Abstract
The aqueous mobility and potential bioavailability of metals and metalloids in road runoff in a ‘wet–dry’ tropical location were assessed by analysing metal and metalloid concentrations in particulate, total dissolved and labile dissolved phases in runoff waters. Road-derived Al, Cu, Pb, Sb and Zn concentrations were substantially elevated in runoff when compared to receiving creek waters. Median dissolved concentrations in road runoff exceeded those in creek waters by up to an order of magnitude. Leaching experiments of road sediments confirmed that several metals and metalloids were released in high concentrations from road sediments. Labile Zn and Cu concentrations measured by diffusion gradients in thin films (DGT) showed that almost all dissolved Zn and up to half of dissolved Cu in runoff waters and in road sediment leachate were potentially bioavailable. Comparisons of dissolved metal concentrations in receiving waters affected by road runoff with ecosystem guideline levels, indicated a risk of reaching toxic levels of Cu and Zn in the receiving waters in the absence of adequate treatment or dilution. Low dilution rates of road runoff are likely to occur during late ‘dry’ season/early ‘wet’ season storms which have the potential to produce high metal concentrations derived from long periods of accumulation of road sediment at a time when creek flow rates are at their annual minimum.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Traffic on roads represents a diffuse source of trace metal emission throughout the world, and investigations have demonstrated that roadside soils and road sediments are important sinks for trace metals (e.g. Westerlund et al. 2003; Morcelli et al. 2005; Westerlund and Viklander 2006; Hjortenkrans et al. 2006). The trace metals hosted by road sediments are commonly mobilised from road surfaces and flushed into the stormwater drainage network during runoff events (e.g. Estebe et al. 1998; Bäckström et al. 2003; Lee et al. 2005; Brown and Peake 2006; Han et al. 2006a; Desta et al. 2007; Flint and Davis 2007; Eriksson et al. 2007). The dissolved fraction of trace metals mobilised from road surfaces likely contains a high proportion of the most labile and potentially bioavailable metal fraction (e.g. Hallberg et al. 2007). As bioavailable metals and other traffic-derived contaminants can impact on ecosystem health, the treatment of road runoff waters is invariably pursued.
The need for, or adequacy of, runoff treatment is increasingly judged by comparing the dissolved metal levels in receiving waters affected by runoff waters to background metal levels in the receiving environment and/or to water quality standards. With the growing understanding of the role of chemical speciation in determining bioavailability and toxicity of metals (e.g. Campbell 1995), metal speciation data in road runoff waters should become increasingly important. However, the measurement of metal speciation and metal bioavailability in runoff waters is complicated by a number of factors. Firstly, analysis and characterisation of episodic road runoff water flows, in particular ‘first-flush’ runoff waters, are inherently difficult (e.g. Mangani et al. 2005; Kim et al. 2005a, b; Han et al. 2006a, b; Kayhanian et al. 2007). Secondly, chemical exchange most likely occurs between runoff waters and the road surface and suspended sediment during the transit of runoff waters into the receiving environment. Such ongoing reactions imply that instantaneous speciation measurements, even if practical, would be of little value in understanding the potential impact on receiving waters. Thus, methods capable of time-integrating measurement of metal speciation may be more appropriate. Diffusion gradients in thin films (DGT) (Davison and Zhang 1994; DGT Research 2008) are one of a few time-integrating techniques for measuring metal speciation in environmental waters (Batley et al. 2004). DGTs are diffusion controlling resin-gel devices that bind only free ions and relatively small labile complexes, which represent bioavailable metal species (Zhang and Davison 2000). The unique advantage of DGTs in most applications lays in the combination of their operational simplicity and low cost as well as their ability to be deployed in situ to provide time-integrated multi-element measurements. However, with respect to analysis of episodic runoff waters, a difficulty with DGTs is that they require constant submergence, an ionic strength >0.2 mmol l−1 (Alfaro-De la Torre et al. 2000; Peters et al. 2003; DGT Research 2008) and relatively long deployment times (several hours) to reach detection limits in the low- to sub-μg/L range.
This research investigated the aqueous mobility and potential bioavailability of trace metals in road runoff in a climatically ‘wet–dry’ tropical location in northern Australia. Specifically, the objectives were: (1) to examine trace metal concentrations in particulate, (total) dissolved and labile dissolved phases in road runoff waters from the Kuranda Range Road; (2) to assess the future need for treatment of road runoff in connection with a planned major upgrade of the Kuranda Range Road; and (3) to investigate the use of the DGT technique to assess potential bioavailability of metals in road runoff. Although DGTs have previously been used to study the metal toxicity of sewage treatment waters (Buzier et al. 2006), there are no published works on the use of DGTs to understand metal bioavailability in road runoff waters.
Site description, sampling and analysis
Study site
This study focused on the Kuranda Range Road, which traverses the Wet Tropics World Heritage Area of north Queensland, Australia (16o50′S, 145o40′E), approximately 15 km northwest of Cairns (Fig. 1). Altitudes within the research area range from near sea level up to 610 m above sea level. The region has a tropical monsoonal climate, with distinct ‘wet’ and ‘dry’ seasons. Approximately 80% of the annual rainfall occurs between November and April. The average annual rainfall in the area is approximately 2,000 mm (Cairns Airport; Bureau of Meteorology 2008) and rainfall intensity can be high during storms events (100 mm/h over a 10 min duration has a 1-year average recurrence interval, Bureau of Meteorology 2009b).
