Abstract
The U-disequilibrium method was utilized to evaluate the velocity of alteration of rocks and fertilizer-derived uranium in the Corumbataí River basin, São Paulo state, Brazil. The Corumbataí River basin is affected by the continuous use of fertilizer-derived uranium utilized in sugar cane crops, increasing the dissolved uranium concentration in the Corumbataí River (Santa Terezinha station) in the wet period to 43%. The weathering rate in the Corumbataí River basin utilizing the U-isotope modeling was 0.0265 mm/year (corresponding to 38,000 years to weather 1 m of rock under actual climatic conditions). However, when the inputs of anthropogenic uranium were considered, then a weathering rate of 0.022 mm/year (corresponding to 45,500 years to weather 1 m of rock) was determined. The removed material in the Corumbataí River basin is mainly from two sub-basins (the Cabeças River and Passa Cinco River), where the sandstones weather easier than the siltstones and claystones in the basin.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The determination of the weathering rate of rocks is of interest to geoscientists, as this phenomenon provides the parameters needed for a better soil exploration, assisting with the establishment of agricultural fields and human settlements. Many studies have used major element concentrations (sodium, calcium, potassium and magnesium) to evaluate the chemical weathering rate (Johnson and others 1968; Gibbs 1970; Moreira-Nordemann 1977; Paces 1986; Koptsik and others 1999; Land and others 1999), despite the difficulties of correcting their intake from rainwater. This research evaluated the chemical weathering rate of rocks using 238U as a natural trace element and the assumption that it is not contained in the rain, following the approach by Moreira-Nordemann (1977).
The natural uranium comprises the isotopes 238U, 234U and 235U, which have the same geochemical behaviour and whose relative proportions, under radioactive equilibrium conditions, are 99.28, 0.0054 and 0.72%, respectively (Cowart and Osmond 1974). U-234 is radiogenic, being generated in the U-238 decay series after one alpha decay and two beta decays, i.e. 234Th (24.1 days) and 234Pa (1.18 min). In the alteration front, 234U is preferentially mobilized to 238U when rock weathers (Ivanovich and Harmon 1992). Measurements of the 234U/238U activity ratio (AR) in rocks, soils and waters have allowed the calculation of the solution coefficient for the uranium characteristic of the region, which allows the evaluation of the time necessary to weather 1 m of rock under actual climatic conditions (Moreira-Nordemann 1980, 1984).
This study reports on the use of U-isotope modeling to evaluate the weathering rate, where the 238U concentration and AR were determined for surface waters, rocks and soils of the Corumbataí River basin, an important sedimentary basin in São Paulo state, Brazil. This basin was chosen because of its unique aspect of comprising all stratigraphic units from the giant Paraná sedimentary basin (Paleozoic–Cenozoic), which covered 70% of the whole São Paulo state. This state produces much of the Brazilian sugar cane, and, consequently, a large quantity of fertilizers is used in agricultural activities. Applications of chemical fertilizers increase the phosphate and uranium concentrations in soils, resulting in impacts on natural systems, such as increasing nutrient and uranium concentration in surface waters, soils and sediments. An additional aspect is that the Corumbataí River basin is populated and affected by domestic and industrial wastes, as well as by agricultural processes and wastes derived from sugar cane crops. Herewith, this work also describes the results obtained in evaluating the fertilizer-derived uranium in surface waters of the Corumbataí River basin, with the purpose of identifying how possible anthropogenic inputs are affecting the dissolved 238U and AR data, as the U-isotope modeling depends on these parameters.
Theoretical background
The U-isotopes method to determine the weathering rate of rocks was first applied in Preto and Salgado River basins (Moreira-Nordemann 1980, 1984). The quantity of removed weathered matter, W (ton km−2 year−1), per unit of surface and per unit of time is determined as follows:
where E E is the weighted average U concentration in river water; E R is mean concentration of U in rocks; D is mean discharge of the river; and S is surface of the basin.
The weathering rate of rocks in the basin (v) depends on the density (ρ) of rocks and of the coefficient of dissolution (k) of the elements partly soluble during weathering, according to the following formula:
where v is in cm/year, E E μg/L, E R μg/g, D L/year, ρ g/m3 and S cm2.
For uranium, the solubility coefficient may be expressed by:
where A R , A E and A S are the mean 234U/238U ratios in rocks, waters and soils, respectively.
Physiographic features
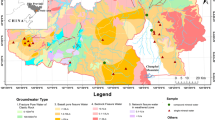
The Corumbataí River basin extends over an area of about 1,581 km2 in the middle-east part of the São Paulo state (Fig. 1). It occurs as an eroded belt in the cuestas zone of the Depressão Periférica geomorphological province (Penteado 1976). Such a province delimits the northeastern edge of the basaltic flows in the Paraná sedimentary basin and the crystalline plateau, having been submitted to smoothing processes during past geological time.
