Abstract
The aim of this paper was to evaluate the chemical weathering rate at Alto Sorocaba basin, São Paulo State, Brazil, as it is an important parameter on geomorphological characterization of continents. Several tools may be used for such evaluation, and this work compares the results obtained from analytical data involving the uranium concentration, the 234U/238U activity ratio and the major cation concentration for samples of waters, soils, and rocks from that basin. The use of the natural uranium as a tracer in weathering rate rocks studies is advantageous since this element is virtually absent in rainwater and also allows obtaining the dissolution rocks coefficient by the 234U/238U activity ratio. The Alto Sorocaba basin has serious environmental problems in terms of the quality of the rainwater and river waters, and, consequently, the geochemical balance using the main cations presented some difficulties. However, measuring of the weathering rate utilizing the U-isotopes method corresponded to 14 μm/year that allows estimating a time of 72,000 years to weather 1 m of rock at Sorocaba River upstream from Itupararanga Reservoir, under the present climatic conditions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The determination of weathering rate of rocks is of interest to geoscientists, as this phenomenon provides the parameters needed for a better soil exploration, assisting with the establishment of agricultural fields and human settlements. Many studies have used major element concentrations to calculate chemical weathering rates despite the difficulties to correct for their intake from rainwater (Jonhson et al. 1968; Gibbs 1970; Moreira-Nordemann 1977; Paces 1986; Clow and Drever 1996; Boeglin and Probst 1998; Grasby and Hutcheon 2000; Semhi et al. 2000; Bain et al. 2001). Some investigations for evaluating the weathering alteration front of rocks have also been carried out in South America, for instance, those held by Moreira-Nordemann (1980, 1984), Bortoletto et al. (2002), Conceição and Bonotto (2003, 2004) and Bonotto et al. (2007).
Under this aspect, major elements (for example, Na+, K+, Ca2+ and Mg2+) and radionuclides (like 238U and 234U) have been used in different geological contexts to evaluate the chemical weathering rate. The natural uranium comprises 238U, 234U, and 235U isotopes which have the same geochemical behavior and whose relative proportions, under radioactive equilibrium conditions, are 99.28, 0.0054, and 0.72%, respectively (Cowart and Osmond 1974). The 234U isotope is radiogenic and the decay chain from 238U through 234U proceeds as follows: 238U (4.46 Ga, α) → 234Th (24.1 days, β−) → 234Pa (1.18 min, α) → 234U (248 ka, α) → ….
In the alteration front, 234U is preferentially mobilized relative to 238U when rock weathers (Ivanovich and Harmon 1982). Measurements of the 234U/238U activity ratio (AR) in rocks, soils, and waters have allowed calculation of the solution coefficient for uranium characteristic of the region, which allows the evaluation of the necessary time to weather 1 m of rock under actual climatic conditions (Moreira-Nordemann 1980, 1984). The technique using the U-isotopes method was successfully used to evaluate the alteration velocity of different rock types occurring in Brazil (Moreira-Nordemann 1980, 1984; Conceição and Bonotto 2003, 2004; Bonotto et al. 2007).
This paper describes the use of the U-isotopes technique at Alto Sorocaba basin, São Paulo State, Brazil, which is an important hydrographic basin that supplies water to about 1,000,000 people in Sorocaba region, close to São Paulo city. One additional aspect concerning the basin is that it is affected by domestic and industrial wastes. This work also describes the results obtained for describing the main parameters related to the quality of rainwater and surface waters of Alto Sorocaba basin, thus demonstrating how possible anthropogenic inputs are affecting them.
General features of the studied area
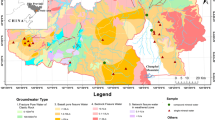
Alto Sorocaba basin is one of the six sub-basins that compose Sorocaba Médio Tietê (SMT) basin, covering an area of approximately 929 km2. It is located in the southeastern part of São Paulo State, between coordinates 23°45′37″ and 23°35′02″ latitude south and 47°21′00″ and 46°57′29″ longitude west. This basin is formed by Una, Sorocabuçu, and Sorocamirim rivers that predominantly flow in the SE-NW direction, crossing Ibiúna, Cotia, Vargem Grande Paulista, and São Roque cities and giving rise to Sorocaba River (Fig. 1).
Simplified geological map of the Alto Sorocaba basin and location of the sampling points for rocks, soils, and waters. Modified from IPT (2005). P1 and P4, Una River; P2 and P5, Sorocabuçu River; P3 and P6, Sorocamirim River; P7 and P8, Sorocaba River
The total population of Alto Sorocaba basin is 110,577 inhabitants, and Alumínio, Cotia, Ibiúna, Mairinque, Piedade, São Roque, Vargem Grande Paulista and Votorantim are the cities composing it. The urbanized area in Alto Sorocaba basin occupies approximately 71 km2, ~55 km2 being characterized by small villages where most of the population is living. With an intense agricultural activity, 393 km2 of this basin is mainly used for growing of vegetables such as cabbage, lettuce, potato, and tomatoes. The vegetal covering of the basin is composed of Dense Rain Forest and Cerrado fields which are inserted in the Atlantic Forest domain.
