Abstract
Purpose
The present study aims to recover value-added products from fresh pineapple processing wastes in an integrated biorefinery.
Methods
Bromelain, a therapeutic protease, was extracted from pineapple by-products via an aqueous, low-temperature process. Bromelain-free biomass was rich in insoluble fibre and was further fractionated into hemicellulose, cellulose and lignin.
Results
The highest content of active bromelain was obtained from pineapple core waste of the different varieties namely, Smooth Cayenne (~ 1.9 ± 0.05 CDU/mg), Giant Kew (1.6 ± 0.05 CDU/mg) and MD2 (1.5 ± 0.1 CDU/mg). The activity of extracted bromelain was close to commercial stem bromelain (2.2 ± 0.1 CDU/mg, % purity of 95–96%). The fractionation of fibrous residue (97.2 ± 0.5 g/100 g of dry mass) was optimised, and maximum yield of hemicellulose (97.5 ± 0.2%) was obtained with 5% (w/v) alkali at the end of 1.5 h. The hemicellulose and cellulose-rich residues were further valorised into xylooligosaccharides (26.1 ± 0.4 g/100 g of hemicellulose) and glucose (85.3 ± 1.7 g/100 g of cellulose-rich residue), respectively. From one ton of fresh pineapple processing waste, ~ 1 kg bromelain, ~ 24 kg xylooligosaccharides, ~ 88 kg glucose and ~ 68 kg residual hemicellulose could be obtained.
Conclusion
The proposed biorefinery concept not only addresses the environmental issues but also creates an opportunity to generate wealth in the form of products required for food and pharmaceutical industries from processing waste. As demand for more natural products is rising and consumers choice are prompted by healthy foods, conversion of pineapple waste into above mentioned valuable products creates a niche in food and therapeutics industry.
Graphic Abstract
Schematic for extraction of bromelain coupled with co-production of xylooligosaccharides and glucose from pineapple waste.

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Statement of Novelty
Pineapple is among the most widely consumed tropical fruits with a global production of 28.3 million metric tons. The fruit is widely processed as canned juice/slices and is known to generate a substantial amount of solid residues as high as 55–65% of the total weight of fresh fruit. In this study, pineapple processing waste has been demonstrated to be a valuable bioresource for the production of biofuels, biochemicals and bioproducts. This is the first attempt to propose a biorefinery approach from pineapple processing waste which offers a sustainable pathway towards the simultaneous recovery of high value products such as bromelain enzyme, xylooligosaccharides (a prebiotic) and glucose which can be further utilized as a substrate to produce high value chemicals.
Introduction
Solid and liquid waste generated by food processing industries is among the major contributors to environmental pollution. Efficient disposal of this waste is one large problem faced by the food processing industries [1, 2]. Large amounts of such wastes, especially from the vegetable and fruit processing industries are diverted as animal feed or as natural composts/fertilizers [3]. The strategies such as (a) reduction of the waste and (b) developing sustainable solutions to manage the residual waste could implement a circular economy in this industrial sector [4]. Waste valorization is often described as an approach of converting waste into value-added products and as a waste management strategy [4,5,6,7]. According to the European Commission [8], the bio-based economy utilizes bio-based renewable resources obtained from both land and sea, processing them into materials and energy for consumption. A fully functional bio-based economy is one of the many routes identified to achieve a circular economy. The principles of circular economy and bio-based economy work together in terms of the goal of attaining a sustainable technological and socio-economic development [9]. Food waste, mainly fruit and vegetable residues, have been demonstrated to be valuable bioresources that can find potential application in obtaining biofuels, biochemicals and bioproducts [4, 10]. The growth in demand for processed and packaged food has led to increased processing of fruits and vegetables, resulting in the generation of organic waste. The generated waste could be in the form of peels, kernels, and leaves as well as edible components that are discarded as wrong size off-cuts. The recent developments in the field of bioprocess engineering and the increasing quantum of food by-products have also increased the interest of stakeholders in the valorization of such wastes [11,12,13]. The potential of various fruit wastes, such as citrus, mango, pomegranate and avocado, have been explored incorporating the biorefinery concept [14,15,16,17].
