Abstract
The present study applied the use of sequential extraction technique and simple bioaccessibility extraction test to investigate the bioavailable fractions and the human bioaccessible concentration of metals collected from nine stations in surface sediment of the Bernam River. The concentrations of total and bioaccessible metals from different stations were in the range of 0.30–1.43 μg g−1 and 0.04–0.14 μg g−1 for total cadmium and bioaccessibility of cadmium, respectively, 6.20–288 μg kg−1 and 2.06–8.53 μg kg−1 for total mercury and bioaccessibility of mercury, respectively, and 9.2–106.59 μg g−1 and 0.4–2.75 μg kg−1 for total tin and bioaccessibility of tin, respectively. The chemical speciation of Cd in most sampling stations was in the order of oxidisable-organic > acid-reducible > residual > exchangeable, while the chemical speciation of Hg was in the order of exchangeable > residual > oxidisable-organic > acid-reducible and the chemical speciation of Hg was in the order of residual > oxidisable-organic > acid-reducible > exchangeable. The principal component analysis showed that the main factors influencing the bioaccessibility of mercury in surface sediments were the sediment total organic matter, cation exchange capacity, and easily, freely, or leachable and exchangeable fraction, and the factors influencing the bioaccessibility of tin were the total tin and cation exchange capacity, while the bioaccessibility of Cd in surface sediments was influenced by the only factor which is the easily, freely, or leachable and exchangeable fraction.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The Bernam River is among one of the most important rivers in Malaysia and has been identified as the ultimate and largest source of water supply from Sungai Bernam, especially for irrigation and supply for domestic use; Bernam River has been posed to be polluted by a variety of contaminant sources such as industrial and domestic wastewater; these are all likely to cause heavy metal pollution at this river which it can reach the water column and accumulate in sediments. Heavy metals from surface sediment may potentially transfer to humans via biota due to that these metals can be released from the surface sediment to overlying water which may cause many problems including health impacts (McCready et al. 2006; Chen et al. 2007; Gu et al. 2014b). Heavy metals can be associated with organic matter present in the thin fraction of the sediments as well as textural characteristic or adsorbed on Fe/Mn hydrous oxides which make aquatic sediments the main sink for toxic metals in the environment, and these parameters may affect the amount of metals bound to sediments (Calmano et al. 1993; Saeedi et al. 2013). In sediments, heavy metals are in existence in a number of chemical forms and generally exhibit different physical and chemical behaviors in terms of chemical interaction, identification of the main binding sites, mobility, biological availability, and potential toxicity (Sundaray et al. 2011). These chemical fractions can be obtained using four stages of the sequential extraction technique (SET) proposed by Yap et al. (2002) (exchangeable, acid-reducible, oxidisable-organic, and residual). Additional studies documented that the bioaccessibility of heavy metals (Bio-HMs), defined as the fraction that is soluble in the gastrointestinal environment and potentially available for absorption, is the major part that may render health risks (Luo et al. 2012b; Sauve et al. 2000). Thus, in this study, surface sediment was addressed to the SET and simple bioavailability tests (SEBT) to assess bioavailability in risk assessment study. Secondly, the occurrence and relative distribution of an element among these various phases, and the physical relation between the phases in the sediment, will control an element’s dissolution properties and, hence, its bioavailability. Several researchers that have widely studied the factors influencing on metal bioaccessibility in the soil and sediment are listed as follows: Jinghua et al. (2015) in the Lake Taihu (China), Devesa-Rey et al. (2008) and Devesa-Rey et al. (2010) in Anllóns River (Spain), Xiaodong et al. (2015, 2016 ) in different soil sites (China), and Kadhum et al. (2016) in Langat River (Malaysia). Each study has its own theory foundation, and various factors influence in bioaccessibility depending on different anthropogenic sites. However, to date and the best of our knowledge, dominant factors which may influence the bioavailability and bioaccessibility for heavy metal analysis in surface sediment of Bernam River remain unknown. Therefore, this study were (1) to estimate the bioavailability and bioaccessibility of Hg and Cd in surface sediment using sequential extraction and SBET and (2) to investigate the potential influencing factors (sediment properties and heavy metal chemical forms) that may affect the bioaccessibility of metals.
Materials and methods
Study area
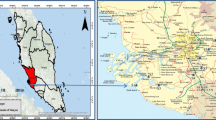
The Bernam River is sited in the west of Peninsular Malaysia between the states of Perak and Selangor, and it covers an area of 3335 km2, as one of the most important in rice cultivation in the lower areas adjacent to peat swamps. Recently, at the upstream of Bernam River, the whole area is embedded with rubber and oil palm plantation. The Bernam River basin is changing from agriculture base to an industrial area where Proton city is located which is rapidly developing. Logging generates pollution through soil erosion, siltation, and sedimentation in the river. Bernam River basin is characterized by high temperatures and relative humidity with small seasonal variations. The mean relative humidity is about 77% on average, while the annual mean air temperature varied from minimum to maximum temperatures which are 26 to 32 °C, respectively. Rainfall maximum flows occur during the northeast monsoon from November to January and southwest monsoon from April to September, with maximum flows occuring from October to December, whereas minimum flows occur in the February, July, and August which are inter-seasonal periods and, hence, recording the lowest rainfall. The mean annual rainfall usually ranges from 2899 mm at the upstream catchment to 1632 mm at the downstream (Jamaluddin 2014).
