Abstract
Purpose
This study implemented strategies to improve citric acid yield by Aspergillus niger through strain improvement and process optimization using under-utilized cashew apple juice.
Methods
A. niger LCFS 5 (MZ448204) earlier isolated from a cashew plantation and produced citric acid on Czapek-Dox agar supplemented with bromocresol green (CZA-BG) was improved through UV mutation (254 nm). The best mutant was grown in cashew apple juice medium. The effects of supplementation of medium with 10% sucrose, metal ions, and biogenic zinc oxide nanoparticles (ZnONPs) were studied and production of citric acid was optimized using Taguchi technique.
Results
The mutant A. niger LCFAn40 (MZ448205) had yellow zonation of 9.0 cm on CZA-BG. It produced citric acid yield of 18.4, 25.03, 34.62 and 92.61%/day for supplementation with sucrose, metal ions, ZnONPs and Taguchi optimization, respectively. These translate to 2.38–11.98 folds improvement in comparison with wild strain.
Conclusion
This study establishes multi-dimensional approach as a viable technique to improve citric acid production in cashew apple juice. To our knowledge, this is the first report of broad-based optimization regime of citric acid production that involves nanoparticles supplementation, which may open a new vista of investigations on the use of nanobiocatalysts in bioprocesses.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Statement of Novelty
The study extended the frontier of substrates that can be exploited to produce citric acid in high yield by employing cashew apple juice that constitutes part of wastes in cashew processing. Also, a new mutant strain, Aspergillus niger LCFAn40 that was developed increased yield of citric acid by 2.38–11.98 folds through innovative optimization. The insight into the influence of zinc oxide nanoparticles on citric acid yield is the first report of its kind. The work is impactful, because a feasible means to expand the scope of fungal production of citric acid was established, and can be part of strategies to meet the increasing demand for citric acid worldwide.
Introduction
Citric acid as a commodity product has been a useful acidulant in many areas of human activities; it is useful in pharmaceutical, textile, food, cosmetic, detergent and photographic industries among others [1]. The demand for citric acid is on the increase due to high utilization in food industry, where it is used as preservative, additive, flavour enhancer, nutrient supplement in foods and as animal feed improver due to its generally regarded as safe (GRAS) status [1, 2]. Citric acid is a good antioxidant, an anticoagulant and a buffer agent to mention just few of the numerous areas of its biomedical applications [2, 3]. The global annual production of citric acid has been put at about 2.1 million tons [3], with estimated value of $3.6 billion in 2020 [4]. However, the market demand estimation is about 3 million tons [2]; thereby necessitating ingenuity at finding means to increase production to meet the demand for citric acid.
Citric acid production has been achieved through the use of microorganisms which include bacteria, yeasts, and moulds. Many research works have used bacteria such as Achromobacter, Aerobacter, Bacillus, Corynebacterium, Klebsiella, Micrococcus, Nocardia, and Pseudomonas spp.; yeasts like Candida parapsilosis, C. fibriae, C. zeylanoides, C. catenulata, C. parapsilosis, Yarrowia lipolytica and Brettanomyces sp., as well as moulds that include Aspergillus flavus, A. niger A. awamari, A. usamil, A. luchensis, A. fumaricus, A. wentii, and A. foetidus [1, 2, 5, 6]. Among the organisms that are utilized for producing citric acid, A. niger has been discovered to be the workhorse for higher yield and safety. It is easy to handle and has a GRAS status [7, 8]. It is a fungus that has been used to produce enzymes, oligosaccharides and nanoparticles among others [9,10,11,12,13,14].
The high cost of production is one of the major problems facing citric acid production, and the yield obtained is directly dependent on the type of substrate employed. Most of the suitable substrates like sugar and starch are much expensive [15]. The by-products of sugar production like molasses that may be cheaper source of substrate for production require rigorous purifications because of high level of chemical contaminants. As for other cellulose-based materials, extra efforts will be needed to hydrolyse the cellulose to simple sugars that will give better yield and fast recovery of citric acid. Most often, hydrolysis of cellulose and some non-cellulose materials involves the use of mineral acid and other chemicals for bleaching which eventually become hazardous to the environment when disposed [16], and does not represents a progress towards green and eco-friendly process. Hence, there is the need to source for simple raw materials that will require less energy and processing as substrates for the production of citric acid [17].
Among those promising substrates of high sugar quality is the cashew apple juice that has not been utilized in such appreciable quantity. Due to astringent taste of cashew juice, over 50–80% of the cashew apple produced in Nigeria is regarded as waste besides the cashew nut that is highly valued for exportation [18, 19]. Nigeria has production of 100,000 tons of cashew nut [20], as the world production stands at 3.96 million tons [21]. However, the cashew apple that constitutes 90% weight of the fruit has not been fully explored biotechnologically as both the cashew apple juice and cashew apple bagasse are largely wasted [22, 23]. The cashew apple juice is composed of valuable nutrients that can support growth of microbes as evaluated by Lowor and Agyente-Badu [24]. It contains (mg/100 mL): tannins (266.0), phenolics (269.5), and Vitamin C (231.4). It is very rich in sugars (12.05 mg/mL). The mineral composition (mg/100 mL) included potassium (76.0) calcium (43.0), magnesium (10.92), phosphorous (0.79) and sodium (0.41), while copper, iron and zinc occurred at 0.05–0.08 mg/100 mL.