Pineapple is among the most widely consumed tropical fruits with a global production of 28.3 million metric tons in 2018 [18]. India is the sixth-largest producer of pineapples with an annual production of 1.7 million metric tons in 2018 [19] while Australia reported annual production of 0.076 million metric tons in 2018 [20]. The fruit is widely processed as canned juice/slices and is known to generate a substantial amount of solid residues as high as 55–65% of the total weight of fresh fruit [1, 11, 21]. The core and peels are a good source of a proteolytic enzyme, bromelain, which shows promising applications in the food and therapeutic sectors [22]. Pineapple waste, particularly peels and crown, are a rich source of hemicellulose. The valorization of hemicellulose in an integrated biorefinery approach could generate valuable co-products from the lignocellulosic biomass in addition to cellulosic ethanol as the major product [23]. Such an integrated process can turn agro-industrial wastes into multiple value-added products and improve the overall economics of the process. Specific valorization strategies have been proposed for pineapple waste for its integral valorization in a biorefinery approach. Gil et al. 2018 have proposed the extraction of bromelain from pineapple wastes followed by co-production of bioethanol with the remaining residue [24]. Similarly, Sepulveda et al., demonstrated the effective recovery of glycosides and polyphenols from pineapple wastes via autohydrolysis [25]. They further proposed the utilization of such value-added molecules in food, cosmetics and health products. Pineapple by-products have also been investigated as low-cost substrates for production of organic acids such as citric, ferulic and lactic acids using fermentation technology due to the commercial value of these products in the food and pharmaceutical sectors [26, 27]. Campos et al. 2020 have recently reported a green approach for valorization of pineapple by-products (peels and stem) [28]. Liquid and solid fractions were obtained from both, peels and stem. The liquid fraction was rich in two ingredients, namely, a enzymatic bromelain-rich fraction and another fraction rich in polyphenols and soluble dietary fibre [28]. In another valorization strategy, solid-state fermentation of pineapple peel biomass with Trichoderma viride produced high protein fungal biomass which could be recommended as suitable animal feed [29]. Casabar et al. 2019 evaluated the effects of alkaline pretreatment and microbial hydrolysis through Trichoderma harzianum of pineapple fruit peel [30]. Bioethanol yield of 5.98 ± 1.01 g/L from pineapple fruit peel was successfully produced at 48 h of fermentation. In another study, de Ramos et al. 2020 recovered bioactive compounds and sugars from pineapple waste extract using spray-drying technology [31]. Teixeira et al. 2021 isolated filamentous fungi compatible-consortia from pineapple wastes for cellulose-degrading enzymes production using pineapple crown waste in solid-state cultivation. Further, the potential of the cocktail enzymes was evaluated for saccharification of pineapple crown waste [32]. Along the same line, the current work focuses upon the utilization of industrial fresh pineapple processing waste in an integrated biorefinery approach. This is the first study to best of our knowledge that explores bromelain, xylooligosaccharides (XOS) and glucose as the major biorefinery products from pineapple processing wastes. Three commonly processed pineapple varieties; Smooth Cayenne (Australian), MD2 (Australian) and Giant Kew (Indian) were used in the study to compare the yields of products and impact of geographical differences on the composition of pineapple waste.
Materials and Methods
Raw Material
Different varieties of pineapples (Smooth Cayenne, MD2 and Giant Kew) were procured from local markets in India and Australia. The pineapples were processed by removing the crown, peeling off the skin and separating the pulp from the core. The waste parts were then frozen at -20˚C until further use.
Chemicals
All the analytical grade chemicals were used as received. Sodium hydroxide, glacial acetic acid, the total dietary fibre assay kit and other chemicals were procured from Sigma Aldrich. Millipore water was used for preparing all other reagents. The enzymes were procured from Megazyme.