Sample collection and preservation
A total of 27 surface sediment (0–5 cm) samples were collected at nine sites from downstream to upstream of Bernam Rivers from January to February in 2015. Figure 1 and Table 1 show the sampling collected locations and the sampling taken from surface sediments (0–5 cm depth); the Ekman grab was washed with distilled water after collecting the sample from each station to avoid possible metal contamination from the grab. They were placed in clean labeled plastic bags and transferred to the laboratory in the icebox. Sediment samples were deep frozen (−20 °C) prior to analysis.
Sequential extraction
Chemical speciation of heavy metal in surface sediments was analyzed by using modified sequential extraction technique described by Yap et al. (2002) and Badri and Aston (1983). The procedure for this method is as follows: Step 1: easily, freely, or leachable and exchangeable (EFLE)—about 10 g of sample was continuously shaken for 3 h with 50 ml of 1.0 M ammonium acetate (NH4CH3COO), pH 7.0 at room temperature. Step 2: acid-reducible—the residue from EFLE was continuously shaken for 3 h with 50 ml of 0.25 M hydroxylammonium chloride (NH2OH·HCl) acidified to pH 2 with HCl, at room temperature. Step 3: oxidisable-organic—the residue from acid-reducible was first oxidized with 15 ml H2O2 (R&M Chemicals 35%) in a water bath at 90 °C. After cooling, the metal released from the organic complexes was continuously shaken for 3 h with 50 ml of 1.0 M ammonium acetate (NH4CH3COO) acidified to pH 2.0 with HCl, at room temperature. Step 4: resistant—the residue from oxidisable-organic was digested in a 10-ml combination (ratio of 4:1) of concentrated HNO3 and HClO4. The samples were analyzed in triplicate, and the analysis was performed using ICP-MS (Perkin Elmer Model Elan DRC-e).
Simplified bioaccessibility extraction test (SBET)
Simplified bioaccessibility extraction test is a one-step in-vitro digestion test without pepsin (Oomen et al. 2002). The procedure for this method was used to assess half gram (63 μm) air-dried sediment placed in a centrifuge tube and mixed with 50 ml of gastric solution (0.4 M glycine; pH = 1.5 pre-adjusted with concentrated HCl). The tube was rotated end-over-end at 30 rpm for 1 h at 37 °C and centrifuged at 3500 g for 15 min, and then, the extracted suspension was filtered through 0.45-μm cellulose acetate disc filter. After determination of pH, the gastric phase samples were stored in a refrigerator at 4 °C. Each sample was analyzed in triplicate, and the analysis was performed using ICP-MS (Perkin Elmer Model Elan DRC-e) within 1 week after the experiments. Relative heavy metal bioaccessibility was calculated by dividing the SBET of heavy metal concentrations by the total heavy metals:
Total heavy metal concentrations
The dried sediment samples (0.5) were digested in a 10-ml solution of a mixture HNO3 (AnalaR grade, R&M 65%) and HClO4 (AnalaR grade, R&M 70%) in the ratio of 4:1 (v/v), into a pre-heated block digester at low temperature (40 °C) for 1 h and then at 140 °C for 3 h (Ismail 1993). The digested samples were then diluted to 40 ml with double-distilled water (DDW) and then filtered through Whatman no.1 filter paper into pre-cleaned 40-ml volumetric flasks. The final samples were measured for metal concentration using an air-acetylene flame Atomic Absorption Spectrophotometer (Perkin-Elmer Model A Analyst 800). The determination of total mercury in sediment samples is carried out by using ultrasound-assisted mercury extraction, which was proposed by Collasiol et al. (2004) and Looi et al. (2014) and analyzed using FIMS-100.
Quality assurance and quality control
To prevent uncertain contaminations, all laboratory equipment used were washed with phosphate-free soap, double rinsed with distilled water, left in 10% HNO3 for 24 h, then rinsed two times with double distilled water, and left semi-closed to dry at room temperature. Certified reference material (CRM) was determined as a precision check used for sediment (PACS-2, Canada). The results for the contents of this experiment in regard to the three replicates and percentage of recoveries for the certified and measured concentration of Cd, Hg, and Sn were found with recovery percentages being about 104, 82, and 107%, respectively. The acceptance of the analytical result for the reference materials and its certified values for each metal was satisfactory. Calibration curves were prepared by making two appropriate dilutions in stock water solution (1000 mg l−1) (BDH Spectrosol®) in 2% HNO3. In addition, five standard solutions were prepared from a different stock standard source with detection limits of 0.1 μg l−. A blank calibration solution was used for a zero. The linearity for the calibration lines of multi-elemental standards shows that the correlation coefficient values were at least 0.999 during analysis and considered as satisfaction.