The Alto Sorocaba basin presents Cwb climate (Köppen classification), i.e., hot and humid climate with dry winter. The annual average temperature is generally 20°C and the evaporation is higher than precipitation in the dry season. The annual average precipitation was 1,492.7 mm between 1960 and 2004, January being the rainiest month (average of 248.1 mm) and August the driest month (average of 42.8 mm) (CBA 2006). The months with the greatest and the lowest average affluent outflow at Itupararanga Reservoir (Sorocaba River) are February (21.7 m3/s) and August (6.9 m3/s), respectively (CBA 2006). Figure 2 shows both the monthly average precipitation (a) and outflow (b) at Itupararanga Reservoir, which indicates that the Sorocaba River regime is directly bounded to rainfall, as expected.
a Monthly average precipitation (mm) and b monthly average discharge (m3/s) into Itupararanga Reservoir, Alto Sorocaba basin, in the period 1960–2004. According to CBA (2006)
The Alto Sorocaba basin comprises diverse geological structures, which formed since Medium-Upper Proterozoic until Quaternary, designated the São Roque and Embu domains (Fig. 1). The São Roque domain presents low-grade metamorphic rocks of the São Roque Group (fillites and metabasics) (Godoy et al. 1996a) and associated granitic complexes like the San Francisco (sienogranites and monzogranites) and São Roque (diorites and granodiorites) granites (Godoy 1989; Godoy and Figueiredo 1991; Hackspacher et al. 1991). Paragneisses and migmatites of the Embu Complex predominate in Embu domain, where Ibiúna (monzogranites and sienogranites) and Caucaia (monzogranites and sienogranites) granites (Godoy et al. 1996b) are also found. Concerning the dominant soil types, the Alto Sorocaba basin presents three major classes: ultisols, oxisols, and litosols (IPT 2005).
Sampling and analytical methods
River water samples for hydrochemical analyses were collected on 08/13/2006, 09/20/2006, 10/18/2006, 11/23/2006, 12/19/2006, and 01/10/2007 from eight monitoring stations in Alto Sorocaba basin, covering dry and rainy periods, i.e., Una River (P1 and P4), Sorocabuçu River (P2 and P5), Sorocamirim River (P3 and P6), and Sorocaba River (P7 and P8) (Fig. 1). The water samples from Sorocaba River (P7) were collected for U-isotope analyses in 08/13/2006 and 10/18/2006. Twenty rainwater samples were collected for analyses (“bulk” collector, wet and dry deposition), from Ibiúna city (Fig. 1), starting in March 2006 and ending in January 2007.
Each water sample was divided into three aliquots and stored in polyethylene bottles under different conditions. Raw water was used for pH, dissolved oxygen, conductivity, and dry residue measurements. Dissolved oxygen was measured in the field, whereas pH and conductivity were measured not more than 6 h after sampling. Filtered water (0.45 μm Millipore membrane) was used for alkalinity and chloride determinations. Filtered and acidified water (HNO3, pH < 2) was used for major ions and uranium determinations.
The dissolved major cations Na+ and K+ were analyzed by atomic absorption spectrometry (AAS) and Ca2+ and Mg2+ by inductively coupled plasma atomic emission spectrometry (ICP-AES). Sulfate (barium sulfate colorimetric method; range 0 to 70 ± 0.9 mg/L), phosphate (cadmium reduction method; range 0 to 3 ± 0.01 mg/L), and nitrate (cadmium reduction method; range 0 to 20 ± 0.4 mg/L) were measured by a Hach DR 2000 spectrophotometer (Hach 1992). Alkalinity was determined by titration with 0.01 M sulfuric acid (Hach 1992) in the range of 1 to 500 ± 0.2 mg/L. Chloride was evaluated by potentiometry (0.1 to 100 ± 0.02 mg/L), according to the procedure described by Tonetto (1996). Dissolved oxygen, pH, and conductivity were measured in the field by potentiometric readings.
Rock (13) and soil (3) samples were collected throughout the whole basin, taking into account the geological context, the types of rocks and their abundance or spatial distribution (Fig. 1). Samples of pulverized rock were quantified in sodium, potassium, calcium and magnesium, among others elements, through the X-ray Fluorescence Phillips spectrometer, model PW 2510.
Uranium concentration (238U) and isotopic composition (234U/238U activity ratio, AR) of water and soil samples were determined using standard alpha spectrometric techniques (Osmond and Cowart 1976; Ivanovich and Harmon 1982; Bonotto 1986), where 232U was the spike utilized. Uranium in water samples was co-precipitated with Fe(OH)3, iron extracted with isopropyl ether, and U separated from Th and other elements by the anion exchange resin. The aliquot containing U was transferred to an electrodeposition cell, and U was deposited on a stainless steel planchet after 3 h at a current density of 1 A/cm2 (Bonotto 1996). The counting of alpha activities was made with a Si(Au) surface barrier detector. The concentration of dissolved uranium was calculated by isotope dilution from the counting rates of 238U and 232U peaks, and AR was calculated from the counting rates of 238U and 234U peaks. About 1.5 g of each soil and rock (R1 and R3) sample was crushed and sieved to 200 mesh, placed in an acid digestion bomb similar to Parr 4575 at 150°C and internal pressures to 1,200 psig, and brought into complete solution with HF, HNO3, and HCl (Bonotto 1996). The solution was heated to dryness, the residue was dissolved in 8 M HCl ,and then, the procedure was the same as utilized for waters. The critical level Lc (Currie 1968) for acceptance of a positive measurement in the 238U and 234U energy region was 0.000827 and 0.00154 cpm, respectively.