Extraction of Bromelain
The method for extraction of bromelain was adapted from the previous study conducted by the same group [33]. Briefly, fresh pineapple processing waste (peels, core and crown) was blended with cold phosphate buffer (50 mM, pH 7.0) in the ratio 1:1 in a laboratory blender for 30 s. The liquid and the solid residue were separated using a cheesecloth. The solid residue was diverted to another valorization stream of the biorefinery. The liquid extract was centrifuged at 4 °C for 20 min at 3260 g to remove the suspended solids. The final supernatant obtained was the enzyme-rich extract and was later tested for its activity. Different precipitants (acetone, acetonitrile, ammonium sulphate, ethanol) were evaluated for precipitation efficiency. The precipitant was added to the crude extract in the ratio 1:5 and kept for overnight precipitation of bromelain. The precipitated enzyme was centrifuged at 4 ˚C and freeze-dried for further characterization and activity test.
Assay to Measure Bromelain Activity
The enzymatic activity of bromelain was expressed in terms of casein digestion unit (CDU) according to the method by Murachi with casein (0.65%, w/v) as the substrate [34]. The principle of the colorimetric assay was based on enzymatic hydrolysis of the peptide bonds in casein which results in the release of a free amino acid (L-tyrosine). Trichloroacetic acid (TCA) was used to quench the hydrolysis reaction and precipitate any unhydrolyzed casein. The precipitate was then removed through centrifugation followed by filtration with 0.45 µm syringe filters. The clear filtrate was used to record absorbance value at 280 nm on a 96-well plate reader. A blank was prepared with the reaction quenching agent (TCA) and casein solution followed by the addition of the enzyme extract. The blank sample contains unreacted casein which was precipitated before the addition of enzyme. The enzyme activity was expressed by the equation described below:
where one casein digestion unit (CDU) is defined as 1 µg of L-tyrosine liberated per min per mL of the sample when casein is hydrolysed for 10 min at 37 °C and pH 7.0. Experiments were conducted in triplicates, and calculations were performed with the average activity value.
Characterization of Extracted Bromelain
Attenuated Total Reflection Infrared Spectroscopy (ATR-IR)
The functional features of the enzyme were determined using attenuated total reflection infrared spectroscopy. The analysis was undertaken with an FT-IR Spectrophotometer model Cary 640, provided with a universal attenuated total reflectance unit. Spectra of the extracted and commercial bromelain samples were recorded from 4000 to 600 cm−1 at 64 scans, with a spectral resolution of 2 cm−1 with a blank window for the background.
Molecular Weight Determination
The molecular weight of the enzyme samples was determined with Liquid Chromatography-Electrospray Ionization-Mass Spectrometry (LC–ESI–MS) using MicroTOFq, a quadrupole TOF mass spectrometer (Bruker Daltonics, Bremen, Germany) coupled online with a 1200 series capillary HPLC (Agilent Technologies, Santa Clara, CA, USA). The enzyme samples were injected onto a MabPac SEC-1 5 µm, 300 Å, 50 × 4 mm (Thermo Scientific) column. The mobile phase constituted of 50% acetonitrile 0.05% trifluoroacetic acid, 0.05% formic acid was adjusted at a flow rate of 50 µL/min. The sample was eluted over a 20 min run-time monitored by UV detection at 254 nm. The Bruker electrospray source with a capillary voltage of 4500 V dry gas was used to nebulize and ionize the eluant at 180 °C, flow rate of 4 L/min, and nebulizer gas pressure at 300 mbar. After 20 min the flow path is switched to infuse low concentration tune mix (Agilent technologies, Santa Clara, CA, USA) to calibrate the spectrum post-acquisition. The spectra were extracted and deconvoluted using Data explorer software version 3.4 build 192 (Bruker Daltonics, Bremen, Germany).
Extraction of Dietary Fibre Concentrate (DFC)
After the extraction of bromelain and removal of adhering free sugars, the residual material was washed thrice with warm water (30 °C) 1:1 (v/w) followed by drying at 60 °C for 12 h in hot air oven before grinding them to a particle size of 100–500 µm. The loss of soluble fibre components as well as bioactive compounds, was minimized by washing the material with water under mild conditions.