Physicochemical properties of sediment analysis
Measurement of pH was conducted according to McLean (1982) by using distilled water with 1:2.5 solid/liquid ratio that is 25 ml of distilled water was added to 10 g of sediment in a glass beaker, which was covered with plastic film, put in orbital shaker for 4 h at 175 rpm, and then read with a digital electrode pH meter model WTW pH 330. The organic matter was expressed as a loss on ignition (LOI) by calculating the difference between the dry weight of sediment samples before and after ashing in a muffle furnace at 550 °C for 5 h (Arain et al. 2008; Kazi et al. 2005). Sediment particle sizes were determined by pipette method (Gee and Bauder 1986). The samples of sediment were classified into sand (>50 μm), silt (2 μm <size< 64 μm), and clay (<2 μm) fraction according to the USDA particle size classification (Soil Survey Staff 1992). Cation exchange capacity (CEC) was determined by measuring the exchangeable cation displaced from sediment and treated with NaCl which was used to determine Ca2+, Mg2+, and K+, while NH4Cl was used to determine the Na+ (Apello and Postma 2005; Aris et al. 2010). The samples were analyzed for Na+, Ca2+, Mg2+, and K+ using ICP-MS (Perkin Elmer Model Elan DRC-e).
Results and discussion
Sediment properties
The soil properties, including pH, particle size, total organic matter content, and CEC, are presented in Table 2. pH and total organic matter values have been reported in the Bernam River (Safaa et al. 2015). The highest pH value was 7.50 (Kampung Bagan), but the lowest pH value was 5.91 (Kampung Tanjung). The mean TOM values were highest in station Bagan Tepi (31.47%) and lowest in station Selisek (9.63%). Organic matter levels in sediments can give a good indicator of metal bioavailability and mobility due to its great affinity for heavy metals (Hu et al. 2013). The particle size plays a vital role in controlling toxic concentrations in sediments whereby the pollutant concentrations tend to increase with declining particle size (Simpson et al. 2005). Thus, investigation of particle size is important to assess the anthropogenic influence on sediment properties. The mean percentage value of clay, silt, and sand fraction in surface sediment of Bernam River were found to range from 0.13 to 52.53%, 0.75 to 41.12%, and 6.24 to 98.39%, respectively. The sum of exchangeable cations (CEC) in the sediment ranged from 23.80 to 184.10 meq/100 g in Bernam River and the order exchangeable cations of decreased Na+ < Mg++ < Ca++ < K+.
The relationships among the examined physicochemical properties and heavy metals were tested using Spearman’s correlation analysis (Table 3). The correlation matrix revealed that total Hg with K+ in all the sampled stations of Bernam River had a negative relationship and total Hg had no significant relationship with other physicochemical properties. CEC and TOM had a strong positive correlation, and CEC in surface sediment of river could increase by increasing the TOM content (Camberato 2001; Shafie et al. 2013). Furthermore, total cadmium in Bernam River had significant relationship with TOM, Na+, Mg++, and CEC, and these physicochemical properties may contribute to increasing Cd concentration in the river. Total Cd had no significant relationship with other metals; this indicated that total Cd came from different sources such as industry effluent, agriculture activities around the rivers, and domestic sewage discharge. The correlation matrix revealed also that total Sn with total Hg in all the sampled stations of Bernam River had a negative relationship.
Total and bioaccessibility of heavy metals
The average concentrations of the total and bioaccessible Cd, Hg, and Sn from nine sampling sites are presented in Table 4. Total Cd, Hg, and Sn values have been reported in Bernam River (Safaa et al. 2015). T-Cd concentrations in the surface sediments from nine stations varied from 0.49 to 1.04 μg g−1, T-Hg concentration was in the range of 3.96 to 65.46 μg kg−1, and T-Sn varied from 106.59 to 9.2 μg g−1. Bio-Cd, Bio-Hg, and Bio-Sn concentrations in sediment varied considerably among different sampling sites; Bio-Cd, Bio-Hg, and Bio-Sn in all nine sampling stations were 0.04–0.14 μg g−1, 5.7–33.3 μg kg−1, and 0.4–2.75 μg g−1, respectively. The highest Bio-Hg and Bio-Sn concentrations were recorded in the sediment collected from Kampung Bagan station while the highest Bio-Cd value was in Kampung Tanjung station. There was an increase of Cd and Sn concentrations from upstream (Slim River) to the downstream (Kampung Bagan); the highest of Cd concentrations in these parts of the sediment profiles may indicate increased metal discharges from upstream to downstream due to settled anthropogenic sources surrounding the rivers into downstream such as local industrial, agricultural development, and oil palm plantation (Chen et al. 2007). Slim River had the lowest T-Cd whereas the lowest Bio-Cd was in Ulu Bernam, while Bio-Hg had the lowest concentrations on in Tanjong Malim station. The relative bioaccessibility of HMs (R-Bio-HMs) was defined as the percentage of bioaccessibility of metals to the total metals; the highest R-Bio-Cd among these sampling stations appeared at Bandar Behrang (39.9%), and the highest R-Bio-Sn was in Sabak Bernam (6.06%). Slim River had the very highest R-Bio-Hg (89.91%). R-Bio-Hg showed higher than R-Bio-Cd and Bio-Sn. There are limited studies determining the bioaccessibility of Cd, Hg, and Sn in sediment or soil from Southeast Asian region. Thus, we compared our results with different universal studies. Relative bioaccessibility of Cd which is showed less than the bioaccessibility of Cd (75.96%) was previously recorded from soil urban parks in Guangzhou, China (Gu et al. 2015), while our report for R-Bio-Hg was higher than R-Bio-Hg of 1.2 and 3.0% for total mercury using PBET (gastric phase and gastric + intestinal phase) methodologies, respectively in residential soils in the Flin Flon, Canada (Safruk et al. 2015). In Malaysia, only one study by Kadhum et al. (2016) reported on R-Bio-Cd and R-Bio-Hg in surface sediment of Langat River (13.47 and 42.23%, respectively). Our results on Bernam River showed that R-Bio-Hg was more than R-Bio-Hg in Langat River while R-Bio-Cd was less than R-Bio-Cd in Langat River.