The Corumbataí River basin and location of sampling points. [Modified from Centro de Análise e Planejamento Ambiental (2001)]
The Corumbataí River basin is a sub-basin of the giant Paraná sedimentary basin which extends over an area of 1,700,000 km2 (1,000,000 km2 in Brazilian surface) (França and Potter 1988). Several stratigraphic units of the Paraná basin (Paleozoic–Cenozoic) crop-out in it [IPT (Technological Research Institute of São Paulo State) 1981]: the Tubarão Group comprising the Itararé Sub-group (sandstones, conglomerates, diamictites, tillites, siltstones, shales and rythmites) and the Tatuí Formation (siltstones, shales, silex and sandstones with local concretions); the Passa Dois Group comprising the Irati Formation (siltstones, mudstones, black betuminous shales and limestones) and the Corumbataí Formation (mudstones, shales and siltstones); the São Bento Group comprising the Pirambóia Formation (sandstones, shales and muddy sandstones), Botucatu Formation (sandstones and muddy sandstones), Serra Geral Formation (basalts and diabases) and related basic intrusives; and different types of Cenozoic covers such as recent deposits, terrace sediments and the Rio Claro Formation (sandstones, conglomerate sandstones and muddy sandstones). Among the major types of soils occurring in the Corumbataí River basin, yellow-red podzols and latossols cover about 65% of the area of the basin (Köffler 1993).
The Corumbataí River flows from the cuestas zone to the confluence with the Piracicaba River. Rio Claro city is the most important municipality in the basin, with 170,000 inhabitants. Monthly measurements of the flow rate over the last 26 years were performed at Santa Terezinha, close to the confluence with the Piracicaba River (Fig. 1). The flow rate frequency distribution (Fig. 2) indicates that 37.4% of the observed values are between 10 and 20 m3 s−1. The average monthly flow is 26.4 m3 s−1, maximum value 168.4 m3 s−1 (February 1995) and minimum value 6.0 m3 s−1 (September 1994) (Conceição 2000).
The tropical climate of the region is characterized by a wet summer (October to March) and dry winter (April to September) (Inácio and Santos 1988). The area often has 55–65 days of rain per year, with more than 80% of the precipitation falling between October to March (Bonotto and Mancini 1992). The mean annual rainfall corresponds to 1,572 mm (Conceição 2000). Figure 3 shows the average monthly rainfall during the last 21 years and the average monthly flow rate in the last 26 years at the Corumbataí River; as expected, flow in the river is directly bounded to rainfall.
Sampling methods
Six water samples from the Corumbataí River at Santa Terezinha station (Fig. 1) were collected for hydrochemical and U-isotope analyses (Table 1) on a monthly basis between 08/13/1998 and 01/20/1999. Water samples from the Cabeças River and Claro stream were collected for U-isotope analyses in 08/13/1998. Rock and soil samples were collected throughout the whole basin, taking into account the geological context, the kinds of rocks and their abundance or spatial representativity (Fig. 1; Tables 2 and 3).
Each water sample was divided into three aliquots and stored in polyethylene bottles under different conditions. Raw water was used for pH, dissolved oxygen, conductivity and dry residue measurements. Dissolved oxygen was measured in the field, whereas pH and conductivity were measured not more than 6 h after sampling. Filtered water (0.45-μm Millipore membrane) was used for alkalinity and chloride determinations. Filtered and acidified water (HNO3, pH <2) was used for major ions and uranium determinations.
The dissolved major cations Na and K were analysed by atomic absorption spectrometry (AAS) and Ca and Mg by inductively coupled plasma atomic emission spectrometry (ICP-AES). Sulfate (barium sulfate colorimetric method; range: 0 to 70 ±0.9 mg/L), phosphate (cadmium reduction method; range: 0 to 3 ±0.01 mg/L) and nitrate (cadmium reduction method; range: 0 to 20 ±0.4 mg/L) were measured by a Hach DR 2000 spectrophotometer (Hach 1992). Alkalinity was measured by titration with 0.02 N sulfuric acid (Hach 1992) in the range of 1 to 500 ±0.2 mg/L. Chloride was evaluated by potentiometry (0.1 to 100 ±0.02 mg/L), according to the procedure described by Tonetto (1996). Dissolved oxygen was measured in the field, whereas pH and conductivity were measured not more than 6 h after sampling.
