0, pp 253–262
Filintas ΑG, Dioudis P, Pateras D, Κoutseris E, Hatzopoulos J, Toulios L (2007a) Irrigation water and applied nitrogens fertilizer effects in soils nitrogen depletion and nitrates GIS mapping. Proc. of 1st international conference on environmental management, engineering, planning and economics (CEMEPE/SECOTOX), June 24–28, vol III. Skiathos Island, Greece, pp 2201–2207
Filintas Α, Dioudis P, Koutseris Ε, Papadopoulos Α (2007b) Soils nitrates GIS mapping, irrigation water and applied N-fertilizer effects in soils nitrogen depletion in a drip irrigated experimental field in Thessaly basin. In: Proc. of 3rd IASME/WSEAS international conference on energy, environment, ecosystems and sustainable development (EEESD’07), July 24–26, Agios Nikolaos, Crete, Greece, WSEAS, ISBN: 978-960-8457-94-2, pp 487–492
Filintas A, Polyzos S, Stamatis G (2008a) Planning, construction and operation of dams and water reservoirs, in interelation with environmental impacts. In: Proc. of 8th international hydrogeological congress of Greece, 8–10 October, Athens, Greece, ISDN 978-960-88816-5-5, pp767–782
Filintas Α, Dioudis P, Stamatis G, Hatzopoulos J, Karyotis Th (2008b) Environmental assessment of groundwater nitrate pollution from agricultural wastes and fertilizers in central Greece watersheds using remote sensing and GIS. In: Proc. of 3rd international conference AQUA 2008 on: water science and technology with emphasis on water & climate, 16–19 October, Athens, Greece, ID-02, p 10
Filintas A, Hatzopoulos J, Polyzos S (2008c) Assessment of surface water springs’ quality by the use of GIS and geostatistical techniques. Chapter 28. In: Arabatzis G., Polyzos S (eds) Natural resources, environment and development. Tziolas Publications, Thessaloniki, Greece, pp 695–728 (in Greek)
Filintas AG, Hatzopoulos J, Parlantzas V (2009) Agriculture spray machinery pattern testing and validation by the use of gis and the use of a dilution of active ingredient in wastewater. In: Proc. of 5th international conference on energy, environment, ecosystems and sustainable development (EEESD’09), pp 334–339
Filintas A, Dioudis P, Prochaska C (2010) GIS modeling of the impact of drip irrigation, of water quality and of soil’s available water capacity on Zea mays L, biomass yield and its biofuel potential. Desalination Water Treat 13(1–3):303–319
Follett RF, Delgado JA (2002) Nitrogen fate and transport in agricultural systems. J Soil Water Conserv 57:402–408
Fytikas M, Kolios N (1979) Preliminary heat flow map of greece. In: Germak V, Rybach L (eds) Terrestrial heat flow in Europe. Springer, Berlin, pp 197–205
Hatzopoulos NJ (2008) Topographic mapping, covering the wider field of geospatial information science & technology (GIS&T). ISBN-10: 1581129866, ISBN-13: 9781581129861, Universal Publishers, 740 pp
Hatzopoulos J, Filintas AG (2009) Integrated urban planning for the safe reuse of municipal wastewater and biosludge from WWT Plants in crops and soils. Research Project Delivarables IV. Dept. of Environment, University of the Aegean, Mitilini, Greece
HNMS (2010) Meteorological data of central Greece, Hellenic National Meteorological Service (HNMS), Greece (in Greek)
Höll K (1979) Wasser: Untersuchung, Beurteilung, Aufbereitung, Chemie, Bakteriologie, Virologie, Biologie. Berlin-New York (Walter de Gruyter), pp 515
Jacobshagen V (1986) Geologie von Griechenland. Berlin-Stuttgart, Borntraeger, p 363
Kallergis G (1970) Hydrogeological investication of Kalambaka basin (Western Thessaly), vol XIV. Institute for Geology and subsurface research, Athens, Greece (in Greek)
Kalavrouziotis I, Filintas A, Koukoulakis P, Hatzopoulos J (2011) Application of multicriteria analysis in the management and planning of treated municipal wastewater and sludge reuse in agriculture and land development: The case of Sparti’s wastewater treatment plant, Greece. Fresen Environ Bull 20(2):287–295
Kallergis G, Morphis A, Papaspyropoulos Ch, Christodoulou Th (1973) Hydrogeological investigations in the Western Thessaly basin. Hydrol Hydrogeol Investig IGME Athens, No 8, pp 166
Katsikatsos G (1992) Geology of Greece. Athens, p 451
Katsikatsos G, Mylonakis E, Triantaphyllis E, Papadeas G, Psonis K, Tsaila-Monopoli S, Skourtsi-Koroneou V (1983) Geological Map of Greece 1:50.000, Velestino Sheet. Publication IGME Athens
Koutseris Ε, Filintas Α, Dioudis P (2010) Antiflooding prevention protection, strategic environmental planning of aquatic resources and water purification: the case of Thessalian basin, in Greece. Desalination 250(1):318–322
Lambrakis N (1991) Elaboration of the hydrochemical data by PC. Miner Wealth 74:53–60