Cadmium speciation
The mean Cd concentrations and percentages of four chemical speciation fractions for each sampling station are tabulated in Table 4 and Fig. 2. The EFLE fraction for Cd ranged from 0.0004 to 0.07 μg g−1 with mean percentage of 7.89%. The acid-reducible fraction ranged from 0.04 to 0.25 μg g−1 with mean percentage of 27.7%. The oxidisable-organic fraction ranged from 0.09 to 0.84 μg g−1 with mean percentage of 37.6%. The residue (resistant) fraction ranged from 0.06 to 0.41 μg g−1 with mean percentage of 26.6%. Cd contained a relatively lower EFLE fraction F1, while the rest fractions displayed greater percentages with Cd. The Cd concentration in most sampling stations was dominated by the non-resistant steps (anthropogenic). The non-resistant fractions (the sum of fractions 1, 2, and 3) are highly potentially toxic for organisms because it is easily removed and used by organisms (fraction 1). Further, fractions 2 and 3 under certain physicochemical conditions, like the presence of oxygen, variations in redox potential, and bacterial activity, can become soluble (Morillo et al. 2004; Yap et al. 2006; Dou et al. 2013). In most of sampling sites, Cd concentrations were mostly in oxidisable-organic fraction, indicating that the oxidisable-organic fraction of Cd in this study was influenced mainly by the organic contents in sediments due to Cd strong positive correlation with phosphate fertilizers and irrigation water discharge (Dou et al. 2013; Liu et al. 2012). Cd was high in non-resistant forms from the most sites, and these results are not in agreement with the literature, which indicates that Cd was mostly present in resistant forms (Sungur et al. 2014; Eyupoglu et al. 2012). However, the results are in agreement with Sungur et al. (2016) which they find that Cd was mostly present in non-resistant forms and easy mobile due to anthropogenic impacts. The percentages of each chemical form of Cd as well as the R-Bio-Cd are presented in Fig. 2. The R-Bio-Cd was evidently less than the non-resistant fractions (F1 + F2 + F3). However, most of resistant forms (F4) in all stations have notably higher content than R-Bio-Cd, indicating that Cd in sediment could be easily mobilized and become easy accessible under anthropogenic condition to aquatic biota and human consumption.
Mercury speciation
The mean concentrations and percentages of sequential extraction of Hg for each sampling station are shown in Table 4 and Fig. 3. Hg in the EFLE fraction was higher than those in the other fractions in most of sampling stations, especially in Kampung Bagan and Ulu Bernam which were approximately 77 and 80%, respectively, implying high potential toxicity for these stations. The EFLE step had high Hg bioavailability in the present study ranged from 2.5 to 18.8 μg/kg with mean percentage of 48.4%; this is due to the relationship between metal adsorption and changes in the ionic composition of the water, which is capable of altering the processes of adsorption–desorption as well as the transport of metals in the soil (Fuentes et al. 2008). Again, this can greatly affect the aquatic environment relative to the other fractions. The acid-reducible fraction ranged from 0.11 to 4.5 μg/kg with the mean percentage of 8.9%. These results are not in agreement with Parthasarathi and Raghunadh (2015) which they found that Hg content was the highest with organic phases of the sediments. The oxidisable-organic fraction ranged from 0.43 to 67.6 μg/kg with the mean percentage of 18.1%, and the residue fraction ranged from 1.0 to 23.1 μg/kg with the mean percentage of 24.3% and the low percentage of acid-reducible, oxidisable-organic, and residue fractions from all stations, suggesting poor bioavailabilities of Hg. The percentages of each chemical form of Hg as well as the R-Bio-Hg are presented in Fig. 3. The R-Bio-Hg was evidently less than the non-resistant fractions (F1 + F2 + F3) in all stations of sediment with exception of Slim River station and a little higher than resistant fractions (F4) in most stations of Bernam River, suggesting that non-resistant forms of Hg could be contributed to the bioaccessibility of T-Hg under anthropogenic conditions in the sediment of Bernam River.