Uranium concentration and isotopic composition of all samples were determined using standard alpha spectrometric techniques (Osmond and Cowart 1976; Bonotto 1986; Ivanovich and Harmon 1992), where 232U was the spike utilized. About 1.5 g of each rock and soil sample was crushed to 200 mesh (0.074 mm), placed in an acid digestion bomb similar to Parr 4575 at 150 °C and under internal pressures of 1,200 psig (84.37 kg cm−2), and brought into complete solution with HF, HNO3 and HCl (Bonotto 1996). The solution was heated to dryness, the residue was dissolved in 8 M HCl, and then the procedure was the same as utilized for waters. Uranium was co-precipitated with Fe(OH)3, iron was extracted with isopropyl ether and U was separated from Th and other elements by the anion exchange resin. The aliquot containing U was transferred to an electrode position cell, and U was deposited on a stainless-steel planchet after 3 h at a current density of 1 A cm−2 (Bonotto 1996). The counting of alpha activities was with an Si(Au) surface barrier detector. The concentration of dissolved uranium was calculated by isotope dilution from the counting rates of 238U and 232U peaks, and AR was calculated from the counting rates of 238U and 234U peaks.
Results
Hydrochemistry
Table 1 shows the physical and chemical parameters for the six sampled waters of Corumbataí River (Santa Terezinha station). The pH values obtained during the 6 months of sampling are close to neutral. No significant relationship was found between the conductivity and dry residue because raw water was analyzed and the dry residue reflected the presence of suspended matter. When the chemical data are plotted on a standard Piper (1944) diagram (Fig. 4), it is possible to verify a variable water chemistry during the sampling period, primarily caused by variable Ca2+ and HCO3 −-SO4 2− contents.
Chemical data for waters from the Corumbataí River basin (Santa Terezinha station) plotted on a standard Piper (1944) diagram
According to CONAMA [CONAMA (National Council for Environment) 1986), the results obtained for dissolved oxygen and major anions suggest that the analysed waters belong to class 2 (fresh waters with salinity equal or lower than 0.50o/oo, which are appropriate to domestic users after conventional treatment). However, in terms of phosphate, all samples exhibited values higher than the maximum permissible concentration limit for fresh waters belonging to class 2 (0.025 mg/L). Data for dissolved nitrate (NO3 −) generally clustered near the maximum permissible concentration limit of 10 mg/L, despite the limited availability of natural phosphorus and nitrogen from sedimentary rocks in the basin. Excess nutrients from fertilizer have been suggested to be the major factor responsible for increased eutrophication of reservoirs or stagnant waters elsewhere (Tundisi 1986).
Uranium in water
Table 1 reports the uranium concentration and AR data for all samples collected at Corumbataí River (Santa Terezinha station). The mean weighted uranium concentration was calculated by the following formula:
where Ui is uranium concentration for the ith measurement and Vi is flow of the river on the day for the ith measurement.
The mean uranium concentration of the river water is 0.25 μg/L, which increased progressively until January 1999. The average weighted 234U/238U activity ratio for the same waters corresponded to 1.82, suggesting the occurrence of preferential leaching of 234U relative to 238U, a process extensively discussed by Dooley and others (1966).
Uranium in rocks
Table 2 contains the uranium concentration and AR in rocks of the Corumbataí River basin, which were sampled taking into account the spatial representativity of the various formations present in the area. The uranium concentration in the analyzed rocks is dependent on the lithology, so that siltstones and claystones (Corumbataí, Tatuí and Irati Formations) have higher uranium concentration than sandstones (Pirambóia and Botucatu Formations), which is compatible with other reported values (Ivanovich and Harmon 1992). The weighted mean U concentration is 8.86 μg/g, whereas the mean weighted AR is 1.08, indicating near secular equilibrium between 238U and 234U isotopes at least over the last 1 million years.
Uranium in soils
Table 3 shows the uranium concentration and AR in the C horizon samples of the four soil profiles. The mean U concentration is 7.60 μg/g, where the lower value was found for the soil cover developed over the Pirambóia Formation. The mean 234U/238U activity ratio corresponded to 0.84, and indicated the preferential leaching of 234U relative to 238U, a typical result for soil/rocks subjected to recent weathering (Dooley and others 1966).
Weathering rate of the Corumbataí River basin
Table 4 summarizes the parameters necessary to perform the calculations at Corumbataí River basin. For this region, the determined k coefficient is equal to 0.245, implying that 24.5% of the available uranium in rocks is taken into solution during the weathering process. Using all the data given in Table 4 and applying the described formula, it is possible to obtain a value of 14.93 ton km-2 year−1 for W, which yields 0.0265 mm/year for v. These results show that one vertical meter of rock needs 38,000 years to be chemically weathered under present climatic conditions.