Gamma spectrometry was also employed to determine the uranium concentration in rock (except R1 and R3) samples. Crushed samples were weighed, sealed in aluminum cans to prevent the escape of radon gas, and stored for a period of 30 days to establish the radioactive equilibrium in the 238U decay series (Bonotto 1996). The gamma spectrometry system consisted of a lead shield where a NaI(Tl) scintillation detector and pre-amplifier were inserted and coupled to a high-voltage power supply. The signals from the pre-amplifier were transferred to an amplifier and processed by Maestro II software from EG&G ORTEC installed on a microcomputer. The determination of the U concentration was made in accordance with the procedure described by Duarte (2002), and the results expressed in μg/g (ppm) corresponded to the equivalent uranium (eU), as read by the 1.76 MeV energy 214Bi photopeak.
Tables 1, 2, 3, and 4 present all data obtained in this investigation which are relevant for the evaluation of the weathering rates. For two soil samples, the alpha readings in 238U and 234U peaks were below the detection limit corresponding to 0.000827 and 0.00154 cpm, respectively. Thus, Table 4 shows the results obtained for the unique soil sample that yielded a positive reading.
Discussion
Chemical analyses of rocks and soils
The surface area, mineralogical composition, and concentration of the major cations (Na+, K+, Ca2+ and Mg2+) in rocks of the Alto Sorocaba basin are shown in Table 1. Thus, considering the distribution of major rock types in the basin, it is possible verify that the area is mostly covered by granites, followed by metamorphic rocks. The average values (g/kg or kg/t) from the chemical analysis indicate that the major cations are present in the area in the following order: K+ > Na+ > Ca2+ > Mg2+.
The bedrock composition is an important factor controlling the weathering processes due to the mineral constituents, texture, and structure, like also are the climate, topography, biosphere, and time. The following are some of the possible reactions involved in weathering processes occurring in the Alto Sorocaba basin (Sardinha 2008): the Na and Ca release by hydrolysis of plagioclase and hornblende; the K release by hydrolysis of biotite, muscovite, and potassium feldspar; and the Mg release by hydrolysis of biotite, muscovite, and hornblende.
The average U content in rocks ranged from 0.7 to 27.9 μg/g (mean 12.06 μg/g), and the 234U/238U activity ratio denotes that both uranium isotopes are in radioactive equilibrium, within experimental errors, which is an expected situation for rocks older than 1 million years, like those occurring in the area studied.
Despite that the samples of litosol and ultisol yielded values below the critical detection limit (Lc) (Currie 1968) for 238U and 234U, the 234U/238U activity ratio corresponding to 0.78 in the sample of oxisol (Table 4) indicates that there is a preferential leaching of 234U relative to 238U (AR < 1) in the soil matrix, suggesting the possibility of its use for estimating weathering rate by the U-isotopes method.
Hydrochemical data for rainwater
The range of the values obtained for the 20 rainwater samples collected between March 2006 and January 2007 is given in Table 2, which also presents the weighted average concentration (VMP) calculated according to the following equation:
where VMP is weighted average concentration (mg/L), C i the element concentration so far in the event i (mg/L), and V i the precipitation volume measured at the event i (mm).
Thus, Eq. 1 takes into account the amount of rain in each event of precipitation, the element concentration, and the total amount of rain. The weighted average concentration is a useful parameter for calculations involving the rainy (between October and March) and dry seasons (from April to September) in the basin, as some thunderstorms may influence the chemical composition of rainwater (Moreira-Nordemann et al. 1997).
The mean pH value (6.3) is slightly higher than the theoretical value of 5.7, reported by Berner and Berner (1996). On the other hand, Lara et al. (2001) found a pH value of 4.4 in rainwater occurring at Piracicaba River basin that is much lower than that obtained in this study.
The most abundant ions in rainwater from Rio de Janeiro were Na+ and Cl−, originating from marine aerosols (Mello 2001). The VMP values in Table 2 (in μeq/L) allow determining the following ratios: Ca2+/Na+ = 2.81, K+/Na+ = 0.29, SO4 2−/Na+ = 1.38, HCO3 −/Na+ = 0.97, and NO3 −/Na+ = 0.38. These ratios are higher than those reported by Wilson (1975) for rainwater samples collected from close to the ocean, i.e., 0.0439, 0.0218, 0.121, 0.0049, and 0.0062, respectively, suggesting a lower marine influence on the atmosphere of the basin studied. These ratios are also higher than those presented by Moreira-Nordemann et al. (1991) for the chemical composition in rainwater from São Paulo coastal area.
The VMP values indicate that the sum of cations in rainwater is greater than the anions sum, as already observed in other areas (Galloway et al. 1982; Moreira-Nordemann et al. 1997; Lara et al. 2001; Conceição and Bonotto 2004). They also show that Ca2+ (65% of the total sum of cations) and SO4 2− (44% of the total sum of anions) are the most abundant ions found in rainwater, as well indicating the following trends: Ca2+ > Na+ > K+ > Mg2+ for the cations and SO4 2− > HCO3 − > NO3 − > Cl− > PO4 3− for the anions.
Considering the seasonal variations, the VMP values found for HCO3 − and Cl− by Sardinha (2008) were higher in the dry season, suggesting that there is no significant rainwater deposition because of the dissolution of soil dust originated by weathering of primary minerals found in this basin, as observed in other watersheds (Conceição and Bonotto 2004).
Like observed elsewhere (Vieira et al. 1988; Berner and Berner 1996; Moreira-Nordemann et al. 1997; Lara et al. 2001; Pelicho et al. 2006), some possible ion sources in rainwater are dissolution of CaCO3 and silicate soil dust from terrestrial origin, anthropogenic inputs like dissolution of agricultural soil dust with fertilizer, biomass (sugar cane) burning, manufacture of building materials, mining activities exploring calcareous rocks, fossil fuel combustion and burning, vehicle emissions, incinerator exhausts, and paper producing industries.