Tin speciation
The distribution of Sn in the different binding phases of the sediments is shown in Table 4 and Fig. 4. The concentrations of EFLE fraction of Sn were observed to be very low bioavailable and were found to vary from 0.12 to 0.92 μg g−1 with mean percentage of 1.91%. The acid-reducible fraction ranged from 0.53 to 4.9 μg g−1 with mean percentage of 4.9%. The oxidisable-organic fraction ranged from 0.17 to 7.76 μg g−1 with mean percentage of 16.75%. Greatest amounts of Sn distribution are found in the residual fraction which ranged from 3.36 to 31.7 μg g−1 with the mean percentage of 76.3%. Tin was mostly present in non-resistant forms across all locations of Bernam River due to natural sources such as chemical weathering of igneous and metamorphic rocks, as well as decomposition of biota detritus (Badri and Aston 1983). These results are in agreement with the literature, which indicates that Sn was mostly present in resistant forms (>80%) (Hussein et al. 2014). Thus, the residual fraction is the most stable with Sn and considered to be long-term stable with a very low risk of contaminant release into the environment. The comparative percentages of each chemical form of Hg as well as the R-Bio-Hg are presented in Fig. 3. The R-Bio-Sn was evidently less than the non-resistant fractions (F1 + F2 + F3) in all stations of sediment with exception of Kampung Bagan and resistant fractions (F4) in all stations of Bernam River, suggesting that non-resistant forms of Hg could be contributed to the bioaccessibility of T-Hg under natural conditions in the sediment of Bernam River.
Principal component analysis
A principal component analysis was run on nine stations where physicochemical properties, T-Hg, T-Cd, Bio-Hg, Bio-Cd, Bio-Sn, and chemical fractions were determined. The suitability of PCA was assessed prior to the analysis. Bartlett’s test of sphericity was statistically significant (p < 0.000), indicating that the data was likely factorizable. PCA for Hg revealed four components that had eigenvalues greater than one and which explained 35.080, 24.527, 13.896, and 9.282% of the total variance, respectively (Table 5). Visual inspection of the scree plot indicated that four components should be retained. The four component solution explained 82.78% of the total variance. A varimax orthogonal rotation was employed to aid interpretability and to further identify the factors responsible for each one. PCA results from Bernam River sediment are represented by loadings and score plot, respectively. In this case, the data set relating to physicochemical properties of sand and pH, represented by PC1, explained 31.86% of the total variance (Fig. 5) and had strong loading on TOM, F1 (EFLE), CEC, and Bio-Hg indicating that F1 (EFLE), CEC, and TOM could play important role in the increasing of Bio-Hg in surface sediment due to anthropogenic activities which can be directly responsible for risks on human health. PC2 explained 20.43% of the total variance, which had strong loadings on T-Hg, silt, and F2 (acid-reducible). PC3 explained 19.02% of the total variance and had strong loadings on pH, clay, sand, and F4 (resistant). PC4 explained 11.45% of the total variance, which had only strong loadings on F4 (resistant) of the total variance.
The T-Cd, fractions, physicochemical properties, and Bio-Cd could mainly be divided into four categories. PCA for Cd revealed also four components that had eigenvalues >1 and which explained 34.693, 18.078, 13.639, and 10.601% of the total variance, respectively.
Visual inspection of the scree plot indicated that four components should be retained. The four component solution explained 77.01% of the total variance. A varimax orthogonal rotation was employed to aid interpretability and to further identify the factors responsible for each one. The PCA (rotated components) presented four PCs with eigenvalues >1, explaining 23.45% (PC1), 19.97% (PC2), 19.46% (PC3), and 12.52% (PC4) of total variance, respectively (Table 6).
To explain the patterns presented by the data, PCA is represented by loadings and score plot, respectively. In this case, the data set relating to TOM, F3 (oxidation-organic), T-Cd, F4 (resistant), and CEC represented by PC1 explained 23.4% of the total variance (Fig. 6). CEC and TOM could play important role in the increasing of T-Cd in F4 (resistant) and F3 (oxidation-organic). Therefore, it was observed that Cd indicates a tendency to accumulate in F4 and F3 along with the increase in the amount of CEC and TOM. PC2 explained 19.9% of the total variance which had strong loadings on clay, and F2 (acid-reducible) PC3 explained 19.4% of the total variance and had strong loadings on silt and sand; PC4 explained 12.5% of the total variance, which had strong loadings on Bio-Cd and F1 (EFLE), indicating that F1 (EFLE) in surface sediment had the largest and positive influence on Bio-Cd.
The T-Sn, fractions, physicochemical properties, and Bio-Sn could mainly be divided into four categories. PCA for Sn revealed four components that had eigenvalues >1 and which explained 30.184, 25.451, 16.647, and 10.500% of the total variance, respectively. Visual inspection of the scree plot indicated that three components should be retained. The four component solution explained 82.78% of the total variance. A varimax orthogonal rotation was employed to aid interpretability and to further identify the factors responsible for each one. The PCA (rotated components) presented four PCs with eigenvalues >1, explaining 23.943% (PC1), 21.637% (PC2), 18.063% (PC3), and 15.604% (PC4) of the total variance, respectively (Table 7). To explain the patterns presented by the data, PCA is represented by loadings and score plot, respectively. In this case, the data set relating to silt, sand, T-Sn, and F2 (acid-reducible), represented by PC1, explained 23.9% of the total variance represented by PC1 and explained 29.99% of the total variance (Fig. 7). It is obvious that silt and sand are responsible to increase T-Sn in acid-reducible fraction. PC2 explained 21.6% of the total variance which had strong loadings on TOM, CEC, and F1 (EFLE). TOM increases with the increasing of CEC in EFLE fraction, and this explains that CEC is entirely due to the organic colloid fractions because the presence of humic substances in these organic materials may enhance CEC in the soil (Lax et al. 1986). PC3 explained 18% of the total variance and had strong loadings on T-Sn, F4 (resistant), and Bio-Sn; it is seen that F4 (resistant) and T-Sn factors can provide significant influencing on Bio-Sn in natural form. In addition, PC4 explained 15.6% of the total variance, which had strong loadings on pH and clay.