It is obvious that the demand of citric acid is far higher than the supply, and optimization of the production process has been identified as a novel way to solve the problem [5]. Several optimization techniques have been reported to enhance bioproducts formation such as Taguchi technique, artificial neural network, genetic algorithm and central composite design [7, 25,26,27,28,29,30,31,32]. In citric acid production, optimization of substrate concentration, temperature, pH, fermentation time, and supplementation of substrates have been reported by many authors [7, 29, 33,34,35,36]. The uses of trace metals and strain improvement through genetic engineering and mutation have also been reported to enhance citric acid production [7, 37,38,39,40]. However, there is no report on the supplementation of fermentation medium with metal nanoparticles to improve the microbial production of citric acid. In this work, the quartet of physical mutation, nutrient supplementation, nanoparticles supplementation and Taguchi optimization have been uniquely used to improve citric acid formation by Aspergillus niger in cashew apple juice-based medium.
Materials and Methods
Isolation and Identification of Fungus
The fungal strain used for this work was isolated from LAUTECH Teaching and Research farm as previously reported [41] and it produced citric in cashew apple juice medium. The isolate was repeatedly screened and sub-cultured on potato dextrose agar (HiMedia Lab. Pvt. Ltd, Mumbai, India) plates to obtain pure culture. The plates were incubated for 7 days at room temperature 30 ± 2 °C. The isolate was identified based on the morphological identity peculiar of Aspergillus niger, which are characteristic black colour due to black pigment production and whitish slender wool-like mycelium at early stage of growth. Microscopic structures of the mould were examined on the plates, stained with lactophenol cotton blue and investigated for microscopic structures. Conventional identification was done following the techniques of Domsch et al. [42]. The molecular identification was based on the use of ITS4 (5′-TCCTCCGCTTATTGATATGC-3′) and ITS5 (5′-GGAAGTAAAAGTCGTAACAAGG-3′) primers [43] for the amplification of the genomic DNA following standard protocols [44]. The products of PCR were analyzed on agarose gel electrophoresis, and also sequenced in a commercial facility. The alignment of sequences was done using BioEdit sequence software, and consensus sequences then deposited in GenBank of National Centre for Biotechnology Information (http://www.ncbi.nlm.nih.gov/) to identify the isolate.
Screening of Fungal Isolates for Citric Acid Production
Screening for the production of citric acid was done on Czapek-Dox agar (HiMedia Lab. Pvt. Ltd, Mumbai, India) embedded with 5 mL of 5% bromocresol green indicator as previously described [41], and the development of yellow halo which indicated citric acid production was monitored and measured [35, 38, 45].
Mutation of the Fungal Strain
The wild strain of A. niger LCFS 5 which produced the highest halo in the screening assay was used for physical mutation through exposure to UV light at 254 nm [7]. The fungus was allowed to produce spores, which were harvested by washing the surface of the culture plates with saline solution into 100 mL stock. Dilutions of 10–3, 10–6 and 10–9 were prepared and distributed into sterile Petri plates. The plates were exposed to UV radiation at a distance of 15 cm for duration of 5, 10, 15, 20, 30, and 40 min. The selected strains from the preliminary exposure were treated with further dose of UV for 60 min at interval of 3 days till the 8th generation. After the UV exposure, the plates were kept in the dark for stability. Thereafter, the spores were incubated on PDA plates, and several mutant strains were isolated and evaluated for the production of citric acid [45, 46]. The best mutant was identified using both conventional and molecular techniques as stated under “Isolation and Identification of Fungus” section.
Substrate Preparation, Production and Quantification of Citric Acid in Cashew Apple Juice-Based Medium
The cashew apples used in preparing the substrate were sorted out, washed and pressed to extract the juice with the aid of juice extractor. The juice was pasteurized and stored at 4 °C until required as previously stated [41]. Both wild and mutant strains of A. niger were investigated for the citric acid production in submerged fermentation as described by Adeoye et al. [7]. The process was monitored for citric acid production and fermentation was terminated when decline in yield was noticed. Other parameters such as pH, total soluble solid and total titratable acidity were determined on daily basis.
Acid production was determined using acid–base titration method [47, 48], where 0.1 M NaOH and 1% phenolphthalein indicator were used to obtain a pink colour that persisted for few seconds when titrated against the fermented broth. Production of citric acid was quantified as follows [49]:
Citric acid equivalent factor is 0.0064 g/L of citric acid.
However, a measure of total performance of the fermentation process under specific cultivation condition was derived thus:
where amount of citric acid produced is in g/100 mL.