Hydrochemical data for river waters
Table 3 reports the mean values of the main parameters analyzed in surface waters of the Alto Sorocaba basin. The lowest electrical conductivity value was obtained in Sorocabuçu River (P2), whereas the highest was found in Una River (P4), downstream from Ibiúna city, that is, a value exceeding the upper limit expected for natural waters, i.e., 100 μS/cm (Hermes and Silva 2004).
The pH values obtained in all sampling points indicate that the river waters are almost neutral. The data are within the range of the values suitable for protection of aquatic life, according to the Brazilian legislation for Class 2 waters (CETESB 2006; CONAMA 2006). In general, higher pH values were detected during the rainy season, contrarily to conductivity (Sardinha 2008). Additionally, more elevated pH values were found in Sorocaba River after Itupararanga Reservoir (P8) (Sardinha 2008), as normally occurs in reservoirs due to biological activity (Esteves 1988).
The lower dissolved oxygen concentration was found in Una River (P4) due to the organic matter decomposition related to the waste disposal at Ibiúna city. However, aerated waters exceeding the minimum value of 5 mg/L established by CONAMA (2006) for Class 2 waters takes place in P7 (Sorocaba River), due to the contribution of waters containing higher levels of dissolved oxygen from Sorocabuçu and Sorocamirim rivers. The higher dissolved O2 content in P8 (Table 3) may be related to the intense photosynthetic activities conducted by algae in Itupararanga Reservoir.
The lowest total dissolved solid (TDS) and total suspended solid (TSS) values were found in Sorocaba River after Itupararanga Reservoir (P8) (Table 3). They tended to increase in the summer, like observed for the flow rate, indicating an increased discharge during this season, like that observed elsewhere (Berner and Berner 1996; Conceição and Bonotto 2004; Conceição et al. 2007). The higher TSS values in the rainy season can be attributed to the increased drag of sediments by laminar erosion.
The trends found for cations (Ca2+ > Na+ > K+ > Mg2+) and anions (SO4 2− > HCO3 − > NO3 − > Cl− > PO4 3−) in rainwater were not generally verified in river waters of the basin mainly due to anthropogenic inputs affecting their chemical composition. The values obtained for bicarbonate, chloride, sulfate, and nitrate (Table 3) are lower than the maximum allowable concentration for class 2 waters (CONAMA 2006). The lowest value for phosphate was found in Sorocaba River after Itupararanga Reservoir (P8), but all other data indicate values exceeding the maximum (0.1 mg/L) permitted for class 2 waters (CONAMA 2006).
The uranium transport in waters occurs as uranyl ions in various soluble complexes often formed with fluoride, phosphate, bicarbonate, and carbonate, under different acidic and basic conditions (Bonotto 2004). Table 4 shows the values obtained for the dissolved uranium in waters of Sorocaba River (P7), and perhaps phosphate may be an important ligand for uranium due to its high dissolved content (2.1 mg/L). The 234U/238U activity ratio exceeding unity in river water (P7) is a consequence of the typical 234U enhancement in the liquid phase due to water–rock/soil interactions (Osmond and Cowart 1976). The ratios reported in Table 4 for Sorocaba River (P7) are like the values obtained from soil samples useful for estimating weathering rates by the U-isotopes method.
The sampling point P7 in Sorocaba River is suitable for focusing calculations of weathering rates in the basin as it receives waters from others tributaries (Una, Sorocamirim and Sorocabuçu rivers). The chemical analyses of water samples collected between August 2006 and January 2007 in this site allowed to identify significant relationships among several parameters, i.e., pH, calcium, sodium, potassium, magnesium, bicarbonate, and TDS (Fig. 3). The pH raise is directly related to the increase of Ca, Na, K, Mg, HCO3 −, and TDS, as expected, and such chemical composition is influenced by geogenic and anthropogenic inputs taking place in the basin.
The relationships among pH, bicarbonate, sodium, potassium, calcium, magnesium, and TDS (total dissolved solids) in water samples collected between August 2006 and January 2007 at Sorocaba River (sampling point P7). According to Spearman’s test values of correlation coefficient r > 0.754 and 0.875 correspond to significance level P = 0.05 and 0.01, respectively
Equations adopted for estimating weathering rates
Garrels and Mackenzie (1967) modeled the abundance and relative proportions of dissolved ions in surface waters, using a mass balance equation. However, because the element/compound entries needed corrections in order to get the fraction from rock dissolution, White and Blum (1995) summarized the chemical dynamics occurring in a river through the following equation:
where F W is the element annual flow in waters of the river (kg/year), F RV the quantified element flow in waters of the river (kg/year), F RW the atmospheric inputs due to wet and dry deposition (kg/year), F IE the dissolved solids from ion exchange on clay minerals (kg/year), F B the dissolved solids due to changes in biomass (kg/year), and F AT the anthropogenic inputs (kg/year).
Some corrections due to anthropogenic inputs from different tributaries may be roughly realized considering another mass balance equation that takes into account the Sorocaba River before its entry into Itupararanga Reservoir (P7) and also its tributaries (Una, Sorocamirim, and Sorocabuçu rivers), i.e.,
where Q RS is the sum of tributaries’ flow (m3/s), C RS the corrected concentration in Sorocaba River (mg/L), Q RU the flow of Una River (m3/s), C RU the concentration of element in Una River (mg/L), Q RSM the flow of Sorocamirim River (m3/s), C RSM the concentration of element in Sorocamirim River (mg/L), Q RSU the flow of Sorocabuçu River (m3/s), and C RSU the concentration of element in Sorocabuçu River (mg/L).