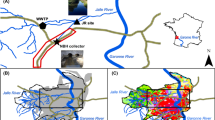
Sketch map of the Kuranda Range Road study area. Road runoff sampling was conducted at the Streets Creek test site; creek water sampling occurred at both the Streets Creek site and the Avondale Creek site. The highlands rise to approximately 600 m above sea level in the western part of the area shown
The Kuranda Range Road carries an average daily traffic (ADT) volume of approximately 7,000 vehicles on two single lanes; the course is convolute and has an average grade of 6%. The surface comprises basalt aggregate and bitumen, and runoff waters enter local catchments via concrete gutters, swales and overland flows. The Kuranda Range Road crosses two main creek systems: Streets Creek and Avondale Creek which drain approximately 1.6 and 1.9 km2, respectively, of mostly undisturbed rainforest. Creek flow rates range from >100 L/s during prolonged monsoonal rainfall evens to almost no flow during late ‘dry’ season conditions.
Road runoff samples were collected from a pilot runoff treatment facility where the Kuranda Range Road crosses Streets Creek. A concrete gully was used to collect road runoff from a 30 m long by 6.6 m wide section of road (Fig. 2). Although direct runoff to Streets Creek from the present road is relatively insignificant (<5 L/s at the test site), an assessment was required of the potential impacts of a much higher runoff rate from a planned bridge with approximately 4,000 m2 of road surface area.
Kuranda Range Road testing site at Streets Creek (during testing of sampling system). Road runoff samples were collected in a concrete gully from a 30 m long by 6.6 m wide section of road. The sample intake point was at the end of the gully (arrow). Sampling events were triggered by a water level probe installed in a V-notch weir downstream of the intake point (not shown)
Road sediment samples and analysis
Pratt (2006) and Pratt and Lottermoser (2007a) presented data for 44 road sediment samples from the Kuranda Range Road and adjacent roads. Three samples (KR1, KR2 and KR3), representing low, medium and high metal concentrations amongst this sample set, were selected for further leaching and DGT experiments.
An amount of road sediment was trapped in a filter bag (100 μm pore-size) in a pit downstream of the collection gully at Streets Creek test site from 27 November 2006 to 2 January 2007 (36 days). The sample represents bulk road sediment, although some fine particulate matter (<100 μm) may have been removed during runoff events. The mass of the sample corresponded to an accumulation rate of 144 g per day for the 198 m2 road surface sampled. In order to determine the solubility of metals, 300 mL of the sediment sample was added to 3 L of freshly collected road runoff water in a plastic basin (2 cm depth) and left exposed to the atmosphere at 23–24°C for 31 h. pH and EC measurements were recorded during this period, and a filtered (0.45 μm) water sample was collected at the start and end of the experiment and analysed by ICPMS at Charles Darwin University (CDU, Darwin, Australia).
Road runoff water sampling and analysis
Road runoff water samples were collected between 8 December 2006 and 25 January 2007 from the Streets Creek test site (Table 1). This period represented the onset of the 2006–2007 ‘wet’ season. Precipitation events during the sampling period (Fig. 3) were from discrete storm cells and did not include monsoonal or cyclonic events. An automated Teledyne ISCO sampler was used to collect flow-weighted samples (one sample per 200 L flow) during storm events. Sampling events were triggered by a water level probe installed in a V-notch weir, receiving the runoff downstream of the sampling point. Sampler programming and data logging (sampling time and flow rate) were performed using ISCO Flowlink® software.
Daily rainfall and road runoff sampling events at the Streets Creek test site. Rainfall shown is the average recorded at 4 stations (Saddle Mountain, Kuranda Hilltop, Kamerunga Bridge and Myola) <5 km from the test site (Bureau of Meteorology 2009a)
Following significant rainfall events, bottles were recovered and recorded data downloaded. The duration between water sampling and processing ranged from 1.5 to 3 days. It is recognised that metal exchange reactions between dissolved and particulate phases are likely to occur relatively rapidly, following the initial contact between road sediment and precipitation, and continually evolve during and after sampling. The implications of this are discussed in “Potential bioavailability of metals in road runoff waters”.
Each sample set was divided into individual storm events (or parts of storms) based on the down-loaded flow data. Composite samples were then produced for each storm event by mixing of equal volumes of individual samples through an acid-washed 500 μm nylon screen into an acid-washed compositing device (USGS Modified Churn Splitter fabricated from Teflon). Consequently, the data for runoff compositions presented below are event mean concentrations.