Supplementation of Cashew Apple Juice with Salts and ZnONPs for the Production of Citric Acid
Further to the procedure in “Substrate Preparation, Production and Quantification of Citric Acid in Cashew Apple Juice-Based Medium” section, cashew apple juice was fortified with K2HPO4 (1%), NaNO3 (1.5%), MgSO4⋅7H2O (1.5%) and KCl (0.3%) and the production of citric acid in submerged fermentation was monitored for 21 days. The effects of these metals were studied on citric acid production and biomass development of the organism.
In the case of ZnONPs supplementation, 5 mL of 1 mM ZnO was reacted with 1 mL of clarified cashew apple juice at 37 °C for 24 h for the bioreduction of Zn2+ to Zno, which was monitored for colour development. In the control experiment, cashew apple juice was not added to the solution of ZnO. The effect of ZnONPs on production of citric acid by A. niger was investigated as follows:
Set-up | Treatment | Concentration of ZnONPs (µg/mL) |
|---|---|---|
A: | Cashew apple juice + ZnONPs (9:1) | 81 |
B: | Cashew apple juice + ZnONPs (8:2) | 162 |
C: | Cashew apple juice + ZnONPs (7:3) | 243 |
Optimization of the Production of Citric Acid Using the Taguchi Technique
Cashew apple juice, fermentation time, pH and inoculum size were the selected parameters to be optimized and designated as A, B, C, D, while the levels of variation designated as 1, 2 and 3, which are the lower, intermediate and upper limit, respectively. The substrates were in the range of 0.5% as the lower limit to 3% as upper limit; fermentation time in the range of 24–144 h, pH was adjusted from 4.5 to 6.5 as described by orthogonal array, and inoculum sizes in the range of 2 to 10% (Table 1). All the investigations were conducted in 100 mL medium under submerged fermentation, using the L9 orthogonal array as described by Taguchi [50].
From the responses that represent citric acid production (R), the signal-to-noise ratios (η) were calculated for the different profiles in the L9 orthogonal array as defined by Chou et al. [51]:
The combinations with the largest value of signal-to-noise ratio give the optimal conditions for the production of citric acid. The main effect of each variable was calculated as the difference between the average of measurement made at the upper limit level and lower limit level of all the variables (Eq. 2).
Thereafter, the optimized condition obtained through Taguchi optimization was then validated through experimentation. Analysis of variance on (ANOVA) was employed to determine the significance of the four parameters on the production of citric acid. The flowchart for the optimization is as presented in Fig. 1 [26].
Results and Discussion
Identification of the Fungus
Different biological, biomedical and computational techniques have been adopted by different authors to classify Aspergillus strains [52]. In this work, the identification was based on macro and micro-morphological examination as previously reported [41, 53]. The early growth of Aspergillus niger LCFS 5 appeared whitish, thread-like and turned black on the plate. The growth pattern is usually radial or circular in feature on agar plate, cotton-like in appearance, turned black when matured and possession of slender stipes bearing conidiophores on elliptical vesicles (Fig. 2). These features have been previously described in literature [53, 54]. Its molecular characterization as earlier reported identified it as a strain of Aspergillus niger with accession number MZ448204 [41].
Qualitative Screening and Quantitative Production of Citric Acid by A. niger LCFS 5
Strains LCFS 5 yielded 7.5 cm zonation on CZA-BG agar after 6 days of cultivation. Similar works reported by many authors have taken the yellow zonation as a measure of citric acid production [33, 49]. The zone of yellow colouration obtained in this work was better when compared with those obtained for strains of Aspergillus niger in our previous works [7, 47]. Therefore, A. niger LCFS 5 was chosen for further work on quantitative production of citric acid, mutation and optimization studies.
As earlier reported, the quantitative evaluation of production of citric acid by LCFS 5 led to the maximal production of 92.8 g/L at 10th day of fermentation in cashew apple juice medium [41], which translates to citric acid yield of 7.73%/day. During the period, pH and total soluble solid reduced from their initial values of 6.5 and 12 obrix to reach 3.44 and 4.2 obrix, respectively on the 10th day of fermentation. However, the total titratable acidity increased gradually from the initial value of 2.5 to 14.5 under the same fermentation period. It was evident from the yield of citric acid that the medium supported good growth of A. niger LCFS 5, thereby establishing the medium as a viable complex medium for the production of citric acid and extends its frontiers of biotechnological valorization as a resourceful agrowaste [41] to develop bioeconomy. Similarly, the new strain LCFS 5 adds to the growing list of viable strains that can be explored for industrial production of citric acid. With the supplementation of cashew apple juice medium with 10% sucrose, the amount of citric produced by LCFS 5 increased to 220.08 g/L at 10th day of fermentation (Table 2), having the citric acid yield of 18.40%/day. The inclusion of sucrose which increased the citric acid yield by 2.38 folds to that of cashew apple juice alone was premised on the fact that it remains the prime choice of source of carbon in chemically-defined media for the production of citric acid [29] and also used to supplement complex media to improve citric acid production [55].