The quantity of removed weathered matter per unit of surface and per unit of time, W (t/km2 year), is determined as follows (Moreira-Nordemann 1980, 1984):
where F W is the element annual flow in river waters (kg/year), E R is the mean element concentration in rocks (kg/t), and S is the surface area of the basin (km2).
The weathering rate of the rocks (v) [LT−1] depends on the rock density (ρ) (kg/m3) and of the dissolution coefficient (k) (dimensionless) of the elements partly soluble during weathering, according to the following expression:
where E E is the mean element concentration in river waters (mg/L) and D is the river discharge (m3/s).
For uranium, the solubility coefficient may be expressed by (Moreira-Nordemann 1980, 1984)
where A R, A E, and A S are the mean 234U/238U activity ratio in rocks, waters, and soils, respectively.
Weathering rates in the basin
In order to estimate chemical weathering fluxes affecting the drainage basins, models utilizing the Na, Ca, K, Mg, and total dissolved load concentrations have been applied to obtain fractions coming from dissolved rocks, after corrections for river waters of the atmospheric inputs, dissolved solids from ion exchange sites in clays minerals, dissolved solids due to changes in biomass, and anthropogenic inputs, temperature, among other factors (Paces 1986; White and Blum 1995; Grasby and Hutcheon 2000; Duan et al. 2002; Millot et al. 2002; Conceição and Bonotto 2004).
The monitoring stations P1, P2, and P3 suffer lower impacts than P4, P5, and P6 due to anthropogenic activities; thus, their chemical data will be initially considered for estimating the Ca, Mg, Na, and K removal in the Alto Sorocaba basin. The mean concentration of these cations in P7 (Table 3) is reported in Table 5, since this sampling point corresponds to the mouth of the basin. The corrected values, given in Table 5, were estimated from Eq. 3 applied to data in Table 3 for Sorocaba River tributaries (P1 Una River, P2 Sorocabuçu River, P3 Sorocamirim River). The most significant difference between mean value and corrected mean value is for the concentration of Na+ ions, 19.2 and 5.8 mg/L, respectively. This is because the mean value includes the sodium contribution from Una River at the monitoring station P4 (67 mg/L), located downstream from Ibiúna city and subjected to its waste disposal. On the other hand, the corrected mean value only considers the monitoring stations suffering lower impacts due to anthropogenic activities (P1 at Una River, but not P4).
The weighted average concentration for Ca2+, Mg2+, Na+, and K+ in rainwater from Alto Sorocaba basin (Table 2) was used for estimating the annual deposition, considering the rainfall between January and December 2006, i.e., 1558.6 mm. Since 1 mm = 1 L/m2, the values shown in Table 2 allow estimating the atmospheric inputs due to wet and dry deposition (F RW) in t/km2 year, as reported in Table 5.
The annual flow of Ca2+, Mg2+, Na+, and K+ in surface waters of Sorocaba River may be calculated using Eq. 2. The dissolved solids from ionic exchange in clay minerals and the dissolved solids due to changes in biomass will not be considered in the calculations, as also performed elsewhere (for instance, White and Blum 1995; Conceição and Bonotto 2004). The anthropogenic inputs, F AT in Eq. 2, are roughly taken into account from the corrected mean element concentration values reported in Table 5 for the surface waters at P7. Thus, the average flow in P7 (12.62 m3/s) and surface area upstream from Itupararanga Reservoir (670.4 km2) allow estimating the annual flow of elements (in t/km2 year) in surface waters of Sorocaba River, which is expressed as F RV in Table 5.
The net annual flow (F w) in the basin may be evaluated on subtracting F RV of the rainwater contribution, F RW. The F w values (in t/km2 year) are also reported in Table 5, where a negative value was found for calcium, which indicates that the element deposition in rainwater is greater than its removal by the superficial waters. One possible explanation for the large Ca2+ inputs in rainfall is the anthropogenic activities occurring in the basin like the intense mining of calcareous rocks and presence of industries producing cement, which cause the release to the atmosphere of particulate matter enriched in CaMg(CO3)2 (Sardinha 2008).
The positive F w values given in Table 5 can be used to estimate the amount of material removed per unit area and time (W, in t/km2 year). The average element concentration in rocks of the basin upstream from Itupararanga Reservoir (Table 5) allows utilizing Eq. 4 to estimate W values corresponding to 120 t/km2 year for magnesium, 82 t/km2 year for sodium, and 32 t/km2 year for potassium.
The average U content in Sorocaba River (P7) was calculated from samples collected in August and October 2006, and corresponded to 0.53 μg/L (=0.00053 mg/L) (Table 5). The average discharge in this period was 9.03 m3/s (Sardinha 2008) and since the average U content in rocks is 12.06 μg/g (=0.01206 kg/t), it is possible to estimate 18.49 t/km2 year as the total amount of material removed in the basin, that is lower than the values obtained from Mg2+, Na+, and K+ data. No corrections for the U presence in rainwater were realized as this element is expected to occur in very low concentrations, like that observed elsewhere (Moreira-Nordemann 1980, 1984).