Total suspended sediment (TSS) concentration was determined by vacuum filtration of a measured sample volume thorough a pre-weighed 0.45 μm membrane filter followed by filter drying at 75°C for 24 h and reweighing. pH and EC were measured electrochemically following the electrode manufacturer’s standard procedures for calibration and measurement. Unfiltered and filtered (0.45 μm Gelman Supor filter) water from the Churn Splitter was collected in acid-washed LDPE bottles and acidified with Suprapur grade nitric acid to a concentration of 1% (vol.). Samples were submitted to CDU for analysis of metal and metalloid concentrations by ICPMS. Procedural blanks (de-ionised ‘Milli-Q’ water), replicate samples, spiked samples and certified reference water (NRC SLRS-4) were included for quality control. Based on data for spike recoveries and certified reference water, the analytical accuracy of metal and metalloid concentration data was estimated to be within ±10% at concentration levels >5 times the detection limit. Based on replicate determinations, precision was generally <10% (2σ relative standard deviation) at concentration levels >5 times the detection limit.
Creek water sampling and analysis
Water samples were collected from Streets and Avondale Creek, approximately 50 m upstream of the Kuranda Range Road in order to characterise the background water quality during wet season (early March, late March and early May) and dry season (early September, early October) flow conditions. For this reason, sampling was carried out on days with no significant rainfall and when no road runoff contributed to creek flows. Water sampling consisted of manual grab samples using acid-washed LDPE bottles. Bottles were rinsed repeatedly with sample water before final sampling. Measurement of pH and EC and sample preparation and analysis of TSS and trace metals were carried out as for road runoff samples.
Diffusion gradients in thin films
The DGT devices (APA2 open diffusive gel type) were purchased from DGT Research (Lancaster, UK). The requirement for DGTs to be continually submerged to avoid drying of the gels meant that DGTs could not be used in situ in episodic runoff waters. Instead, DGT measurements were conducted on a selection of recently collected runoff samples in the laboratory. DGT measurements were carried out in 3 L of stirred samples in acid-washed 4 L plastic containers for durations of approximately 24 h. The ionic strength of all samples was adjusted by addition of 1 mmol/L NaNO3. Separate experiments to measure the effect of NaNO3 addition on metal and metalloid concentrations were carried out in a similar set-up using metal-spiked unfiltered creek water with NaNO3 additions of nil, 0.90, 1.79 and 5.38 mmol/L.
Three road sediment samples (KR1, KR2 and KR3) were leached in de-ionised water with 1 mmol/L NaNO3. DGT measurements were conducted on these leachates. In all DGT experiments, pH, EC and temperature were determined and unfiltered and filtered (0.45 μm) water samples were taken at the beginning and the end of each experiment and analysed by ICPMS (CDU).
Following deployment, DGTs were disassembled, the resin gels eluted in nitric acid and analysed by ICPMS and concentrations calculated according to Davison and Zhang (1994) and DGT Research (2008). Several unused DGTs were analysed as blanks for each experiment. Metal uptake by the type of DGT used in this study is based on binding to chelex-100 chelating resin and, anionic metalloids, such as As and Sb, are not taken up by Chelex based DGTs.
Results
Road sediment
Kuranda Range Road sediment is characterised by an abundance of coarse-grained particles (1–4 mm) whilst the finer grained fraction (<106 μm) constitutes <10 wt% (Pratt and Lottermoser 2007a). The sediment has distinctly elevated total trace metal concentrations (Table 2) with large quantities of Cd, Cu, Pb and Zn bound to the fine-grained (<250 μm) sediment fraction which includes abundant Zn-rich tyre rubber shreds and metal-rich shavings (Pratt 2006; Pratt and Lottermoser 2007a).
During the 31 h leach experiment of the bulk road sediment from the Streets Creek test site, EC values increased from 24.1 to 118 μS/cm and pH values decreased from 7.11 to 6.38. Leaching released major (>1,000 mg/L) amounts of Al, minor (>5 mg/L) Cu, Ni and Zn, and traces (<5 mg/L) of As, Cd, Sb and Pb (Table 3). Aluminium is likely to predominantly have been derived from soil clay minerals on the road and is not considered a major traffic-related metal contaminant. The leachate concentrations shown in Table 3 were derived by subtracting the concentrations in the initial leachate solution (i.e. before sediment addition) from the concentrations in the final leachate (0.45 μm filtered samples).
Road runoff water
The road runoff waters were characterised by near-neutral pH, low EC and variable TSS concentrations (Table 1). There is no clear relation between 3-day antecedent rainfall and the pH, EC or TSS concentrations (Table 1). Such correlations may occur where road sediment accumulates during long dry spells before precipitation leads to runoff events with elevated TSS, EC (due to dissolution of TSS) and pH (due to increased TSS buffering capacity). Distinctly elevated metal levels have been reported for such runoff waters (e.g. Kim et al. 2005a). The storm event on 22/1/07 (sampled in three sequential parts: #9, #10 and #11, Table 2) displayed a typical pattern of initial high intensity (as measured by runoff flow rate) followed by declining intensity during the later part of the storm. This decline in intensity was correlated with a decreasing TSS concentration as the road surface became progressively cleaned of sediment during the storm. The absence of a more general relationship between pollutant concentration and antecedent rainfall for all samples is likely to be a function of the sampling design, which sought to obtain event mean concentrations. This was done to provide an overall assessment of the effects of road runoff on stream catchments, including any dilution of initially elevated concentrations that may occur in the waning portions of storm events.