Discussion
The phosphate effect on U release
Palma-Silva (1999) monitored dissolved phosphate at the Corumbataí River basin and identified a large increase downstream from Rio Claro city, which was attributed to residential and industrial wastes discharged in the Rio Claro urban area. However, the total coliform data did not confirm this because a non-significant correlation was found with the phosphate data. Ferreira and others (1997) reported radiometric anomalies (238U, 232Th and 40K) in soils developed on basic rocks (diabase sills) in the heavily cultivated Araras region (near the Corumbataí River basin). These soils are known to normally contain low concentration of radionuclides and the radiometric anomalies were attributed to impurities in fertilizers. Long-continued application of U-bearing fertilizers can elevate the uranium concentration in fertilized soil profiles (Rothbaum and others 1979) and in irrigation runoff/drainage from fertilized lands (Spalding and Sackett 1972).
Sugar cane is extensively cultivated in the Corumbataí River basin by industries that produce alcohol and sugar. Fertilizers are widely used in this type of agriculture, particularly in tropical regions, where weathering of soil nutrients is more intense. The phosphate rocks contain uranium (Altschuler and others 1958), which is incorporated in calcium phosphate minerals during their original deposition (Guzman 1992). The original uranium is largely retained in superphosphate and phosphoric acid during the manufacture of fertilizers produced from phosphate rocks (Guimond and Windham 1975; Roessler and others 1979). Most of the fertilizers used in the Corumbataí River basin come from the Araxá region (Minas Gerais), where the phosphate rocks typically have a U content of 180 ppm, whereas simple or concentrated (triple) superphosphates reach up to 90–100 ppm (Paschoa and others 1984). They are of the NPK 5:25:25 type, i.e. 5% of nitrogen, 25% of phosphate and 25% of potassium (Fertiza 1986), exhibiting a U concentration of 47.7 ppm and AR of 1.00 ±0.10 (Table 5).
Pfister and others (1976) reported that the uranium does not accumulate in soils, and that it is lost by leaching. Guimond (1978) considered that uranium is lost by erosion of the sediments together with the phosphate. Uranium is soluble under oxidizing conditions as uranyl ion, and in soils with low organic matter it can have the mobility equivalent to that of phosphate (Rothbaum and others 1979). Agricultural practices can elevate uranium concentration in shallow soils and co-existing water (Zielinski and others 1995), but in another study Zielinski and others (1997) attributed minimal impact of fertilizer-U compared to natural uranium leached from the local soils.
The dissolved uranium concentration at Santa Terezinha station increased progressively from August 1998 (0.05 μg/L) to January 1999 (0.48 μg/L). Except for November 1998, the discharge of the Corumbataí River basin at the same site also increased continuously from 13.33 to 41.71 m3 s−1. The same occurred with dissolved phosphate, which increased from 0.15 to 2.72 mg/L. The monthly variation of these parameters from August 1998 to January 1999 is shown in Fig. 5. Thus, a significant positive correlation (r=0.96) exists between the uranium concentration and the river discharge. The most important uranyl complexes are formed with fluoride, phosphate and carbonate under acid, near-neutral and alkaline conditions, respectively (Langmuir 1978). Among these anions, there was a significant correlation only between dissolved uranium and phosphate (r=0.84) (Fig. 5), implying that the uranyl ions must be forming complexes with phosphate, a situation favored by their near-neutral conditions.
Thus, these data suggest that the presence of dissolved phosphate affects the migration of uranium into the waters of the Corumbataí River basin, and another way to confirm this is to use two different multivariate statistical approaches. The first methodology is the cluster analysis (CA) in r-mode (results cluster variables) which was performed using Ward's hierarchical agglomerative method (unweighted pair-group method, UPGM) and the squared Euclidean distance measured. CA was applied to the ranked data, with Table 6 presenting the dissimilarity among variables and Fig. 6 the obtained dendrogram. The results indicate that dissolved uranium and phosphate have the smaller dissimilarity (2.55), exhibiting a similar behavior. The second statistical treatment applied to the same ranked data set was the principal component analysis (PCA), which is an ordination method utilizing values and vectors from a variance–covariance matrix. The obtained results plotted in Fig. 7 indicate that dissolved uranium and phosphate have the same ordination and, therefore, a similar behavior. Thus, these two multivariate statistical approaches are also compatible with the evaluation performed by the use of the statistical correlation tests.