The solubility coefficient in the basin from U-isotopes may be estimated through Eq. 6 and the following values: A R = 1.0 (mean value from data in Table 1), A S = 0.78 (Table 4) and A E = 1.21 (mean value from data in Table 4). Thus, k = 0.51, meaning that 51% of the uranium in rocks is carried out by the Sorocaba River (P7) in soluble form. The weathering rate (v) can be evaluated from Eq. 5, utilizing 2.6 g/cm3 as a representative value for the rock density in Alto Sorocaba basin. Therefore, the chemical weathering rate in Sorocaba River (P7) upstream from Itupararanga Reservoir is 0.014 mm/year (Table 5), implying that it would take approximately 72,000 years to weather 1 m of rock under the present climatic conditions. The uncertainty/reliability of this value is coupled to the analytical uncertainty of ±10% corresponding to 1σ standard deviation and also to the development of further studies in order to improve the uranium database in rocks, soils, and waters of the studied area.
The weathering rate (v) cannot be estimated from Na+, K+, and Mg2+ data like realized considering the uranium values, due to the lack of the dissolution coefficient k for rocks related to these elements. However, a rough way to obtain v from them could be to compare the values obtained for the amount of material removed (W, in t/km2 year) as reported in Table 5. Thus, the following rates would be obtained: 0.062 mm/year for Na+ (weathering of 1 m of rock in ~16,000 years), 0.024 mm/year for K+ (weathering of 1 m of rock in ~42,000 years), and 0.090 mm/year for Mg2+ (weathering of 1 m of rock in ~11,000 years).
The corrected mean element concentration values reported in Table 5 for the surface waters at P7 may not be reasonable enough to represent the natural conditions associated with the weathering processes involving the bedrock composition. For instance, let us consider K that is an element often utilized as fertilizer in agricultural activities. Table 5 reports a value of 3.7 mg/L for its corrected mean concentration, which may also be including some K fertilizer-derived. A lower value of 2 mg/L could be representative of the “non-anthropogenic” potassium. Under this assumption, it would be 1.19 t/km2 year (K flow in surface waters of Sorocaba River) and 3.4 t/km2 year (total amount of material removed per unit area and time in the basin). Such amount could imply a weathering rate of 0.0026 mm/year (weathering of 1 m of rock in ~385,000 years), that is, ~9 times lower than the previous estimate.
These data suggest that the evaluation of the chemical weathering rates based on uranium as a tracer is more appropriate, since it appears to be closer of the natural conditions. For instance, Bonotto et al. (2007) also utilized the U-isotopes method in Morro do Ferro, Poços de Caldas plateau, Minas Gerais State, Brazil, and determined a weathering rate of 0.015 mm/year (67,000 years to weather 1 m of rock) that is much similar to the value found in this investigation. It is also very reasonable if compared with the range of 0.015–0.05 mm/year based on land surface lowering within the entire Poços de Caldas caldera (Holmes et al. 1992). The weathering rate estimated by the U-isotopes method in this paper is as well compatible with that corresponding to 0.013 mm/year determined by Moreira-Nordemann (1984) for the Salgado River basin in a semi-arid region in Bahia State, Brazil. The major ions Na, K, Ca, and Mg are very susceptible to anthropogenic inputs occurring in Alto Sorocaba basin, and the use of the uranium concentration and 234U/238U activity ratio in the calculations eliminates certain premises that are required by the other method, among them the subtraction of inputs due to the presence of elements/compounds in rainwater.
Since it has been often pointed out that the bedrock, climate, topography, biosphere, and time are the major factors controlling the weathering processes, different scenarios in the future may be expected if the climate changes are taken into account. Because the global warming implies temperature rising, the weathering rates probably would increase due to the enhancement of the silicate dissolution during the water–rock interactions (Paces 1986; Faure 1998), but to an extent that is difficult to properly evaluate in the present days.
Finally, several studies have been recently realized to determine the parameters controlling the denudation, chemical weathering, and physical erosion under different climatic conditions. For instance, cosmic-ray-produced radionuclides (like 7Be, 10Be and 26Al) coupled to geochemical mass balance methods have been used for investigating long-term denudation/erosion/weathering processes affecting hydrographic basins. Thus, further studies integrating different techniques can provide new insights into this important subject.
Conclusion
In managing our river basin systems increasing use is being made of information generated from studies made for understanding the controls on chemical weathering. This is a consequence of its importance in affecting the evolution of the Earth’s surface, shaping landscapes, determining nutrient supply to ecosystems, and regulating global chemical cycles, thus, implying on the present/future design of the hydrographic basins. The interaction among rainwaters, the atmosphere, and the continental crust controls the chemical evolution of the Earth’s surface, and, consequently, of the hydrographic basins. Thus, of late, many studies have tried to determine the parameters controlling the denudation, chemical weathering, and physical erosion under different climatic conditions. In this investigation, chemical weathering rates were determined by different approaches in Alto Sorocaba basin, São Paulo State, Brazil. Among the major cations, it was not possible to utilize calcium as a natural tracer in the basin since its deposition in rainwater is greater than its removal by the superficial waters. One possible explanation for the large Ca2+ inputs in rainfall are the anthropogenic activities occurring in the basin, like the intense mining of calcareous rocks and presence of industries producing cement. The adoption of simplified assumptions allowed the use of Na, K, and Mg as natural tracers for estimating the amount of material removed per unit area and time. Thus, it was possible to obtain the following values: 120 t/km2 year for Mg, 82 t/km2 year for Na, and 32 t/km2 year for K. The U-isotopes method was successfully utilized to evaluate the chemical weathering rate, yielding a value of 0.014 mm/year from the total amount of material removed in the basin (18.49 t/km2 year). The estimate based on the use of uranium as tracer appeared to be closer of the natural weathering conditions, being much similar to the value found in another Brazilian geological context, i.e., the alkaline massif in Poços de Caldas plateau in Minas Gerais State.