The metal chemistry of filtered and unfiltered road runoff waters is listed in Table 3. In general, filtered waters contained significantly lower trace metal values than unfiltered samples. The difference in metal concentrations in filtered and unfiltered samples represents the labile particulate metal concentration (M P) desorbed from the particulate matter in unfiltered samples upon acidification to 1% HNO3:
where M UF and M F [μg/L] represents the unfiltered and filtered metal concentrations, respectively, and TSS [mg/L] represents the suspended sediment concentration.
Figure 4 shows the strong linear correlations recorded for TSS and concentrations of Zn, Cu, Pb and Cd in unfiltered road runoff samples. The linear slopes (1,185, 157, 133 and 1.2 mg/kg dry wt., for Zn, Cu, Pb and Cd, respectively) correspond approximately to the maximum labile particulate metal concentrations shown in Table 3 (1,140, 169, 134 and 1.1 mg/kg dry wt. for Zn, Cu, Pb and Cd, respectively). The strong correlations (R > 0.96) shown in Fig. 4 suggests that the labile portion of metals in road sediment remained approximately constant during the 7 week sampling period. The recorded levels of labile Zn, Cu, Pb and Cd were elevated by factors of up to approximately 10 compared to local bedrock, soil and stream sediments (Pratt 2006; Pratt and Lottermoser 2007a).
Relationship between total suspended sediment (TSS) concentration and concentrations of Zn (Fig. 4a), Cu (Fig. 4b), Pb (Fig. 4b) and Cd (Fig. 4c) in unfiltered Kuranda Range Road runoff samples. The slopes and correlation coefficients (R) are shown at the upper end of the linear regression lines (slope, R)
A comparison of the median labile metal concentrations in runoff particulates shown in Table 3 (Zn = 743, Cu = 101, Pb = 100, Ni = 47 and Cd = 0.6 mg/kg dry wt.) with the total metal concentrations in the <500 μm sediment grain size fraction (Zn = 1,540, Cu = 66, Pb = 87, Ni = 47 and Cd = 0.3 mg/kg dry wt.; Pratt and Lottermoser 2007a) suggest preferential transport of some metals during runoff events. For example, labile particulate Zn is lower than total road sediment Zn while labile particulate Cd, Cu and Pb exceed total road sediment concentrations of these metals. These differences are likely to reflect physical sorting of road sediment containing grains of variable size and density. For example, dense Zn-rich steel particles may not be easily transported from the road surface by runoff flow and therefore become underrepresented in suspended particulates. By contrast, Pb is likely to have been derived in part from fine-grained automotive air emissions (Bäckström et al. 2003) that is relatively easily transported by runoff waters and therefore will be overrepresented in the suspended particulates relative to in situ sediment.
The receiving creek water samples collected upstream from the Kuranda Range Road are likely to contain near-natural background concentrations of metals and metalloids (Table 3) given their near-pristine rainforest catchments and absence of any substantial urban or industrial emission sources. The median road runoff dissolved metal and metalloid concentrations (0.45 μm filtrate) exceed the median element concentrations in the receiving creek waters by the following approximate factors: Al ×4, As ×1.5, Cd ×2, Cu ×10, Pb ×13, Sb >×7 and Zn ×8. Nickel concentrations were not appreciable elevated in runoff waters relative to creek waters.
DGT experiments
The potential bioavailability of metals in road runoff waters was evaluated by DGT measurements following addition of NaNO3. While there were no substantial changes to unfiltered and filtered metal concentrations with addition of NaNO3, there were small consistent trends of declining DGT-labile concentrations for all five metals analysed (i.e. ~10–20% decrease in metal concentrations with increasing NaNO3 addition; Table 4). This observation accords with earlier observations of a tendency of DGTs to overestimate metal concentrations in low-ionic strength waters (<0.2 mmol/L; Alfaro-De la Torre et al. 2000; Peters et al. 2003; DGT Research 2008). However, the experiments demonstrated that small additions of NaNO3 up to approximately 1 mmol/L did not substantial change metal speciation, consistent with the very limited formation of metal-nitrates in most natural waters.
Filtered and DGT-labile Mn, Cu and Zn concentrations and the DGT/filtered concentration ratios measured for creek and runoff waters are shown in Table 5. Manganese was included to help evaluate DGT performance. Road runoff waters have DGT-labile/filtered metal ratios of approximately 100%, 25–46% and 47–77%, for Zn, Cu and Mn, respectively. By contrast, creek water had DGT-labile/filtered metal ratios of approximately 53 and 34%, for Cu and Mn, respectively. Therefore, almost all Zn and a substantial proportion of Cu in the road runoff waters were in potentially bioavailable forms. Nickel, Cd and Pb concentrations could not be reliably measured by DGTs in road runoff and creek waters due to high detection limits for Ni (1 μg/L for a 24 h deployment) and low concentrations for Cd (<0.02 μg/L) and Pb (<0.03 μg/L). DGT-labile Zn could not be measured in creek waters due to the low dissolved Zn concentration.