These results reinforce what was suggested by Conceição and Bonotto (2000), indicating that the Corumbataí River can be receiving from laminar erosion (of the acid soils in the basin with low organic matter) large quantities of radionuclides, which are associated with phosphate fertilizers extensively used in agrichemical activities. In general, the cultivation of sugar cane starts in September/October, when about 28.5–30.5 g/m2 of fertilizers is applied (Cotia Agricultural Cooperative 1991) and, in many areas, an additional 12.2 g/m2 of superphosphate is applied. The higher concentrations of dissolved uranium and phosphate in the Corumbataí River (Santa Terezinha station) are more pronounced during wet periods when natural and fertilizer-derived U are more easily released from the soil cover. The presence of uranium and its radioactive daughters in added fertilizer may enhance the natural gamma radiation emitted by the soils (Menzel 1968; Mortvedt 1986; Guimond and Hardin 1989; Todorovsky and Kulev 1993).
Fertilizer-derived U and the natural weathering rate
Non-natural inputs of uranium isotopes 238U and 234U in the drainage due to agrichemical activities can affect the modeling of weathering rates, because the equations used in the calculations are dependent on the concentration and 234U/238U activity ratio of dissolved uranium. The preferential release of 234U to the liquid phase is sustained during water–rock/soil/fertilizer interactions, and, thus, the AR values used in the calculation are not greatly affected by a fertilizer component. In contrast, the calculations are very sensitive to the value of the dissolved uranium concentration.
To properly correct such influence, it is necessary to know the amount of dissolved U from laminar erosion of soils enriched with phosphate fertilizers. It is expected there will be little influence of fertilizer-derived U during the dry season due to the poor U-leaching from soils. Thus, utilizing a weighted AR of 2.08, it is possible to obtain k=0.194 for the period of August to November 1998, implying that 19.4% of available uranium in rocks is taken into solution during the weathering process. Because waters collected during the rainy season are certainly affected by fertilizer-derived U, higher k values are generated from dissolved U data for this period, i.e. 0.276 (December 1998) and 0.342 (January 1999), indicating that 70 and 57% of dissolved U is naturally originated in December 1998 and January 1999, respectively. Consequently, the fertilizer-derived U corresponds to 30 and 43%, respectively, in December 1998 and January 1999. Therefore, the natural dissolved U content should be 0.19 and 0.27 μg L−1 during December 1998 and January 1999, respectively (Table 1).
The AR value of waters collected in the rainy season is also expected to be affected by fertilizer-derived U (Zielinski and others 2000), and if the equilibrium value of 1.00 ±0.10 found for commercial phosphate fertilizer is taken into account (Table 5), then lower AR values should be obtained for samples collected during the rainy season, which effectively happens (see Table 1). Thus, one mass-balance equation can be written describing the complementary nature of the two phases (natural water and fertilizer-derived water):
where U M is uranium in mixed water; U F is uranium in fertilizer-derived water; U N is uranium in natural water; A M is 234U/238U in mixed water; A F is 234U/238U in fertilizer-derived water; and A N is 234U/238U in natural water.
The use of Eq. (5) allows the estimation of 2.01 and 1.96 as the natural AR, respectively, in December 1998 and January 1999 (Table 1). After evaluating the fertilizer effects on the calculations, it is possible to use the weighted mean uranium concentration and AR values from the whole period (Table 1), i.e. U E =0.17 μg L−1 instead of 0.25 μg L−1 and A E =2.02 instead of 1.82. Now, the quantity of removed material in the Corumbataí River basin corresponds to 10.12 ton km−2 year−1, having a coefficient k of 0.204, which yields a velocity of alteration v of 0.022 mm/year, which implies that one vertical meter of rock needs 45,500 years to be weathered under present climatic conditions.
These results clearly show that the evaluation of the weathering rate based on the U-isotope disequilibrium method depends on any factor affecting the dissolved U concentration. The "fertilizer corrected" evaluation of the velocity of alteration (0.022 mm/year) is 55% lower than that reported by Moreira-Nordemann (1977, 1980) for the rocks of the Preto River basin, Brazil (0.04 mm/year). The Corumbataí River basin is large, contains many inhabitants, and exhibits several environmental problems related to residual, agricultural and industrial wastes; in contrast, the basin studied by Moreira-Nordemann (1977, 1980) is smaller and comparatively undeveloped. The rocks of the Preto River basin are metamorphic, more susceptible to erosion than the sedimentary rocks of the Corumbataí River basin (in general, metamorphic rocks have minerals that weather more easily than sedimentary rocks). Another dominant factor justifying the different values is the great climatic variability of the two basins.