Generation of Mutants and Evaluation for Production of Citric Acid
To further enhance the potential of LCFS 5 in producing higher yield of citric acid, the fungus was exposed to UV radiation to produce mutants via random mutation. Several mutants that were generated produced yellow zonation ranging from 4.5 to 9.0 cm on the 6th day on CZA-BG agar showing their capabilities at producing citric acid. The width of growth and spread were more expansive and similar in both mutants designated LCFAn25 and LCFAn40 that produced 9.0 cm zonation (Table 3). Thus, mutant strain LCFAnM40 was selected for further studies, and it was identified using molecular biology technique as a strain of Aspergillus niger with accession number MZ448205. The PCR products of both LCFS 5 (wild) and LCFAn40 (mutant) were electrophoresed on agarose as shown in Fig. 3, and the phylogenetic construct showed the relatedness with the wild type (Fig. 4). Mutants of Aspergillus niger have shown improved production of citric acid compared with the wild strains [7, 38, 56], showing that mutation is a viable means to improve the strains for enhanced productivity as obtained in this study.
Production of Citric Acid by LCFAn40 in Cashew Apple Juice Medium Supplemented with Salts and ZnONPs
The effects of supplementation of cashew apple juice with K2HPO4 (1%), NaNO3 (1.5%), MgSO4·7H2O (1.5%) and KCl (0.3%) on the production of citric acid by LCFAn40 are as stated in Table 4. The amount of citric acid produced by the mutant ranged from 4.4 g/L at day 1 to 85.1 g/L at day 20 with corresponding reduction in the pH of the fermenting broth from 5.6 to 2.0. The medium also supported good growth of the mutant with the biomass steadily increasing from 0.36 g at day 1 to reach maximum of 1.17 g at day 19 before it reduced to 1.01 g at 21st day. In our earlier investigation, the cashew apple juice has been described as a good medium for the propagation of Aspergillus niger for citric acid production [41]. The citric acid production which peaked at 85.1 g/L at the 20th day of fermentation translates to citric acid yield of 25.03%/day which is a 3.24-fold improvement compared to the maximum value obtained for the wild strain. It also gave 1.36-fold improvement compared to the yield obtained for supplementation of cashew apple juice with sucrose. Therefore, it means that scope exists to increase the citric acid yield by the fungus with supplementation of the medium with salts instead of sucrose. The sustenance of citric acid production by the organism under this condition for extended period of 21 days as investigated in this study showed that the medium formulation can be ideal for continuous fermentation to produce citric acid with acceptable yield.
Biofabrication of ZnONPs was undertaken using the cashew apple juice which produced yellowish colloidal solution after 24 h, and absorbed maximally at 240 nm with particle sizes of 30.21–67.37 nm (Fig. 5). Its use to supplement cashew apple juice afterwards led to the improved production of citric acid at lower sugar content (6.5 obrix) and shorter days of fermentation (Table 5). Specifically, between 3.2 and 9.0 g/100 mL of citric acid was produced within 5 days of fermentation, with the best production (9.0 g/100 mL) at 4 days which translated to yield of 34.62%/day. This nano-supplementation is suggestive of the importance and application of nanomaterials in microbial physiology, where such materials can stimulate the synthesis of bioproducts that may open a new vista in bioprocess optimization. Sanusi et al. [31] reported the positive influence of nickel oxide nanoparticles with improved yield of 18% achieved in bioethanol production by Saccharomyces cerevisiae BY4743 in potato peel-based medium, and enhanced fermentation efficiency from 40 to 60%. The citric acid yield obtained using ZnO nanobiocatalyst in this study led to 4.48-fold improvement compared with non-supplemented cashew apple juice and 1.38-fold of the supplementation with K2HPO4, NaNO3, MgSO4·7H2O and KCl. To the best of our knowledge, there is no report of nano-supplementation of media to increase citric acid production via fermentation. The inclusion of nanobiocatalyst in fermentation medium may be a new springboard to increase citric acid yield via fermentation. In previous works, Mg2+, Zn2+, and K+ among others have been identified as important metal ions necessary for increased formation of citric acid by Aspergillus niger [57, 58].
Optimization of Production of Citric Acid by A. niger LCFAn40 in Cashew Apple Medium Using Taguchi Technique
The strain with evidence of stability and higher yield among the mutants LCFAn40 was further explored for optimization using Taguchi technique [26] which produced variations of 2.30–11.80 g/100 mL in the production of citric acid and signal–noise ratio of 7.23–21.43 (Table 6). Citric acid production of 11.80 g/100 mL was therefore regarded as the local optimum. The signal–noise ratio was used to obtain the effect values (Table 7) to show the influence of each investigated parameter on the production of citric acid. It has been previously stated that the higher the signal–noise ratio, the more influential is such parameter on the production process [59]. The optimum condition, A3B3C1D1 (substrate concentration, 3%; fermentation time, 144 h; pH, 4.5; and inoculum size, 2%) was therefore obtained as combinations of parameters that gave the highest signal–noise ratio (Table 7). The experimentation of this profile yielded citric acid production of 16.67 g/100 mL as against 3.2 and 11.80 g/100 mL for the experimental lower and higher values, respectively (Table 5). It is evident that the improvement must have resulted from the interactive effects of the different parameters to produce the global optimum value. Further, from ANOVA (Table 8), substrate concentration, fermentation time, initial pH and inoculum size contributed 61.08, 22.14, 9.50, and 7.28%, respectively to the global optimal citric yield recorded. Taguchi technique is gaining wider applications in the optimization of bioprocesses that include production of citric acid [26, 59,60,61].