The road sediment leaching experiments with DGT measurement of metal concentrations were carried out in de-ionised water containing 1 mmol l−1 NaNO3, and hence, any capacity to form inert metal complexes in the leachate would have been derived from the road sediment itself. The proportions of metals that were leached from the sediment over 4.5 h were 2–5% for Zn, 7–11% for Cu and 2–4% for Mn (0.45 μm filtered samples). Compared to road runoff waters, the DGT-labile/filtered concentrations in the leachate were slightly lower for Zn but substantially higher for Mn and Cu (Table 5). The data derived from the leaching experiments added to the evidence from runoff waters that Kuranda Range Road sediment is prone to release substantial concentrations of potentially bioavailable Cu and Zn.
Discussion
Comparison to guideline values
The background water quality of the Kuranda Range creek waters is compared to the Australian water quality guideline values for freshwater systems (NWQMS 2000) in Table 3 in order to assess the capacity of the creek system to dilute road runoff waters without reaching concentration levels that are likely to constitute a toxicity risk to the aquatic ecosystem. The Australian water quality guidelines contain default concentration ‘trigger values’ for metal toxicants for alternative levels of protection (99, 95, 90, and 80%), depending on ecosystem conditions. Here, the 95% guideline values (for slightly to moderately disturbed aquatic systems) has been selected as appropriate for the Kuranda Range creek waters. The data in Table 3 show that the guideline values most likely to be exceeded in receiving creek waters, which receive road runoff, are those for Al, Cu and Zn. While the creek background concentrations for Al, Cu and Zn were factors of 7.6, 4.7 and 10 below the guideline levels, respectively, the maximum concentrations of these metals in road runoff were relatively high (exceeding the guideline levels). Therefore, in the absence of treatment or dilution, Al, Cu and Zn concentrations in creek waters affected by road runoff could potentially rise to levels that would pose an ecotoxicity risk. It should be noted that during storm events in the late ‘dry’ season/early ‘wet’ season, dilution of runoff waters entering the creek systems may be almost absent as creek base flow is very low (see “Assessment of environmental impact”).
Potential bioavailability of metals in road runoff waters
Although a generalised quantitative measurement of bioavailable forms of metals has proved to be an elusive goal (see Meyer 2002 for a discussion), Zhang and Davison (2000) argued that biological responses to dissolved metals may, depending on the particular biological species, be a function of both the free ion activity as well as some portion of dissolved labile inorganic and organic metal complexes. The ‘open-pore’ gel version of DGTs (as used in the present study) have been shown to measure free metal ions and labile inorganic and organic metal complexes, specifically those complexes that are <5 nm in size and are sufficiently labile to dissociate and be bound to the Chelex gel layer within the effective measurement time of the DGT device (approx. 13.5 min, Zhang and Davison 2000). An alternative ‘restricted-pore’ DGT gel (pore-size approximately 1 nm) is capable of excluding a higher proportion of large metal–organic complexes (Zhang and Davison 2000). However, colloidal-bound metals and inert dissolved metal complexes are excluded from measurement by all DGTs.
In natural waters, humic and fulvic acids derived from transformed organic matter form supra-molecular structures and have the ability to chelate positively charged metal ions (Piccolo 2001). The runoff waters from the Kuranda Range Road are likely to contain, or potentially generate, humic and fulvic acids due to the abundance of leaf litter derived from the surrounding rainforest. Pratt and Lottermoser (2007a) reported a median organic carbon content in the <75 μm fraction of Kuranda Road sediment of 9.38 wt% (n = 9). Other road sources of organic ligands with a potential to form metal–organic complexes include bitumen, tyre fragments and automotive fuel and lubricants (Wik and Dave 2005).
Metal uptake by ‘open-pore’ DGTs can include substantial portions of metals bound to humic and fulvic acids (Zhang and Davison 2000), although in some cases such complexes were found not to be acutely toxic in toxicity tests (Tusseau-Vuillemin et al. 2004; Buzier et al. 2006). Therefore, in the present study metal measurement by ‘open-pore’ gels is likely to constitute a conservative estimate of the maximum potential bioavailability consistent with the requirements for a conservative management of environmental threats in the high-value World Heritage Area surrounding the Kuranda Range Road.
The DGT-measured concentration data for the Kuranda Range Road runoff waters (Table 5) showed that Zn in the dissolved phase (0.45 μm filtered sample) was potentially highly bioavailable (DGT/filtered = approximately 100%), whereas Cu was only partly bioavailable (DGT/filtered = 25–46%). Consequently, the ecotoxicity risk of elevated levels of dissolved Cu in runoff waters is reduced by the indicated lower bioavailability. Buzier et al. (2006) reported similar levels of DGT-measured bioavailable Cu in waste water, namely 41–42 and 29–37% of dissolved Cu in raw and treated waste water, respectively.