Weathering rate at two sub-basins
To investigate the effect of lithology and topography on weathering rates, two sub-basins of the Corumbataí River basin were chosen: Cabeças River and Claro stream. The Cabeças River sub-basin consists mainly of sandstones and the Claro stream sub-basin is composed predominantly of siltstones, basic rocks and claystones (Fig. 1).
Table 7 reports the parameters necessary to obtain the weathering rates, i.e. area and discharge of each sub-basin, as well as the dissolved U content and AR during August 1998, which was the month less affected by fertilizers. The Cabeças River sub-basin has a smaller area and discharge than the Claro stream sub-basin. An inverse relationship is indicated between dissolved uranium concentration and AR. The uranium concentration and AR in rocks of the two sub-basins also must be known, and the weighted mean values in Table 8 were evaluated by graphically estimating the percentage of area covered by each formation in each sub-basin, and using uranium concentration for each formation from Table 2.
The calculated quantity of removed weathered material from the Claro stream sub-basin is 1.05 ton km−2 year−1 and from the Cabeças River sub-basin is 16.8 ton km−2 year−1. Utilizing these values and considering that sandstones covered 63% of the basin, it is possible to estimate the removal of 10.86 ton km−2 year−1 for the whole basin, which practically coincides with the "fertilizer corrected" evaluation (10.12 ton km−2 year−1). Equation (3) generated values of k=0.08 and 0.324 for the Ribeirão Claro stream and Cabeças River sub-basins, respectively, implying that 8 and 32.4% of the uranium is taken into solution during the respective weathering processes. The velocity of weathering of the rocks determined by Eq. (2) yielded 0.0058 mm/year for the Claro stream sub-basin and 0.023 mm/year for the Cabeças River sub-basin. Therefore 172,000 and 40,000 years are necessary to weather 1 m of rock in present climatic conditions, respectively, at the Claro stream and Cabeças River sub-basins.
Wedepohl (1969), Gabelman (1977) and Krauskopf (1979) report that normally the uranium concentration is greater in siltstones and claystones than in sandstones. A higher mean weighted value of uranium concentration (9.99 μg/g) was found for rocks of the Claro stream sub-basin, consisting predominantly of siltstones and claystones. These rock types are less susceptible to weathering and leaching than coarse-grained sandstones of the Cabeças River sub-basin and this contributes to generating a lower value of dissolved uranium (Table 6) and coefficient of dissolution (k=0.08).
The Claro stream sub-basin has clayey-dominated soils, low average slope (6–12%), much protection due to the contour levels of the cultivated areas, and middle susceptibility to erosion (Bacci 1994), as confirmed by the weathering rate obtained in this paper. The Cabeças River sub-basin has more superficial sandy soils, higher slope (12–20%), not much protection due to the pasture, and high susceptibility to erosion (Bacci 1994), as also confirmed by the weathering rate obtained in this investigation. The sub-basin calculations indicate that much more material is supplied from the Cabeças River than from the Claro stream sub-basin. The Passa Cinco River sub-basin (Fig. 1) was not studied but has similar geology and similar susceptibility to erosion as that of the Cabeças River sub-basin.
Conclusions
The weathering rate of the rocks in the Corumbataí River basin, São Paulo state, Brazil, was estimated using the method of U-isotope disequilibrium. A value of 0.0265 mm/year indicated that it would take 38,000 years to weather 1 m of rock under present climatic conditions. Significant correlations between uranium and phosphate (r=0.84), discharge and phosphate (r=0.94) and discharge and uranium (r=0.96), as well as multivariate statistical approaches, suggested that the presence of dissolved phosphate may be linked to the release of uranium in the basin. Fertilizers containing uranium and other impurities are used in the Corumbataí River basin in support of the important sugar cane agriculture. Estimates of the weathering rate that are based on assumed natural abundances of dissolved uranium may be affected by anthropogenic inputs of fertilizer-derived uranium, which may reach 43% in the wet period. After corrections, the weathering rate corresponded to 0.022 mm/year (45,500 years to weather 1 m of rock), demonstrating that the evaluation of the weathering rate utilizing the U-isotope disequilibrium method is affected by environmental factors acting in the Corumbataí River basin. The methodology was also useful for indicating relative differences in susceptibilities to erosion, related to different lithologies and topography.