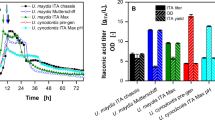
Comparative Analysis of Citric Acid Yield (%/Day)
In Table 9, the performance of the different optimization strategies were compared, which showed gradual improvement in the yield from 18.40%/day for mutant grown with sucrose supplementation to 92.61%/day for Taguchi approach. Correspondingly, the yield witnessed 2.38–11.98 folds in improvement. In the same vein, a comparative analysis of the optimal yield obtained in this study with those obtained in the literature (Table 10) showed that A. niger LCFAn40 produced higher yield of citric acid in cashew apple juice via Taguchi optimization. The greater results obtained in this study might have been influenced by the strain, the richness of cashew apple juice and the regimen of optimization techniques that were used. Specifically, this study reports the effect of nanobiocatalyst for citric acid production for the first time, and adds to the few reports on the use of the robust Taguchi technique for the optimization of production of citric acid. These adventures may serve as new inputs into investigations aiming at increasing citric acid production via fungal fermentation.
Conclusion
In this work, multi-dimensional techniques have been employed in achieving higher yield of citric acid up to 92.61%/day through fungal fermentation in cashew apple juice-based medium. A mutant strain, Aspergillus niger LCFAn40 which yielded yellow zonation of 9.0 cm CZA-BG had its performance at producing citric acid improved by nutrient and nanobiocatalyst supplementation as well as Taguchi optimization. At each step of the optimization, the yield of citric acid increased to give 2.38–11.98 folds improvement with the optimum obtained through Taguchi technique. These efforts have positioned both A. niger LCFAn40 and cashew apple juice as valuable inputs in the production of citric acid at high titers. For the first time, the employment of nanobiocatalyst in the form of biogenic ZnONPs to improve citric acid production in A. niger is reported, and this may open a new frontier aiming at improved bioprocess operations.
Availability of Data and Material
Data for the work are available with the authors.
Code Availability
Not applicable.
References
Behera, B.C.: Citric acid from Aspergillus niger: a comprehensive overview. Crit. Rev. Microbiol. 46, 727–749 (2020)
Sawant, O., Mahale, S., Ramchandran, V., Nagaraj, G., Bankar, A.: Fungal citric acid production using waste materials: a mini-review. J. Microbiol. Biotechnol. Food Sci. 8, 821–828 (2018)
Ciriminna, R., Meneguzzo, F., Delisi, R., Pogliaro, M.: Citric acid: emerging applications of key biotechnology industrial product. Chem. Central J. 11, 1–9 (2017)
Börekçi, B.S., Kaban, G., Kaya, M.: Citric acid production of yeasts: an overview. EuroBiotech. J. 5, 79–91 (2021)
Behera, B.C., Mishra, R., Mohapatra, M.S.: Microbial citric acid: production, properties, application and future perspectives. Food Front. 2, 62–76 (2021)
Cavallo, E., Charreau, H., Cerrutti, P., Foresti, M.L.: Yarrowia lipolytica: a model yeast for citric acid production. FEMS Yeast Res. 17, fox084 (2017)
Adeoye, A.O., Lateef, A., Gueguim-Kana, E.B.: Optimization of citric acid production using a mutant strain of Aspergillus niger on cassava peel substrate. Biocatal. Agric. Biotechnol. 4, 568–574 (2015)
Tong, Z., Tong, Y., Wang, D., Shi, Y.C.: Whole maize flour and isolated maize starch for production of citric acid by Aspergillus niger: a review. Starch-Stärke (2021). https://doi.org/10.1002/star.202000014
Elegbede, J.A., Lateef, A.: Valorization of corn-cob by fungal isolates for production of xylanase in submerged and solid state fermentation media and potential biotechnological applications. Waste Biomass Valor. 9, 1273–1287 (2018)
Elegbede, J.A., Lateef, A., Azeez, M.A., Asafa, T.B., Yekeen, T.A., Oladipo, I.C., Adebayo, E.A., Beukes, L.S., Gueguim-Kana, E.B.: Fungal xylanases-mediated synthesis of silver nanoparticles for catalytic and biomedical applications. IET Nanobiotechnol. 12, 857–863 (2018)
Elegbede, J.A., Lateef, A., Azeez, M.A., Asafa, T.B., Yekeen, T.A., Oladipo, I.C., Abbas, S.H., Beukes, L.S., Gueguim-Kana, E.B.: Silver-gold alloy nanoparticles biofabricated by fungal xylanases exhibited potent biomedical and catalytic activities. Biotechnol. Progr. 35, e2829 (2019)