Chemical Analysis of DFC
Moisture, fat, protein and ash content of the DFC were determined by AOAC methods [35]. Briefly, moisture (g water/100 g sample) was determined by drying 5 g of the sample at 105 ˚C until a constant weight was achieved. Ash content (g ash/100 g dry matter) was determined by heating the sample in a muffle furnace at 525 ± 25 °C for 6 h. Protein (g protein/100 g dry matter) was calculated by determining the total N content of the sample on a dry basis. Fat (g fat/100 g dry matter) was determined by weight loss after a 10-cycle extraction with hexane in a Soxhlet apparatus. Total dietary fibre (TDF) and insoluble dietary fibre (IDF) were determined by the TDF assay kit based upon the enzymatic gravimetric method [36]. Soluble dietary fibre (SDF) was calculated by subtracting the IDF content from the TDF. Each assay was carried out in triplicate.
Functional Properties of DFCs
Functional properties namely, water-holding capacity (WHC), swelling capacity (SWC) and oil-holding capacity (OHC) were investigated according to the method described by Martínez et al. with few modifications [37]. Briefly, 25 mL of phosphate buffer (1 M, pH 6.3) or commercial cooking oil (canola oil in this case) were added to 0.25 g of dry sample, stirred and left at room temperature for 1 h. The residue was weighed after centrifugation. WHC was calculated as g of water held by 1 g of sample, while OHC was articulated as g of oil held by 1 g of sample. Swelling capacity was estimated by accurately weighing 0.2 g of dry sample in a graduated measuring cylinder and adding 10 mL of phosphate buffer (1 M, pH 6.3) to it. The final volume attained by the fibre was measured after the mixture was hydrated for a period of 18 h. All the estimations were carried out in triplicate.
Extraction of Hemicellulose from Pineapple Pomace Fibre
The cellulose, hemicellulose and lignin content of the pineapple pomace fibre were estimated by the Van Soest and NREL protocols [38,39,40]. Hemicellulose along with cellulose-rich residue was extracted from pineapple pomace by the hydrothermal-assisted alkali-based method [23]. Briefly, the extraction was carried out at 121 °C (without any previous incubation) with varying concentrations of sodium hydroxide (5, 10, 15% w/v) and solid to liquid ratio of 1:10 for 30, 60 and 90 min. Dried pineapple pomace powder was mixed with alkali solution of different concentrations at 121 °C in the hydrothermal reactor at 15 psi pressure. After the desired reaction time, the reactor was depressurized, and the solution was filtered. The residual matter was washed with hot water and acetone to remove the adhering sugars and alkali. The pH of the liquor was set to pH 5 with glacial acetic acid followed by overnight precipitation of hemicellulose using 95% (v/v) ice-cold ethanol [23].
Scanning Electron Microscopy (SEM)
Morphological features of the residual biomass left after extraction of hemicellulose were analyzed using a Hitachi SU8030 Scanning Electron Microscope. Powdered samples were coated over carbon tape mounted over the sample holder. The cellulosic residue left after pre-treatment was sputtered with gold plasma to improve SEM images by reducing electrical charging of the samples.
Enzymatic Production of XOS
The hemicellulose extracted from pineapple pomace was enzymatically hydrolyzed using endo-1,4-β-Xylanase M1 obtained from Trichoderma viride (Megazyme, U.S.A.). The optimized enzymatic method (50 °C, pH 5.0 and 15 U enzyme dose) for production of XOS was adapted from Banerjee et al., 2019. The XOS (xylobiose and xylotriose) and sugar monomers produced by enzymatic hydrolysis were analyzed using HPLC (Agilent Technologies 1200 Infinity series). The sugar oligomers and monomers were eluted using 5 mM sulfuric acid in HPLC grade water as mobile phase at a flow rate of 0.7 mL/min. The samples were analyzed using Hi-Plex H column (300 × 7.7 mm) attached to a refractive index detector (RID), operating at 65 °C and 50 °C, respectively.
Enzymatic Production of Glucose
The dried cellulose-rich residue was enzymatically hydrolyzed with cellulase from Trichoderma reesei. The optimized conditions for enzymatic hydrolysis were obtained from previous studies with slight modifications [41, 42]. Briefly, 60 U enzyme/g substrate was incubated with 10% (w/v) solid loading of substrate at pH 5.5, 50 °C, 120 rpm and 72 h to obtain glucose. The enzyme was inactivated by heating the hydrolysate at 95 °C followed by centrifugation at 2840 g for 10 min to settle down the enzyme and the unreacted cellulose. The supernatant was filtered using 0.2 µm pore size filter followed by determination of glucose concentration using high performance liquid chromatography.