It seems that F1 (EFLE) in surface sediment had the largest and positive influence on Bio-Hg, while F4 (resistant) had the largest and positive influence on the Bio-Sn. The bioaccessibility of Hg and Cd was generally more affected by non-resistant fractions (F1-F3) than resistant fraction (F4). In contrast, the bioaccessibility of Sn was generally more affected by resistant fraction (F4) than non-resistant fractions (F1-F3).
Bio-Hg was more affected by TOM, F1 (EFLE), and CEC of sediment. In addition, Bio-Cd was affected more by F1 (EFLE) of sediment and F4 (resistant) and T-Sn factors can provide significant influence on Bio-Sn, which represents metals largely embedded in the crystal lattice in the minerals in sediment (Ikem and Nyavor 2003). Thus, bioavailability is related to be mobilized (Luoma and Rainbow 2008; Luo et al. 2012a), suggesting that bioaccessibility of Hg and Cd in the sediment can contribute to the anthropogenic input in the sediment of Bernam River.
Conclusion
This study made use of the sequential extraction technique (SET) and simple bioaccessibility extraction test (SBET) to quantify the concentration of bioavailable metals and the human bioaccessible fractions collected from nine stations in the surface sediment of Bernam River. The Cd and Hg in the Bernam River surface sediments were greater in the non-resistant fractions (anthropogenic) than in the resistant fractions (natural origin). The relative bioaccessibility of Hg and Cd concentrations were greater than the resistant fraction concentrations, indicating that Cd and Hg were highly accessible in surface sediment of Bernam River. The principal component analysis showed that the main factors influencing the bioaccessibility of mercury in surface sediments were the sediment total organic matter, cation exchange capacity, and easily, freely, or leachable and exchangeable fraction, and the factors influencing the bioaccessibility of tin were the total tin and cation exchange capacity, while the bioaccessibility of Cd in surface sediments was influenced by the only factor which is the easily, freely, or leachable and exchangeable fraction. The results can be useful to build models in the future through incorporating these factors to predict the bioaccessibility of Cd and Hg in the surface sediment based on physicochemical properties and fractions. Therefore, these results could improve ecological risk assessment and pollution control.
References
Apello J, Postma D (2005) Geochemistry, groundwater and pollution, 2nd edn. Balkema, CRC Press, Roterdam
Arain B, Kazi G, Jamali K, Afridi I, Jalbani N, Sarfiraz A, Baig A, Kandhro A, Memon MA (2008) Time saving modified BCR sequential extraction procedure for the fraction of Cd, Cr, Cu, Ni, Pb and Zn in sediment samples of polluted lake. J Hazard Mater 160:235–239
Aris Z, Abdullah H, Praveena M, Yusoff K, Juahir H (2010) Extenuation of saline solutes in shallow aquifer of a small tropical island: a case study of Manukan Island, North Borneo. Environment Asia 3:84–92
Badri MA, Aston SR (1983) Observation on heavy metal geochemical associations in polluted and nonpolluted estuarine sediments. Environmental Pollution (Series B) 6:181–193
Calmano W, Hong J, Fo¨rstner U (1993) Binding and mobilization of heavy metals in contaminated sediments affected by pH and redox potential. Water Science Technology 28:223–235
Camberato J (2001). Cation exchange capacity—everything you want to know and much more. South Carolina Turfgrass Foundation News. Retrieved on 8 May 2011 from http://ebookbrowse.com/cation-exchange-capacity-pdf-d19788599
Camusso M, Gasparella A (2006) Measuring bioavailable trace metals from freshwater sediments by diffusive gradients in thin films (DGT) in monitoring procedures for quality assessment. Ann Chim 96:205–213
Chen W, Kao M, Chen F, Dong D (2007) Distribution and accumulation of heavy metals in the sediments of Kaohsiung Harbor, Taiwan. Chemosphere 66(8):1431–1440
Collasiol A, Pazebon D, Maia M (2004) Ultrasound assisted mercury extraction from soil and sediment. Anal Chim Acta 518:157–164
Devesa-Rey R, Paradelo R, Díaz-Fierros F, Barral MT (2008) Fractionation and bioavailability of arsenic in the bed sediments of the Anllóns River (NW Spain). Water Air Soil Pollut 195:189–199
Devesa-Rey R, Diaz-Fierros F, Barral MT (2010) Trace metals in river bed sediments: an assessment of their partitioning and bioavailability by using multivariate exploratory analysis. Journal Environment Management 91:2471–2477
Dou Y, Zhao J, Hu B, Yang S (2013) Distribution, enrichment and source of heavy metals in surface sediments of the eastern Beibu Bay, South China Sea. Mar Pollut Bull 67:137–145