In the present study, DGT measurements were performed on unfiltered runoff waters, which were 1.5–3 days old. It is recognised that metal partitioning between dissolved and particulate species is likely to have continually evolved both prior to, and after sampling. Therefore, DGT-labile concentrations are likely to represent an evolved stage of metal exchange where reaction rates have slowed considerably since the initial phase of precipitation runoff from the road surface. Previous DGT measurements of reactions between particulate and dissolved phases have shown that initial reaction rates are relatively high followed by slowing rates and an approach to exchange equilibrium after approximately 36 h (Munksgaard and Parry 2003). Consequently, it is reasonable to assume that the DGT-labile metal concentrations in road runoff waters measured in this study equates to the maximum potential bioavailability that would prevail in the receiving environment with no or little dilution.
The high DGT/filtered Zn concentration ratios in road sediment leachates (90–93%) confirmed that Zn potential bioavailability in road runoff is likely to be high and suggests that there was no substantial Zn complexing capacity derived from the sediment itself. By contrast, a much greater proportion of Cu was bound in inert complexes unavailable to DGTs (DGT/filtered = 62–82%). The lower DGT/filtered Cu ratios in runoff waters compared to sediment leachates suggest that some complexing capacity was derived from the precipitation itself, possibly due to organic ligands acquired from the overhanging forest canopy.
Assessment of environmental impact
Traffic-derived metals may be taken up by flora and fauna (e.g. Schäfer et al. 1998; Garcia and Millan 1998; Viard et al. 2004; Pratt and Lottermoser 2007b), and the discharge of metal enriched road runoff waters can adversely impact on aquatic organisms (e.g. Day et al. 1993; Pitt et al. 1995; Forman and Alexander 1998; Trombulak and Frissell 2000; Wik and Dave 2005; Pettigrove et al. 2007). Creek dependent fauna in the Kuranda Range include threatened frog species.
The relatively high solubility of metals, recorded for both suspended particles in runoff waters and road sediment, combined with the DGT evidence of high potential bioavailability for some metals, suggests that Kuranda Range Road runoff waters could pose an ecotoxicity risk to the receiving creek systems under adverse conditions, i.e. if large volumes were discharged without adequate treatment or dilution. The potentially bioavailable Cu and Zn concentrations in road runoff samples in the present data set can be estimated at up to approximately 20 times that in the receiving creek water (based on maximum filtered concentrations and average DGT/filtered ratios, Tables 3 and 5). Receiving creek waters, if dominated by road runoff during times of minimal dilution, may exceed guideline levels for Cu and Zn concentrations (Table 3). Since these guideline levels are based on ecotoxicity data (albeit not for local species) and hence represent bioavailable concentrations above which some detrimental response may occur, it appears that Kuranda Range Road runoff waters may have the potential to impart toxic effects in the receiving creek systems. A recent road runoff study by Kayhanian et al. (2007) identified Zn and Cu as the principal cause of toxicity to a range of freshwater and marine species; however, the recorded runoff concentrations of dissolved Zn and Cu were more than an order of magnitude higher than those recorded for the Kuranda Range Road.
Figure 5 shows the estimated median monthly flow rate for Streets Creek and the median monthly rainfall for Cairns Airport (10 km SE of study site, Bureau of Meteorology 2008). As no hydrographic data were available for Streets Creek, flow rates were calculated from long-term data records (QNRW 2008) from the upper part of the nearby Clohesy River catchment by adjusting for catchment size. Although much larger, the upper Clohesy River catchment’s main hydrographic characteristics are likely to be broadly similar to those of the Streets Creek catchment. The data highlight two important observations pertaining to dilution of road runoff water into Streets Creek. Firstly, creek base flow is likely to be very low (<5 L/s) towards the end of the ‘dry’ season (September–December). Secondly, creek flow rates respond slowly to increase in rainfall at the onset of the ‘wet’ season as a consequence of a high infiltration capacity in rainforest catchments. This is consistent with the observed slow response of flow in Streets and Avondale Creeks during storm events. By contrast, road runoff is near instantaneous during intense storm events. Therefore, at the beginning of the ‘wet’ season, low flow rates in the receiving creeks (<5 L/s) may coincide with high road runoff rates during storms. Runoff rates of >100 L/s can be expected with an average recurrence interval of 1 year from a 4,000 m2 bridge surface for a 100 mm/h rainfall intensity event over a 10 min duration, Bureau of Meteorology (2009b). This may lead to the discharge of almost undiluted road runoff waters with excessive bioavailable metals into local creek systems. However, metal levels will vary depending on the preceding length of time available for build-up of road sediment at the close of the ‘dry’ season. This risk to the Kuranda Range aquatic ecosystems must be adequately managed by treatment of road runoff waters from the large bridge structures planned for the upgrade of the Kuranda Range Road.
Calculated median monthly flow rate for Streets Creek and observed median monthly rainfall for Cairns Airport (15 km SE of the study site, Bureau of Meteorology 2008). Streets Creek flow rates were calculated from long-term data records (QNRW 2008) from the upper part of the nearby Clohesy River catchment by adjusting for catchment size (see text)
Conclusions
Aluminium, Cu, Pb, Sb and Zn were identified as the most significantly elevated metals and metalloids released from road sediment during storm events when compared to the concentrations in receiving creek waters. The median dissolved concentrations in road runoff waters (<0.45 μm filtrate) exceeded the median concentrations in the creek waters by up to an order of magnitude. Copper and Zn were assessed as the metals most likely to reach ecotoxic concentration levels.