Statistical Analysis
All the experiments in the study were carried out in triplicates and results have been expressed as mean ± standard deviation. One way Analysis of variance (ANOVA) was conducted to determine the statistical significance of the response (p < 0.05). The difference between bromelain content (and enzymatic activity) present in different pineapple by-products (crown, peels, core) of the three different varieties and commercial stem bromelain was evaluated using a one-way ANOVA. The compositional difference among pineapple by-products (core, pomace and peels) for the three varieties was evaluated using two-way ANOVA. Means were compared using Tukey’s test at 95% confidence interval. Minitab Statistical software version 18 (Pennsylvania State University, USA) was used for analyzing the data.
Results and Discussion
Effect of Precipitant on the Activity of Bromelain
The efficacy of the precipitant (acetone, acetonitrile, ammonium sulphate, ethanol) was determined by evaluating the activity of the precipitated enzyme (Figure ES1). Saturated ammonium sulphate (60% w/v) and ethanol (95%, v/v) were among the best precipitants for bromelain. Ethanol, being a green solvent, was then chosen as the precipitating agent for further experiments. Ethanolic precipitation was chosen as the purification method for its ease to scale up [43, 44]. All the by-products, namely, crown, peels and core from pineapple processing, were found to contain bromelain (Table 1). However, the highest content of active bromelain was obtained from pineapple core waste (Smooth Cayenne variety) (~ 1.9 ± 0.1 CDU/mg) which is at par with the activity of commercial pineapple stem bromelain (2.2 ± 0.1 CDU/mg). Similarly, core waste contains the maximum amount of active bromelain in case of other varieties, namely, Giant Kew (1.6 ± 0.1 CDU/mg) and MD2 (1.5 ± 0.1 CDU/mg). The enzymatic activity is lower than that of commercial stem bromelain but is better than the bromelain obtained from pineapple peels and crown waste.
Characterization of Extracted Bromelain
The bromelain extracted from pineapple core was then characterized using ATR-IR and molecular weight determination to ascertain its similarity with commercial stem bromelain.
Attenuated Total Reflection Infrared Spectroscopy (ATR-IR)
Typical broad bands at 3350–3400 cm−1 were observed for both commercial and extracted bromelain. These bands represent O–H stretching vibrations arising due to hydrogen bonds or a contribution from an N–H stretching mode. The bands between 3000 and 2800 cm−1 contributed to C–H stretching vibrations. The most signature spectral region for protein structure was observed between 1800 and 1500 cm−1 and corresponds to amide I and amide II (Fig. 1). The characteristic absorption bands at ~ 3380 cm−1 and ~ 1600 cm−1 contributed to the –NH2 and –COO group in the enzyme [45].
Molecular Weight Determination
The molecular weight of commercial and extracted bromelain was estimated with Liquid Chromatography-Electrospray Ionization-Mass Spectrometry (LC–ESI–MS). The protein in the samples was detected and is seen as multiple species with a delta mass of approx. 75 Da and 88 Da (a mass difference of an unknown modification). Both samples contained this spread of masses in the 24 kDa region (Fig. 2) [46]. The main difference between the two samples is that a species around 26 kDa was observed in the extracted bromelain but it was negligible in the commercial bromelain sample. The difference could be attributed to residual impurity in the extracted bromelain.
Extraction of Dietary Fibre Concentrate
Chemical Composition of Dietary Fibre Concentrates
Table 2 shows the proximate composition of the residual fibrous material left after extraction of bromelain from pineapple wastes. For all the three varieties of pineapple considered in this study, the core and pomace fibres are reported to have lower ash content in comparison to the peel. Table 3 shows the quantification of TDF, IDF and SDF in the dietary fibre concentrate. A large amount of IDF present in pineapple waste is an indicator of the significant amounts of lignocellulosic matter present in it. The soluble dietary fibre content is relatively low (~ 2 g per 100 g of DFC). Hu & Zhao have reported that a maximum of 8.76% of soluble dietary fibre can be obtained from pineapple pomace by shear homogenization-assisted extraction [47]. A high proportion of IDF in the dietary fibre concentrate could be considered an advantage because IDF could be used in the food industry to increase the bulking effect of the food [48,49,50]. Besides, a higher IDF content could have beneficial health effects such as increase in satiety, increase in the volume and weight of the faecal mass, promoting better functioning of the digestive system.