Elegbede, J.A., Lateef, A., Azeez, M.A., Asafa, T.B., Yekeen, T.A., Oladipo, I.C., Aina, D.A., Beukes, L.S., Gueguim-Kana, E.B.: Biofabrication of gold nanoparticles using xylanases through valorization of corncob by Aspergillus niger and Trichoderma longibrachiatum: antimicrobial, antioxidant, anticoagulant and thrombolytic activities. Waste Biomass Valor. 11, 781–791 (2020)
Ganaie, M.A., Lateef, A., Gupta, U.S.: Enzymatic trends of fructooligosaccharides production by microorganisms. Appl. Biochem. Biotechnol. 172, 2143–2159 (2014)
Lateef, A., Oloke, J.K., Gueguim-Kana, E.B., Raimi, O.R.: Production of fructosyltransferase by a local isolate of Aspergillus niger in both submerged and solid substrate media. Acta Aliment. 41, 100–117 (2012)
Wang, B., Li, H., Zhu, L., Tan, F., Li, Y., Zhang, L., Shi, G.: High-efficient production of citric acid by Aspergillus niger from high concentration of substrate based on the staged-addition glucoamylase strategy. Bioprocess Biosyst. Eng. 40, 891–899 (2017)
Ji, H., Xiang, Z., Qi, H., Han, T., Pranovich, A., Song, T.: Strategy towards one-step preparation of carboxylic cellulose nanocrystals and nanofibrils with high yield, carboxylation and highly stable dispersibility using innocuous citric acid. Green Chem. 21, 1956–1964 (2019)
Özüdoğru, H.R., Nieder-Heitmann, M., Haigh, K.F., Görgens, J.F.: Techno-economic analysis of product biorefineries utilizing sugarcane lignocelluloses: xylitol, citric acid and glutamic acid scenarios annexed to sugar mills with electricity co-production. Ind. Crops Prod. 133, 259–268 (2019)
Monteiro, F., Catarino, L., Batista, D., Indjai, B., Duarte, M.C., Romeiras, M.M.: Cashew as a high agricultural commodity in West Africa: insights towards sustainable production in Guinea-Bissau. Sustainability 9, 1666 (2017)
Tola, J., Mazengia, Y.: Cashew production benefits and opportunities in Ethiopia: a review. J. Agric. Crop Res. 7, 18–25 (2019)
FAOSTAT. http://www.fao.org/faostat/en/#data/QC. Accessed 13 June 2021
STATISTA.: Production of cashew nuts (in shell) worldwide from 2010 to 2019. https://www.statista.com/statistics/967702/global-cashew-nut-production/. Accessed 13 June 2021
de Araujo Padilha, C.E., da Costa Nogueira, C., Oliveira Filho, M.A., de Santana Souza, D.F., de Oliveira, J.A., dos Santos, E.S.: Valorization of cashew apple bagasse using acetic acid pretreatment: production of cellulosic ethanol and lignin for their use as sunscreen ingredients. Process Biochem. 91, 23–33 (2020)
Jeyavishnu, K., Thulasidharan, D., Shereen, M.F., Arumugam, A.: Increased revenue with high value-added products from cashew apple (Anacardium occidentale L.)—addressing global challenges. Food Bioprocess Technol. 14, 985–1012 (2021)
Lowor, S.T., Agyente-Badu, C.K.: Mineral and proximate composition of cashew apple (Anarcadium occidentale L.) juice from northern savannah, forest and coastal savannah regions in Ghana. Am. J. Food Technol. 4, 154–161 (2009)
Adeeyo, A.O., Lateef, A., Gueguim-Kana, E.B.: Optimization of the production of extracellular polysaccharide from the Shiitake medicinal mushroom Lentinus edodes (Agaricomycetes) using mutation and a genetic algorithm-coupled artificial neural network (GA-ANN). Int. J. Med. Mushrooms 18, 571–581 (2016)
Elegbede, J.A., Lateef, A.: Optimization of the production of xylanases in corncob-based media by Aspergillus niger and Trichoderma longibrachiatum using Taguchi approach. Acta Biol. Szeged. 63, 51–58 (2019)
Gueguim-Kana, E.B., Oloke, J.K., Lateef, A., Adesiyan, M.O.: Modeling and optimization of biogas production on saw dust and other co-substrates using Artificial Neural Network and Genetic Algorithm. Renew. Energy 46, 276–281 (2012)
Gueguim-Kana, E.B., Oloke, J.K., Lateef, A., Donfack-Kana, A.F.: Pro-optimizer: a novel web enabled optimization engine for microbial fermentations. Biotechnol. Biotechnol. Equip. 24, 2137–2141 (2010)
Gueguim-Kana, E.B., Oloke, J.K., Lateef, A., Oyebanji, A.: Comparative evaluation of artificial neural network coupled genetic algorithm and response surface methodology for modelling and optimization of citric acid production by Aspergillus niger MCBN 297. Chem. Eng. Transact. 27, 397–402 (2012)