Dundar MS, Altundag H, Eyupoglu V, Keskin SC, Tutunoglu C (2012) Determination of heavy metals in lower Sakarya river sediments using a BCR-sequential extraction procedure. Environ Monit Assess 184:33–41
Fuentes A, Lorens M, Saez J, Aguilar I, Ortuno F, Meseguer F (2008) Comparative study of six different sludges by sequential speciation of heavy metals. Bioresour Technol 99:517–525
Gee G, Bauder J (1986) Particle-size analysis. Madison, Wisconsin
Gu G, Lin Q, Jiang J, Wang H (2014b) Metal pollution status in Zhelin Bay surface sediments inferred from a sequential extraction technique, South China Sea. Marina Pollution Bulletin 81:256–261
Gu Y, Yan G, Qin L (2015) Contamination, bioaccessibility and human health risk of heavy metals in exposed-lawn soils from 28 urban parks in southern China’s largest city, Guangzhou. Appl Geochem 67:52–58
Hoanninen O, Knol A, Jantunen M, Lim T, Conrad A, Rappolder M, Carrer P, Fanetti A, Kim R, Buekers J, Torfs R, Iavarone I, Classen T, Hornberg C, Mekel O (2014) Environmental burden of disease in Europe: assessing nine risk factors in six countries. Environmental Health Perspective 122:439–446
Hu B, Li G, Li J, Bi J, Zhao J, Bu R (2013) Spatial distribution and ecotoxicological risk assessment of heavy metals in surface sediments of the southern Bohai Bay. Environ Sci Pollut Res 20:4099–4110
Hussein K, Okoro S, Fatoki A, Adekola J, Ximba Reinette G (2014) Fractionation, mobility and multivariate statistical evaluation of metals in marine sediments of Cape Town Harbour, South Africa. Chem Speciat Bioavailab 26(3)
Ikem A, Egiebor O, Nyavor K (2003) Trace elements in water, fish and sediment from Tuskegee Lake, Southeastern USA. Water Air Soil Pollut 149:51–75
Ismail A (1993) Heavy metal concentrations in sediments off Bintulu, Malaysia. Marine Pollution Bulletin 26:706–707
Jamaluddin I B (2014) Analysis of trend in hydrologic system for Sungai Bernam Basin. Faculty of Engineering. Universiti Putra Malaysia. Master thesis
Jinghua R, Paul N, Williams Jun L, Hongrui M, Xiaorong W (2015) Sediment metal bioavailability in Lake Taihu, China: evaluation of sequential extraction, DGT, and PBET techniques. Environmental Science Pollution Research 22:12919–12928
Kadhum AS, Mohd I, Syaizwan Z (2016) Estimation and influence of physicochemical properties and chemical fractions of surface sediment on the bioaccessibility of Cd and Hg contaminant in Langat River. Malaysia, Environment Geochemical Health, pp 1–14
Kazi G, Jamali K, Kazi H, Arain B, Afridi I, Siddiqui A (2005) Evaluating the mobility of toxic metals in untreated industrial wastewater sludge using a BCR sequential extraction procedure and a leaching test. Anal Bioanal Chem 383:297–304
Kim Y, Kim W, Lee U, Lee S, Cook J (2002) Assessment of As and heavy metal contamination in the vicinity of Duckum Au-Ag mine, Korea. Environment Geochemical Health 24:213–225
Lax A, Roig A, Costa F (1986) A method for determining the cation-exchange capacity of organic materials. Plant Soil 94(3):349–355
Li G, Zhang S, Liu Y, Wan Y, Zhang H, Chen F. (2002). Soil nutrient assessment for urban ecosystems in Hubei, China. Soil Nutrient of Urban Ecosystems. Plos (8) 9
Li N, Kang Y, Pan W, Zeng L, Zhang Q, Luo J (2015) Concentration and transportation of heavy metals in vegetables and risk assessment of human exposure to bioaccessible heavy metals in soil near a waste-incinerator site, South China. Science Total Environment 521–522:144–151
Lim WY, Aris AZ, Ismail THT, Zakaria MP (2013a) Elemental hydrochemistry assessment on its variation and quality status in Langat River, Western Peninsular Malaysia. Environmental Earth Sciences 70(3):993–1004
Liu X, Lou C, Xu L, Sun L (2012) Distribution and bioavailability of cadmium in ornithogenic coral-sand sediments of the Xisha archipelago, South China Sea. Environment Pollution 168:151–160
Looi L, Zaharin A, Yusoff F, Hashim H (2014) Mercury contamination in the estuaries and coastal sediments of the Strait of Malacca. Environment Monitoring Assessment 187:4099
Luo S, Ding J, Xu B, Wang J, Li B, Yu S (2012a) Incorporating bioaccessibility into human health risk assessments of heavy metals in urban park soils. Science Total Environment 424:88–96
Luo S, Yu S, Zhu G, Li D (2012b) Trace metal contamination in urban soils of China. Science Total Environment:421–422
Luoma N, Rainbow S (2008) Metal contamination in aquatic environments. Cambridge University Press, New York
Madrid F, Biasioli M, Ajmone-Marsan F (2008) Availability and bioaccessibility of metals in fine particles of some urban soils. Arch Environ Contam Toxicol 55:21–32
Martin R, Arana D, Ramos-Miras J, Gil C, Boluda R (2015) Impact of 70 years urban growth associated with heavy metal pollution. Environment Pollution 196:156–163
McLean O (1982) Method of soil analysis. In: Page AL et al (eds) Soil pH analysis. Madison, Wisconsin, pp 199–224