Measurement of DGT-labile metal concentrations showed that almost all dissolved Zn and approximately half of dissolved Cu in road runoff waters were potentially bioavailable. DGT measurements were carried out after addition of NaNO3 to the sampled runoff waters in order to raise the ionic strength as required by the DGT technique. Experiments showed that NaNO3 addition to approximately 1 mmol/L had negligible effects on metal speciation.
During the early ‘wet’ season there is the potential for large accumulations of road sediment to coincide with minimal creek flows and hence minimal dilution potential. Consequently, in the ‘wet–dry’ tropics, the highest ecotoxicity risk associated with road runoff is likely to occur during the first storm events of the early ‘wet’ season.
References
Alfaro-De la Torre MC, Beaulieu PY, Tessier A (2000) In situ measurement of trace metals in lakewater using the dialysis and DGT techniques. Anal Chim Acta 418:53–68
Bäckström M, Nilsson U, Hakansson K, Allard B, Karlsson S (2003) Speciation of heavy metals in road runoff and roadside total deposition. Water Air Soil Pollut 147:343–366
Batley GE, Apte SC, Stauber JL (2004) Speciation and bioavailability of trace metals in water: progress since 1982. Aust J Chem 57:903–919
Brown JN, Peake BM (2006) Sources of heavy metals and polycyclic aromatic hydrocarbons in urban stormwater runoff. Sci Total Environ 359:145–155
Bureau of Meteorology (2008) Climate statistics for Australian locations, summary statistics Cairns Aero. http://www.bom.gov.au/climate/averages/tables/cw_031011.shtml. Accessed 10 Mar 2008
Bureau of Meteorology (2009a) Daily rainfall data for Saddle Mountain, Kuranda Hilltop, Kamerunga Bridge and Myola stations. Data provided by Bureau of Meteorology, 24 March 2009
Bureau of Meteorology (2009b) Design IFD rainfall. http://www.bom.gov.au/hydro/has/ifd.shtml. Accessed 19 Mar 2009
Buzier R, Tusseau-Vuillemin M-H, dit-Meriadec CM, Rousselot O, Mouchel JM (2006) Trace metal speciation and fluxes within a major French wastewater treatment plant: impact of the successive treatments stages. Chemosphere 65:2419–2426
Campbell PGC (1995) Interactions between trace metals and aquatic organisms: a critique of the free-ion activity model. In: Tessier A, Turner DR (eds) Metal speciation and bioavailability in aquatic systems. Wiley, Chichester, pp 45–102
Davison W, Zhang H (1994) In situ speciation measurement of trace components in natural waters using thin film gels. Nature 367:546–548
Day KE, Holtze KE, Metcalfe-Smith JL, Bishop CT, Dutka BJ (1993) Toxicity of leachate from automobile tires to aquatic biota. Chemosphere 27:665–675
Desta MB, Bruen M, Higgins N, Johnston P (2007) Highway runoff quality in Ireland. J Environ Monit 9:366–371
DGT Research (2008) DGT—for measurements in waters, soils and sediments. http://www.dgtresearch.com/dgtresearch/dgtresearch.pdf. Accessed 5 Oct 2008
Eriksson E, Baun A, Scholes L, Ledin A, Ahlman S, Revitt M, Noutsopoulos C, Mikkelsen PS (2007) Selected stormwater priority pollutants—a European perspective. Sci Total Environ 383:41–51
Estebe A, Mouchel JM, Thevenot DR (1998) Urban runoff impacts on particulate metal concentrations in River Seine. Water Air Soil Pollut 108:83–105
Flint KR, Davis AP (2007) Pollutant mass flushing characterization of highway stormwater runoff from an ultra-urban area. J Environ Eng 133:616–626
Forman RTT, Alexander LE (1998) Roads and their major ecological effects. Ann Rev Ecol Sys 29:207–231
Garcia R, Millan E (1998) Assessment of Cd, Pb and Zn contamination in roadside soils and grasses from Gipuzkoa (Spain). Chemosphere 37:1615–1625
Hallberg M, Renman G, Lundbom T (2007) Seasonal variations of ten metals in highway runoff and their partition between dissolved and particulate matter. Water Air Soil Pollut 181:183–191
Han YH, Lau SL, Kayhanian M, Stenstrom MK (2006a) Correlation analysis among highway stormwater pollutants and characteristics. Water Sci Technol 53:235–243
Han Y, Lau SL, Kayhanian M, Stenstrom MK (2006b) Characteristics of highway stormwater runoff. Water Environ Res 78:2377–2388
Hjortenkrans D, Bergbäck B, Häggerud A (2006) New metal emission patterns in road traffic environments. Environ Monit Assess 117:85–98
Kayhanian M, Suverkropp C, Ruby A, Tsay K (2007) Characterization and prediction of highway runoff constituent event mean concentration. J Environ Manag 85:279–295
Kim LH, Kayhanian M, Lau SL, Stenstrom MK (2005a) A new modeling approach for estimating first flush metal mass loading. Water Sci Technol 51:159–167