References
Altschuler ZS, Clarke RS Jr, Young EJ (1958) Geochemistry of uranium in apatite and phosphorite. US Geol Surv Prof Pap 314D:45–90
Bacci D de La C (1994) Sand extraction in Corumbataí River Basin (SP). Ms Diss, IGCE, UNESP, Rio Claro
Bonotto DM (1986) Hydrogeochemical applications of natural isotopes from U (4n+2) and Th (4n) series in Morro do Ferro, Poços de Caldas (MG). PhD Thesis, Instituto Astronômico e Geofisico, Universidade de São Paulo
Bonotto DM (1996) Hydrogeochemical behavior of 222Rn and uranium isotopes 238U and 234U under controlled conditions in the laboratory and in natural systems. Post PhD Thesis, IGCE, UNESP, Rio Claro
Bonotto DM, Mancini LH (1992) Hydrochemical and isotopic evaluation in aquifers from Rio Claro (SP). Geochim Brasil 6(2):153–167
Centro de Análise e Planejamento Ambiental (2001) Environmental atlas of Corumbataí River basin. http://www.rc.unesp.br/igce/ceapla/atlas/index.html
CONAMA (National Council for Environment) (1986) CONAMA resolution no. 20. National register for freshwaters, 4th edn.. Instituto Brasileiro do Meio Ambiente e dos Recursos Naturais Renováveis, Brasília
Conceição FT (2000) The U-isotopes disequilibrium method applied to the weathering evaluation at Corumbataí River basin (SP). Ms Diss, IGCE, UNESP, Rio Claro
Conceição FT, Bonotto DM (2000) Anthropogenic influences on the uranium concentration in waters of the Corumbataí River basin (SP), Brazil. Rev Bras Geociências 30:551–553
Cotia Agricultural Cooperative (1991) Compendium for fertilization and neutralization. CAC, São Paulo
Cowart JB, Osmond JK (1974) 234U and 238U in the Carrizo sandstone aquifer of south Texas. In: Isotope techniques in groundwater hydrology. Publ 2. International Atomic Energy Agency, Vienna, pp 131–149
Dooley JR, Granger HC, Rosholt JN (1966) Uranium-234 fractionation in sandstones-type uranium deposits of the Ambrosia Lake District, New Mexico. Econ Geol 61:1362–1382
Ferreira FJF, Souza JL, Rocha HO, Mantovani LE (1997) Airborne gamma-ray spectrometry and remote sensing to map uranium accumulation in soils from long-continued application of fertilizers in Araras region, Brazil. In: Proc 12th Int Conf on Applied Geologic Remote Sensing, Publ 1, pp 223–330
Fertiza (1986) Fertilization compendium. Fertiza (Fertilizers National Company), São Paulo
França AB, Potter PE (1988) Stratigraphy, depositional environment and reservoir analysis from Itararé Group, Paraná basin (part 1). Petrobrás Geosci Bull 2(2/4):147–192
Gabelman J (1977) Migration of U and Th exploration significance. Study in geology no 3. American Association of Petroleum Geologists, Cincinnati, Ohio
Gibbs RJ (1970) Mechanisms controlling world water chemistry. Science 170:1088–1090
Guimond RJ (1978) The radiological aspects of fertilizer utilization. Radioactivity in consumer products. US Nuclear Regulatory Commission, Washington, pp 380–392
Guimond RJ, Hardin JM (1989) Radioactivity released from phosphate-containing fertilizers and from gypsum. Radioat Phys Chem 34(2):309–315
Guimond RJ, Windham ST (1975) Radioactivity distribution in phosphate products, by-products, effluents and wastes. Tech Note ORP/CSD-75-3. US Environmental Protection Agency, Office of Radiation Programs, Washington, DC
Guzman ETR (1992) Recovery of uranium in phosphate rocks and their products. Monograph (Bachelor in Chemistry), Universidad Autónoma del Estado de México, Toluca
Hach (1992) Water analysis handbook, 2nd edn. Hach Company, Loveland, Colorado
Inácio A, Santos MJZ (1988) Rio Claro climatic characteristics. Theor Geogr Bull 18(35–36):87–104
IPT (Technological Research Institute of São Paulo State) (1981) Geological map from São Paulo state. Monographs. IPT, São Paulo
Ivanovich M, Harmon RS (1992) Uranium series disequilibrium: applications to environmental problems, 2nd edn. Oxford University Press, Oxford
Johnson NM, Likens GE, Borman FH (1968) Rate of chemical weathering of silicates minerals in New Hampshire. Geochim Cosmochim Acta 32:531–545
Köffler NF (1993) Diagnosis of the agricultural use of soils from Corumbataí River basin, SP. IGCE, UNESP, Rio Claro
Koptsik G, Teveldal S, Aamlid D, Venn K (1999) Calculations of weathering rate and soil solution chemistry for forest soils in the Norwegian–Russian border area with PROFILE model. Appl Geochem 14:173–185