Morillo J, Usero J, Gracia I (2004) Heavy metal distribution in marine sediments from the southwest coast of Spain. Chemosphere 55:431–442
Oomen AG, Hack A, Minekus M, Zeijdner E, Cornelis C, Schoeters G, Verstraete W, Van de Wiele T, Wragg J, Rompelberg CJ, Sips AJ (2002) Comparison of five in vitro digestion models to study the bioaccessibility of soil contaminants. Environ Sci Technol 36(15):3326–34
Parthasarathi C, Raghunadh V (2015) Environmental controls on the speciation and distribution of mercury insurface sediments of a tropical estuary, India. Mar Pollut Bull 95:350–357
Ramirez M, Massolo S, Frache R, Correa JA (2005) Metal speciation and environmental impact on sandy beaches due to El Salvador copper mine, Chile. Mar Pollut Bull 50:62–72
Saeedi M, Li LY, Karbassi AR, Zanjani AJ (2013) Sorbed metals fractionation and risk assessment of release in river sediment and particulate matter. Environment Monitoring Assessessment 185:1737–1754
Safaa K, Mohd I, Syaizwan Z (2015) Evaluation and assessment of baseline metal contamination in surface sediments from the Bernam River, Malaysia. Environment Science Pollution Research 23:6312–6321
Safruk M, Robert G, Blair J, Celine P, Alan T, Elliot A (2015) The bioaccessibility of soil-based mercury as determined by physiological based extraction tests and human biomonitoring in children. Sci Total Environ 518–519:545–553
Sarmani S (1989) The determination of heavy metals in water, suspended materials and sediments from Langat River, Malaysia. Hydrobiologia 176(177):233–238
Shafie A, Ahmad A, Nadzhratul P (2013) Influential factors on the levels of cation exchange capacity in sediment at Langat river. Arab J Geosci 6(8):3049–3058
Sheng J, Fan F, Yang Y, Qi L, Xu L (2008) Distribution patterns of heavy metals in surface sediments of the Yangtze Estuary and its adjacent areas and environmental quality assessment. Environmental Science 29:2405–2412
Simpson S, Batley E, Chariton A, Stauber L, King K, Chapman J, Hyne V, Gale A, Roach A, Maher A (2005) Handbook for sediment quality assessment. CSIRO Publishing, North South Wales
Soil Survey Staff, Soil survey laboratory methods manual (1992). Ver. 2.0. USDA/NRCS, Soil Survey Investigations Report No. 42. U.S. Government Printing Office, Washington, DC
Sundaray SK, Nayak BB, Lin S, Bhatta D (2011) Geochemical speciation and risk assessment of heavy metals in the river estuarine sediments—a case study: Mahanadi basin, India. Journal Hazard Material 186:1837–1846
Sungur A, Soylak M, Yilmaz S, Özcan H (2014) Determination of heavy metals in sediments of the Ergene River by BCR sequential extraction method. Environ Earth Sci 72:3292–3305
Sungur A, Soylak M, Özcan H (2016) Chemical fractionation, mobility and environmental impacts of heavy metals in greenhouse soils from C¸ anakkale, Turkey. Environ Earth Sci 75:334
Tzoulas K, Korpela Venn KS, Yli-Pelkonen SV, Ka Z, Niemela A, James JP (2007) Promoting ecosystem and human health in urban areas using green infrastructure, a literature review. Landsc Urban Plan 81:167–178
Wan Y, Ahmad Z, Mohamad Z (2012) Spatial variability of metals in surface water and sediment in the Langat River and geochemical factors that influence their water-sediment interactions. Sci World J 14
Xiaodong Z, Fen Y, Chaoyang W (2015) Factors influencing the heavy metal bioaccessibility in soils were site dependent from different geographical locations. Environment Science Pollution Research 22:13939–13949
Xiaodong Z, Fen Y, Chaoyang W (2016) Bioaccessibility of heavy metals in soils cannot be predicted by a single model in two adjacent areas. Environment Geochemical Health 38:233–241
Yap CK, Ismail A, Tan SG, Omar H (2002) Concentrations of Cu and Pb in the offshore and intertidal sediments of the west coast of Peninsular Malaysia. Environ Int 28:467–479
Yap CK, Choh MS, Edward FB, Ismail A, Tan SG (2006) Comparison of heavy metal concentrations in surface sediment of Tajung Piai wetland with other sites receiving anthropogenic inputs along the southwestern coast of Peninsular Malaysia. Wetland Science 4(1):48–57
Acknowledgements
The authors wish to thank the Universiti Putra Malaysia for supporting this study (Vot: 9458700) by the Ministry of Higher Education Malaysia. The authors are very thankful to the chief editor and the reviewers of this article for their valuable information and contribution.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Kadhum, S.A., Ishak, M.Y., Zulkifli, S.Z. et al. Investigating geochemical factors affecting heavy metal bioaccessibility in surface sediment from Bernam River, Malaysia. Environ Sci Pollut Res 24, 12991–13003 (2017). https://doi.org/10.1007/s11356-017-8833-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-017-8833-8