Gueguim-Kana, E.B., Oloke, J.K., Lateef, A., Zebaze-Kana, M.G.: Novel optimal temperature profile for acidification process of Lactobacillus bulgaricus and Streptococcus thermophilus in yoghurt fermentation using Artificial Neural Network and Genetic Algorithm. J. Ind. Microbiol. Biotechnol. 34, 491–496 (2007)
Sanusi, I.A., Suinyuy, T.N., Lateef, A., Gueguim-Kana, E.B.: Effect of nickel oxide nanoparticles on bioethanol production: process optimization, kinetic and metabolic studies. Process Biochem. 92, 386–400 (2020)
Sewsynker, Y., Gueguim-Kana, E.B., Lateef, A.: Modelling of biohydrogen generation in microbial electrolysis cells (MECs) using a committee of artificial neural networks (ANNs). Biotechnol. Biotechnol. Equip. 29, 1208–1215 (2015)
Dutta, A., Sahoo, S., Mishra, R.R., Pradhan, B., Das, A., Behera, B.C.: A comparative study of citric acid production from different agro-industrial wastes by Aspergillus niger isolated from mangrove forest soil. Environ. Exp. Biol. 17, 115–122 (2019)
Francisco, J.C.E., Rivera, W.L., Vital, P.G.: Influences of carbohydrate, nitrogen, and phosphorus sources on the citric acid production by fungal endophyte Aspergillus fumigatus P3I6. Prep. Biochem. Biotechnol. 50, 292–301 (2020)
Hesham, A.E.L., Mostafa, Y.S., AlSharqi, L.E.O.: Optimization of citric acid production by immobilized cells of novel yeast isolates. Mycobiol. 48, 122–132 (2020)
Papadaki, E., Mantzouridou, F.T.: Citric acid production from the integration of Spanish-style green olive processing wastewaters with white grape pomace by Aspergillus niger. Bioresour. Technol. 280, 59–69 (2019)
Hu, W., Li, W.J., Yang, H.Q., Chen, J.H.: Current strategies and future prospects for enhancing microbial production of citric acid. Appl. Microbiol. Biotechnol. 103, 201–209 (2019)
Ozdal, M., Kurbanoglu, E.B.: Citric acid production by Aspergillus niger from agro-industrial by-products: molasses and chicken feather peptone. Waste Biomass Valor. 10, 631–640 (2019)
Zhang, L., Zheng, X., Cairns, T.C., Zhang, Z., Wang, D., Zheng, P., Sun, J.: Disruption or reduced expression of the orotidine-5′-decarboxylase gene pyrG increases citric acid production: a new discovery during recyclable genome editing in Aspergillus niger. Microb. Cell Factories 19, 1–12 (2020)
Zhang, N., Jiang, J.C., Yang, J., Wei, M., Zhao, J., Xu, H., Yu, L.: Citric acid production from acorn starch by tannin tolerance mutant Aspergillus niger AA120. Appl. Biochem. Biotechnol. 188, 1–11 (2019)
Adeoye, A.O., Lateef, A.: Biotechnological valorization of cashew apple juice for the production of citric acid by a local strain of Aspergillus niger LCFS 5. J. Genet. Eng. Biotechnol. 19, 137 (2021)
Domsch, K.H., Gams, W., Anderson, T.H.: Compendium of soil fungi. 2nd Edition. Eching, IHV-Verlag. 672 p. (2007)
White, T.J., Bruns, T., Lee, S., Taylor, J.: Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J. (eds.) PCR Protocols: A Guide to Methods and Applications. Academic Press, New York (1990)
Adebayo, E.A., Oloke, J.K., Yadav, A., Barooah, M., Bora, T.C.: Improving yield performance of Pleurotus pulmonarius through hyphal anastomosis fusion of dikaryons. World J. Microbiol. Biotechnol. 29, 1029–1037 (2013)
Bhattacharjee, I., Baruah, P.K.: Comparison of locally available carbon rich substrates for augmented microbial production of citric acid with mutant Aspergillus niger S-6 strain. J. Pharmacog. Phytochem. 8, 2961–2964 (2019)
Kamalambigeswari, R., Alagar, S., Sivvaswamy, N.: Strain improvement through mutation to enhance pectinase yield from Aspergillus niger and molecular characterization of polygalactouronase gene. J. Pharmaceut. Sci. Res. 10, 989–994 (2018)
Ajala, A.S., Adeoye, A.O., Olaniyan, S.A., Fasonyin, O.T.: A study on effect of fermentation conditions on citric acid production from cassava peels. Sci. Afr. 8, e00396 (2020)
Umarov, U.A., Maslov, O.Y., Kolisnyk, S.V., Fathullaeva, M.: Development and validation of the conductometric titration method of quantitative determination of free organic acids in the anise fruits. Eur. J. Mol. Clin. Med. 7, 3874–3883 (2020)
Dienye, B.N., Ahaotu, I., Agwa, O.K., Odu, N.N.: Citric acid production potential of Aspergillus niger using Chrysophyllum albidum peel. Adv. Biosci. Biotechnol. 9, 190–203 (2018)
Taguchi, G.: Introduction to Quality Engineering: Designing Quality into Products and Processes. American Supplier Institute, Dearborn (1986)