References
Bain DC, Roe MJ, Duthie DML, Thomson CM (2001) The influence of mineralogy on weathering rates and processes in an acid-sensitive granitic catchment. Appl Geochem 16:931–937
Berner EK, Berner RA (1996) Global environment. Water, air and geochemical cycles. Prentice Hall, Upper Saddle River, p 367
Boeglin JL, Probst JL (1998) Physical and chemical weathering rates and CO2 consumption in a tropical lateritic environment: the upper Niger basin. Chem Geol 148:137–156
Bonotto DM (1986) Hydrogeochemical applications of natural isotopes from U (4n + 2) and Tn (4n) decay series at Morro do Ferro, Poços de Caldas (MG). Ph.D. thesis, USP-Universidade de São Paulo, São Paulo, 378 pp
Bonotto DM (1996) Hydrogeochemical behavior of 222Rn and uranium isotopes 238U and 234U under laboratory controlled conditions and in natural systems. Post Ph.D. thesis, UNESP-Universidade Estadual Paulista Júlio de Mesquita Filho, Rio Claro, 203 pp
Bonotto DM (2004) Radioactivity in waters: from England to Guarani. In: UNESP (ed), São Paulo, 251 pp
Bonotto DM, Fujimori K, Moreira-Nordemann LM (2007) Determination of weathering rate of the Morro do Ferro Th-REEs deposit, Brazil using U-isotope method. Appl Radiat Isot 65:474–481
Bortoletto MJ, Mortatti J, Probst JL (2002) Chemical erosion in Corumbataí River (SP) hydrographic basin. Geochim Brasil 16:99–111
CBA (Brazilian Company of Aluminum) (2006) Discharge and rainfall data at Itupararanga Reservoir meteorological station. CBA, Votorantim, São Paulo
CETESB (Technological Company for Environmental Sanitation) (2006) Rule 8468 published in 8th September 1976 for the Prevention and Control of Pollution in Environment. http://www.cetesb.sp.gov.br/Institucional/portugues_leis_dec.asp
Clow DW, Drever JI (1996) Weathering rates as function of flow through an alpine soil. Appl Geochem 132:131–141
CONAMA (National Council of Environment) (2006) Rule 357 published in 17th March 2005. http://www.mma.gov.br/port/conama/legiano
Conceição FT, Bonotto DM (2003) Use of U-isotopes disequilibrium to evaluate the weathering rate and fertilizer-derived uranium at São Paulo State. Environ Geol 44:408–418
Conceição FT, Bonotto DM (2004) Weathering rates and anthropogenic influences in a sedimentary basin, São Paulo State. Brazil Appl Geochem 19:575–591
Conceição FT, Sardinha DS, Souza ADG, Bonotto DM (2007) Hydrochemical relationships at Meio Stream Watershed, São Paulo State, Brazil. Rev Brasileira Geociências 37:389–400
Cowart JB, Osmond JK (1974) 234U and 238U in the Carrizo sandstone aquifer of south Texas. In: IAEA (International Atomic Energy Agency) (ed) Isotope Techniques in groundwater hydrology II. IAEA, Vienna, pp 131–149
Currie LA (1968) Limits for Quantitative Detection and Quantitative Determination. Anal Chem 40:586–593
Duan L, Hao J, Xie S, Zhou Z, Ye X (2002) Determining weathering rates of soils in China. Geoderma 110(3–4):205–225
Duarte CR (2002) Natural radioelements in the area of Rio Preto (GO) project. Ph.D. thesis, UNESP-Universidade Estadual Paulista Júlio de Mesquita Filho, Rio Claro, 169 pp
Esteves FA (1988) Fundamental limnology. Interciência, Rio de Janeiro, p 602
Faure G (1998) Principles and applications of geochemistry, 2nd edn. Prentice-Hall, Upper Saddle River, NJ, p 600
Galloway JN, Likens GE, Keene WC, Miller JM (1982) The composition of precipitation in remote areas of the world. J Geophys Res 87:8771–8786
Garrels RM, Mackenzie FT (1967) Origin of the chemical composition of some springs and lakes. In: ACS (American Chemical Society) (ed) Equilibrium concepts in natural waters systems, Advances in Chemistry Series. ACS, Washington, pp 1–67
Gibbs RJ (1970) Mechanisms controlling world water chemistry. Science 170:1088–1090
Godoy AM (1989) Characterization of the faciology, petrography and geochemistry of Sorocaba and São Francisco (SP) massifs. Ph.D. thesis, USP-Universidade de São Paulo, 221 pp
Godoy AM, Figueiredo MCH (1991) Characterization of the faciology and petrography of Sorocaba (SP) granitoid massif. In: SBG (Brazilian Society of Geology) Proc. Symp. Southeast Geology, São Paulo, pp 131-138
Godoy AM, Oliveira MAF, Hackspacher PC (1996a) Geochemistry of granitoids rocks in São Roque sheet. Geociências 15:67–88
Godoy AM, Hackspacher PC, Oliveira MAF (1996b) Geology of Sorocaba region. Geociências 15:89–110
Grasby SE, Hutcheon I (2000) Chemical dynamics and weathering rates of a carbonate basin Bow River, southern Alberta. Appl Geochem 15:67–77
Hach (1992) Water Analysis Handbook, 2nd edn. Hach Company, Loveland, Colorado, p 831