Kim LH, Kayhanian M, Zoh KD, Stenstrom MK (2005b) Modeling of highway stormwater runoff. Sci Total Environ 348:1–18
Lee BC, Matsui S, Shimizu Y, Matsuda T (2005) Characterizations of the first flush in stormwater runoff from an urban roadway. Environ Technol 26:773–782
Mangani G, Berloni A, Bellucci F, Tatano F, Maione M (2005) Evaluation of the pollutant content in road runoff first flush waters. Water Air Soil Pollut 160:213–228
Meyer JS (2002) The utility of the terms ‘bioavaliability’ and ‘bioavailable fraction’ for metals. Mar Environ Res 53:417–423
Morcelli CPR, Figueiredo AMG, Sarkis JES, Enzweiler J, Kakazu M, Sigolo JB (2005) PGEs and other traffic-related elements in roadside soils from Sao Paulo, Brazil. Sci Total Environ 345:81–91
Munksgaard NC, Parry DL (2003) Monitoring of labile metals in turbid coastal seawater using diffusive gradients in thin-films. J Environ Monit 5:145–149
NWQMS (National Water Quality Management Strategy) (2000) Australian and New Zealand Guidelines for Fresh and Marine Water Quality. Paper No. 4. Australian and New Zealand Environment and Conservation Council, Agriculture and Resource Management Council of Australia and New Zealand, Canberra, Australia
Peters AJ, Zhang H, Davison W (2003) Performance of the diffusive gradients in thin films technique for measurement of trace metals in low ionic strength freshwaters. Anal Chim Acta 478:237–244
Pettigrove V, Marshall S, Ryan B, Hoffmann A (2007) A field microcosm method to determine the impact of sediments and soils contaminated by road runoff on indigenous aquatic macroinvertebrates. In: Morrison GM, Rauch S (eds) Highway and urban environment: proceedings of the 8th highway and urban environment symposium, Nicosia, Cyprus, 11–14 June 2006. Springer, New York, pp 385–398
Piccolo A (2001) The supramolecular structure of humic substances. Soil Sci 166:810–832
Pitt R, Field R, Lalor M, Brown M (1995) Urban stormwater toxic pollutants: assessment, sources, and treatability. Water Environ Res 67:260–275
Pratt C (2006) The environmental fate of traffic-derived metals in a section of wet tropics world heritage area (WTWHA), Far North Queensland (FNQ). PhD thesis, James Cook University
Pratt C, Lottermoser BG (2007a) Mobilisation of traffic-derived trace metals from road corridors into coastal stream and estuarine sediments, Cairns, North Queensland, Australia. Environ Geol 52:437–448
Pratt C, Lottermoser BG (2007b) Trace metal uptake by the grass M. repens from roadside soils and sediments, tropical Australia. Environ Geol 52:1651–1662
QNRW (Queensland Natural Resources and Water) (2008) Historical Monitoring Data, Clohesy River at Main Road. http://www.nrw.qld.gov.au/watershed/precomp/110013a/mvr.htm. Accessed 5 Oct 2008
Schäfer J, Hannker D, Eckhardt JD, Stuben D (1998) Uptake of traffic-related heavy metals and platinum group elements (PGE) by plants. Sci Total Environ 215:59–67
Trombulak SC, Frissell CA (2000) Review of ecological effects of roads on terrestrial and aquatic communities. Conserv Biol 14:18–30
Tusseau-Vuillemin M-H, Gilbin R, Bakkaus E, Garric J (2004) Performance of the diffusive gradient in thin films in evaluating the toxic fraction of copper to Daphnia magna. Environ Toxicol Chem 23:2154–2161
Viard B, Pihan F, Promeyrat S, Pihan JC (2004) Integrated assessment of heavy metal (Pb, Zn, Cd) highway pollution: bioaccumulation in soil, Graminaceae and land snails. Chemosphere 55:1349–1359
Westerlund C, Viklander M (2006) Particles and associated metals in road runoff during snowmelt and rainfall. Sci Total Environ 362:143–156
Westerlund C, Viklander M, Bäckström M (2003) Seasonal variations in road runoff quality in Luleå, Sweden. Water Sci Technol 48:93–101
Wik A, Dave G (2005) Environmental labeling of car tires—toxicity to Daphnia magna can be used as a screening tool. Chemosphere 58:645–651
Zhang H, Davison W (2000) Direct in situ measurements of labile inorganic and organically bound metal species in synthetic solutions and natural waters using Diffusive Gradients in Thin films. Anal Chem 72:4447–4457
Acknowledgments
The study was funded by the Queensland Department of Main Roads and Stormwater360 provided the ISCO samplers. Dr. Chris Pratt is thanked for contributing data on road sediment compositions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Munksgaard, N.C., Lottermoser, B.G. Mobility and potential bioavailability of traffic-derived trace metals in a ‘wet–dry’ tropical region, Northern Australia. Environ Earth Sci 60, 1447–1458 (2010). https://doi.org/10.1007/s12665-009-0280-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12665-009-0280-5