Krauskopf KB (1979) Introduction to geochemistry, 2nd edn. McGraw Hill, New York
Land M, Johan I, Öhland B (1999) Past and present weathering rates in northern Sweden. Appl Geochem 14:761–774
Langmuir D (1978) Uranium solution-mineral equilibria at low temperatures with applications to sedimentary ore deposits. Geochem Cosmochim Acta 42:547–569
Menzel RG (1968) Uranium, radium and thorium content in phosphate rocks and their possible radiation hazard. J Agric Food Chem 16:231–284
Moreira-Nordemann LM (1977) Evaluation of the alteration velocity of rocks by uranium as a natural tracer: application to two basins of northeast Brazil. PhD Thesis, University of Paris VI
Moreira-Nordemann LM (1980) Use of 234U/238U disequilibrium in measuring chemical weathering rate of rocks. Geochim Cosmochim Acta 44:103–108
Moreira-Nordemann LM (1984) Salinity and weathering rate of rocks in a semi-arid region. J Hydrol 71:131–147
Mortvedt JJ (1986) Effects of calcium silicate slag application on radium-226 concentrations in plant tissues. Commun Soil Sci Plant Anal 17:75–84
Osmond JK, Cowart JB (1976) The theory and uses of natural uranium isotopic variations in hydrology. Atom Energy Rev 14:621–679
Paces T (1986) Rate of chemical weathering in small drainage basins. In: Coleman S, Dethier D (eds) Rates of chemical weathering of rocks and minerals. Academic Press, New York, pp 531–550
Palma-Silva GM (1999) Environmental diagnosis, water quality and purification index of the Corumbataí River—SP. Ms Diss, CEA, UNESP, Rio Claro
Paschoa AS, Mafra OY, Cardoso DO, Rocha ACS (1984) Applications of SSNTD to the Brazilian phosphate fertilizer industry to determine uranium concentrations. Nucl Tracks Radiat Meas 8(1–4):469–472
Penteado MM (1976) Geomorphology of the central-occidental sector from "Depressão Periférica" in São Paulo state. Thesis and Monogr Ser 22. Faculdade de Filosofia, Letras e Ciências Humanas, Universidade de São Paulo
Pfister RJ, Philipp G, Pauly H (1976) Population dose from natural radionuclides in phosphate fertilizers. Radiat Environ Biophys 13:247–261
Piper AM (1944) A graphic procedure in the geochemical interpretation of water-analyses. Trans Am Geophys Union 25:914–928
Roessler CE, Smith ZA, Bolch WE, Prince RJ (1979) Uranium and radium-226 in Florida phosphate materials. Health Phys 37:269–277
Rothbaum HP, Mc'Gaveston DA, Wall T, Johnston AE, Mattingly GEG (1979) Uranium accumulation in soils from long-continued applications of superphosphate. J Soil Sci 30:147–153
Spalding RF, Sackett WM (1972) Uranium runoff from the Gulf of Mexico distributive province: anomalous concentrations. Science 175:629–631
Todorovsky D, Kulev I (1993) On the uranium content in some technogenic products: potential environmental pollutants. J Radioanal Nucl Chem 5:404–413
Tonetto EM (1996) The thorium in groundwaters from Águas da Prata (SP). Ms Diss, IGCE, UNESP, Rio Claro
Tundisi JG (1986) Environment and dams. Ciênc Hoje 5(27):48–55
Wedepohl KH (1969) Handbook of geochemistry. Springer, Berlin Heidelberg New York
Zielinski RA, Asher-Bolinder S, Meier AL (1995) Uraniferous waters of the Arkansas River valley, Colorado, USA: a function of geology and land use. Appl Geochem 10:133–144
Zielinski RA, Asher-Bolinder S, Meier AL, Jonhson CA, Szabo B (1997) Natural or fertilizer-derived uranium in irrigation drainage: a case study in southeastern Colorado, USA. Appl Geochem 12:9–21
Zielinski RA, Simmons KR, Oerm WH (2000) Use of 234U and 238U isotopes to identify fertilizer-derived uranium in the Florida Everglades. Appl Geochem 15:369–383
Acknowledgements
This investigation was performed under a scholarship from FAPESP-Brazil (Process no. 98/02217-5). The authors especially thank Dr. Jairo Roberto Jimenez-Rueda, Dr. Lycia Maria Moreira-Nordemann and Dr. Jorge Luis Nepomuceno de Lima for their general help.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Conceição, F.T., Bonotto, D.M. Use of U-isotope disequilibrium to evaluate the weathering rate and fertilizer-derived uranium in São Paulo state, Brazil. Env Geol 44, 408–418 (2003). https://doi.org/10.1007/s00254-003-0775-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00254-003-0775-4