Hackspacher PC, Oliveira MAF, Godoy AM (1991) Control of the metamorphic level and disposition of the granitoids bodies by Dn+3/transcurrent tectonics in Cabreúva sheet. In: SBG (Brazilian Society of Geology) Proceedings of Symposium on Southeast Geology, São Paulo, pp 151–154
Hermes LC, Silva AS (2004) Evaluation of the water quality: practical manual. Embrapa, Brasília, p 55
Holmes DC, Pitty AE, Noy DJ (1992) Geomorphological and hydrogeological features of the Poços de Caldas caldera analogue study sites. J Geochem Explor 45:215–247
IPT (Technological Research Institute of São Paulo State) (2005) Report of the situation of the hydrological resources at the Sorocaba and Médio Tietê basin (Report Zero) as an aid to the elaboration of the basin plan. IPT, São Paulo, Tech. Rep. 80401-205, 420 pp
Ivanovich M, Harmon RS (1982) Uranium Series Disequilibrium: Applications to Environmental Problems. Oxford University Press, Oxford, p 571
Jonhson NM, Likens GE, Bormann FH, Pierce PS (1968) Rate of chemical weathering of silicate minerals in New Hampshire. Geochim Cosmochim Acta 32:531–545
Lara LBLS, Artaxo P, Martinelli LA, Victoria RL, Camargo PB, Krusche A, Ayers GP, Ferraz ESB, Ballester MV (2001) Chemical composition of rainwater and anthropogenic influences in the Piracicaba River basin, Southeast Brazil. Atmos Environ 35:4937–4945
Mello WZ (2001) Precipitation chemistry in the coast of the metropolitan region of Rio de Janeiro. Brazil. Environ. Pollut. 114:235–242
Millot R, Gaillardet J, Dupré B, Allègre CJ (2002) The global control of silicate weathering rates and the coupling with physical erosion: new insights from rivers of the Canadian Shield. Earth Planet Sci Lett 196:83–98
Moreira-Nordemann LM (1977) Study of the alteration velocity of rocks by the use of uranium as a natural tracer: application to two basins from northeast Brazil. Ph.D. thesis, University of Paris VI, 162 pp
Moreira-Nordemann LM (1980) Use of 234U/238U disequilibrium in measuring chemical weathering rate of rocks. Geochim Cosmochim Acta 44:103–108
Moreira-Nordemann LM (1984) Salinity and weathering rate of rocks in a semi-arid region. J Hydrol 71:131–147
Moreira-Nordemann LM, Danelon OM, Magalhães AL, Ferreira C, Panitz CMN, Santiago MM, Silva Filho E, Souza CF (1991) Environmental impacts in rainfall at the brazilian coast. In: SBGq (Brazilian Society of Geochemistry) Proceedings of 3rd Brazilian Congress of Geochemistry, São Paulo, vol 1, pp 406–409
Moreira-Nordemann LM, Girard P, Poppi N (1997) Chemistry of the atmospheric precipitation in Campo Grande city (MS). Rev Bras Geofis 15:35–44
Osmond JK, Cowart JB (1976) The theory and uses of natural uranium isotopic variations in hydrology. At. Energy. Rev. 14:621–679
Paces T (1986) Rate of chemical weathering in small drainage basins. In: Colman SM, Dethier DP (eds) Rates of chemical weathering of rocks and minerals. Academic Press, Orlando, pp 531–550
Pelicho AF, Martins LD, Nomi SN, Solci MC (2006) Integrated and sequential bulk and wet-only samplings of atmospheric precipitation in Londrina, South Brazil (1998–2002). Atmos Environ 40:6827–6835
Sardinha DS (2008) Evaluation of the chemical weathering and anthropogenic influences in Alto Sorocaba (SP) basin. Ms. Dissertation, UNESP-Universidade Estadual Paulista Júlio de Mesquita Filho, Rio Claro, 103 pp
Semhi K, Suchet PA, Clauer N, Probst JL (2000) Impact of nitrogen fertilizers on the natural weathering-erosion process and fluvial transport in the Garonne basin. Appl Geochem 15:865–878
Tonetto EM (1996) Thorium in groundwaters from Águas da Prata (SP). Ms. Dissertation, UNESP-Universidade Estadual Paulista Júlio de Mesquita Filho, Rio Claro, 121 pp
Vieira BM, Forti MC, Tavares MFM, Moreira-Nordemann LM (1988) Atmospheric Na, Ca, K, Mg and Cl at eastern region in São Paulo State. Rev Bras Geofis 6(1–2):17–23
White AF, Blum AE (1995) Effects of climate on chemical weathering in watersheds. Geochim Cosmochim Acta 59:1729–1747
Wilson TRS (1975) Salinity and the major elements of sea water. In: Riley JP, G Skirrow (eds) Chemical oceanography, vol 1, 2nd edn. Academic Press, Orlando, pp 365–413
Acknowledgments
FAPESP (Foundation Supporting Research in São Paulo State) in Brazil is thanked for financial support for this investigation (Process No. 06/54884-3). Two anonymous referees are greatly thanked for helpful comments that improved the readability of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sardinha, D.S., Bonotto, D.M. & da Conceição, F.T. Weathering rates at Alto Sorocaba basin, Brazil, using U-isotopes and major cations. Environ Earth Sci 61, 1025–1036 (2010). https://doi.org/10.1007/s12665-009-0424-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12665-009-0424-7