Lambrakis N, Stamatis G, Giannoulopoulos P, Voivonta A (2001) Groundwater quality and estimation of rehabilitation time of the Argolid plain’s aquifers under artificial recharge conditions. Bull Geol Soc Greece XXXIV(5):1819–1826
Lazaridis L, Kalaouzis G, Koutsoyiannis D, Marinos P (1996) Basic engineering and economic characteristics regarding water resources management of Thessaly. Proc. of the Intern. Conf. on Water Resources Management, Larissa, Technical Chamber of Greece
Lloyd JW, Heathcote JA (1985) Natural inorganic hydrochemistry in relation to groundwater. Clarendon Press, Oxford, p 296
Marinos P, Thanos B, Perleros K, Kavadas M (1995) Groundwater in Thessaly plain (origin in Greek). In: Proc. of the 3rd Hydrogeological Congress in Greece, Heraklion – Crete, pp 468–480
Mariolakos E, Lekkas S, Alexopoulos A, Fountoulis I, Spyridonos E, Badekas I, Mariolakos D, Andreadakis E (2001a) Artificial Recharge of a karstic aquifer in Fillion mountain in Farsala basin – Thessaly (Orig. in Greek). In: Proc. of the 9th Intern. Congr. Athens, September 2001, Bull Geol Greece, vol XXXIV/5, pp 1843–1850
Mariolakos E, Lekkas S, Papadopoulos T, Alexopoulos A, Fountoulis I, Alexopoulos I, Spyridonos E, Badekas I, Mariolakos D,. Andreadakis E (2001b) The subsurface tectonic structure of the Farsala basin (Thessaly) as determining factor of the Hydrogeological conditions of the region. In: Proc. of the 9th Intern. Congr. Athens, Sept. 2001, Bull Geol Greece, vol XXXIV/5, pp 1851–1858
Mpliatsos K (2009) Agricultural and irrigated land of Nikaia region in 2008. G.A Larissa, Greece (In Greek)
NSSG (2009) Agriculture statistics of Greece-Data for 2008, National statistical Service of Greece (NSSG), Pireas, Greece
Plastiras B, Karantasi S (1985) Geological Map of Greece 1:50.000, Larissa Sheet. Publication IGME Athens
Postma D, Boesen C, Kristiansen H, Larsen F (1991) Nitrate reduction in an unconfined sandy aquifer: water chemistry, reduction processes, and geochemical modeling. Water Resour Res 27(8):2027–2045
Rhoades JD (1977) Potential for using saline agricultural drainage waters for irrigation. In: Proc. water management for irrigation and drainage. ASCE, Reno, Nevada. 20–22 July, pp 85–116
Richards LA (1954) Diagnosis and improvement of saline and alkali soils. USDA Agricultural Handbook No. 60. US Department of Agriculture, Washington DC, p 160
Silva RG, Cameron KC, Di HJ, Hendry T (1999) A lysimeter study of the impact of cow urine, dairy shed effluent and nitrogen fertilizer on nitrate leaching. Aust J Soil Res 37:357–369
Soil Survey Staff (1975) Soil taxonomy: a basic system of soil classification for making and interpreting soil surveys, USDA Natural Resources Conservation Service, USA
Solley WB, Pierce RR, Perlman HA (1990) Estimated use of water in the United States in 1990. US Geological Survey Circular 1081
Soulios G (1996) General hydrogeology, vol I. University Studio Press, Thessaloniki Greece, p 295
Soulios G, Toubektsi M, Tzevelekis G, Ezeigbo H (1991) Water balance of basins in central Greece: comparison with other circum-Mediterranean basins and validity of empirical methods. Environ Geol Water Sci 18(2):85–94
Stamatis G, Boudouris K (2003) Marine and human activity influences on the groundwater quality of southern Korinthos area. Int J Hydrol Process 17:2327–2345
Stamatis G, Lambrakis Ν, Alexakis D, Zagana E (2006) Groundwater quality in Neocene and quaternary deposits of Mesogea basin in Eastern Attica (Greece). Int J Hydrol Process 20:2803–2818
Stamatis G, Parpodis K, Lambrakis N, Zagana E (2007) Origin and quality of thermal groundwaters in the region of Farsala (E. Thessaly/Greece). Bull Geol Soc Greece XXXX:570–571
Tan CS, Drury CF, Reynolds WD, Groenevelt PH, Dadfar H (2002) Water and nitrate loss through tiles under a clay loam soil in Ontario after 42 years of consistent fertilization and crop rotation. Agric Ecosyst Environ 93:121–130
USDA-SCS (1970) Irrigation water requirements. Technical Release No. 21. USDA Soil Conservation Service, Washington, DC
Voudouris K (1995) Hydrogeological conditions of the NW part of Achaia (NW Peloponnesus, Greece). Phd thesis, University of Patras, Patras, Greece (in Greek), pp 237
WHO (1984) Guidelines for drinking-water quality, vol 2. Health criteria and other supporting information, Geneva
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Stamatis, G., Parpodis, K., Filintas, Α. et al. Groundwater quality, nitrate pollution and irrigation environmental management in the Neogene sediments of an agricultural region in central Thessaly (Greece). Environ Earth Sci 64, 1081–1105 (2011). https://doi.org/10.1007/s12665-011-0926-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12665-011-0926-y