Chou, W.J., Sun, C.H., Yu, G.P., Huang, J.H.: Optimization of the deposition process of ZrN and TiN thin films on Si(1 0 0) using design of experiment method. Mater. Chem. Phys. 82, 228–236 (2003)
Tsang, C.C., Tang, J.Y., Lau, S.K., Woo, P.C.: Taxonomy and evolution of Aspergillus, Penicillium and Talaromyces in the omics era–past, present and future. Comput. Struct. Biotechnol. J. 16, 197–210 (2018)
Zakaria, L.: Microscopic characterization of two black Aspergillus, A. niger and A. aculeatus from different substrates and indoor environment. Malays. J. Microsc. 16, 30–36 (2020)
Barwant, M., Lavhate, N.: Isolation and maintenance of fungal pathogens Aspergillus niger and Aspergillus flavus. Int. J. Appl. Nat. Sci. 9, 47–52 (2020)
Khandagale, A.B., Gangavane, S.C., Kulkarni, G.Y., Mandle, G.S., Upadhye, V.J.: Comparative analysis of citric acid production by Aspergillus niger using different media. Plant Cell Biotechnol. Mol. Biol. 22, 77–85 (2021)
Khoshroo, S.M.R.: Investigation of increased production of citric acid by Aspergillus niger mutant native strains. Appl. Biol. 33, 46–60 (2020)
Angumeenal, A.R., Venkappayya, D.: An overview of citric acid production. LWT-Food Sci. Technol. 50, 367–370 (2013)
Show, P.L., Oladele, K.O., Siew, Q.Y., Zakry, F.A.A., Lan, J.C., Ling, T.C.: Overview of citric acid production from Aspergillus niger. Front. Life Sci. 8, 271–283 (2015)
Rani, G.B., Chiranjeevi, T., Chandel, A.K., Satish, T., Radhika, K., Narasu, M.L., Uma, A.: Optimization of selective production media for enhanced production of xylanases in submerged fermentation by Thielaviopsis basicola MTCC 1467 using L16 orthogonal array. J. Food Sci. Technol. 51, 2508–2516 (2014)
Das, S.P., Gupta, A., Das, D., Goyal, A.: Enhanced bioethanol production from water hyacinth (Eichhornia crassipes) by statistical optimization of fermentation process parameters using Taguchi orthogonal array design. Int. Biodeter. Biodegr. 109, 174–184 (2016)
Yadegary, M., Hamidi, A., Alavi, S.A., Khodaverdi, E., Yahaghi, H., Sattari, S., Bagherpour, G., Yahaghi, E.: Citric acid production from sugarcane bagasse through solid state fermentation method using Aspergillus niger mold and optimization of citric acid production by Taguchi method. Jundishapur J. Microbiol. 6, e7625 (2013)
Yu, D., Shi, Y., Wang, Q., Zhang, X., Zhao, Y.: Application of methanol and sweet potato vine hydrolysate as enhancers of citric acid production by Aspergillus niger. Bioresour. Bioprocess. 4, 35 (2017)
Ayeni, A., Daramola, M.O., Taiwo, O., Olanrewaju, O.I., Oyekunle, D.T., Sekoai, P.T., Elehinafe, F.B.: Production of citric acid from the fermentation of pineapple waste by Aspergillus niger. Open Chem. Eng. J. 13, 88–96 (2019)
Vidya, P., Annapoorani, A.M., Jalalugeen, H.: Optimization and utilisation of various fruit peel as substrate for citric acid production by Aspergillus niger isolated from orange and carrot. Pharma Innov. J. 7, 141–146 (2018)
Zafar, M., Bano, H.S., Anwar, Z.: Orange peels valorization for citric acid production through single and co-culture fermentation. Jordan J. Biol. Sci. 14, 261–266 (2021)
Chergui, D., Akretche-Kelfat, S., Lamoudi, L., Al-Rshaidat, M., Boudjelal, F., Ait-Amar, H.: Optimization of citric acid production by Aspergillus niger using two downgraded Algerian date varieties. Saudi J. Biol. Sci. (2021). https://doi.org/10.1016/j.sjbs.2021.08.013
Acknowledgements
The assistance of Dr. T.B. Asafa of the Department of Mechanical Engineering, LAUTECH, Ogbomoso, Nigeria on the Taguchi optimization technique is duly acknowledged.
Funding
This work was not funded by any private or public entity.
Author information
Authors and Affiliations
Contributions
AL conceived, supervised, interpreted the results, wrote part of the manuscript and edited the manuscript; AOA carried out all the laboratory investigations, collected and analyzed the data and wrote part of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Authors declare that there is no conflict of interest.
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Adeoye, A.O., Lateef, A. Improving the Yield of Citric Acid Through Valorization of Cashew Apple Juice by Aspergillus niger: Mutation, Nanoparticles Supplementation and Taguchi Technique. Waste Biomass Valor 13, 2195–2206 (2022). https://doi.org/10.1007/s12649-021-01646-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-021-01646-0