Functional Properties of Dietary Fibre Concentrate
WHC is a very significant functional property of the dietary fibre concentrate. It depicts the capacity of a moist substrate to hold water when subjected to an external centrifugal gravity force or compression [50]. WHC technically correlates with the quantity of insoluble fibre present in the dietary fibre concentrate. Among the different pineapple by-products, the fibre concentrates from pomace waste were found to possess the highest WHC i.e. 14.4 ± 1.5 g water/g sample (Fig. 3). Selani et al. reported lower WHC of pineapple pomace i.e. 5.3 ± 0.6 g water/g sample [50]. This variation in the values of WHC could be attributed to the differences in the material used for the analysis. The present study evaluated the dietary fibre concentrate extracted from the pineapple pomace and core while Selani et al. evaluated the pomace as a whole. Prakongpan et al. reported the WHC of dietary fibres extracted from pineapple pomace to be in the range 10.3–12.2 g water/g sample [51]. Since the dietary fibres from pineapple pomace and core were rich in the insoluble fibre content, their WHC value was found to be higher than orange peel (1.6 g water/g sample), lemon peel (1.7–1.8 g water/g sample) and apple pomace (1.6–1.8 g water/g sample) [52]. Swelling capacity is another significant hydration property correlating the cellulosic components present in the fibre. The dietary fibres from core (5.8 ± 1.2 mL/g) and pomace (5.5 ± 0.7 mL/g) have reported higher swelling capacity when compared to those from the peels (3.6 ± 0.6 mL/g).
OHC is another functional property related to the chemical structure of the plant polysaccharide. However, the OHC values for the fibre concentrate is quite low when compared to other fibrous materials such as coconut fibre (5.3 g oil/g fibre) [53]. Hence, the foods supplemented with these co-products will not be capable of retaining high amounts of oil. Hence, the specific use of dietary fibre in food products is largely determined by its functional properties.
Pineapple Pomace as a Source of Hemicellulose, XOS and Glucose
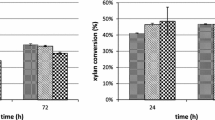
The pineapple pomace was found to be rich in hemicellulose content (48.4 ± 1.6 g per 100 g of dried extractive-free pomace) with low lignin content (2.1 ± 0.6 g per 100 g of dried extractive-free pomace), hence it was explored as a source of hemicellulose for production of XOS. The maximum relative recovery of 98.7 ± 0.8% of hemicellulose was obtained with 10% (w/v) alkali at the end of 1.5 h of hydrothermal extraction (121 ˚C and 15 psi pressure). A significant yield of hemicellulose (97.5 ± 0.2%) was obtained with 5% (w/v) alkali at the end of 1.5 h (Table 4). The statistical model for recovery of hemicellulose contains two main effect terms, such as time (h) and alkali concentration (%) along with one interaction term. Statistical results of the model showed that the individual terms and interaction terms were significant (Table 5, 6). The adjusted R2 for this model was 98.7% which indicates a good fit of the model. The contour plot (Fig. 4) shows that the extraction time is the most influential factor in the recovery of hemicellulose from pineapple pomace waste. Above 1 h of extraction time, more than 90% of the hemicellulose could be recovered with all concentrations of alkali. No significant increase was observed in the % recovery of hemicellulose when the alkali concentration was increased at extraction times more than 1 h. This could be attributed to the synergistic effect of time-alkali concentration combination on the % recovery of hemicellulose. Higher alkali concentrations are often associated with higher costs and downstream challenges. In addition to this, another disadvantage associated with higher alkali dosage is the significant loss of polysaccharides [23]. Therefore, in the present study, a combination of 5% (w/v) alkali and 1.5 h duration was selected to pretreat pineapple pomace for hemicellulose recovery for further processing into XOS. Under the optimal experimental conditions of 50 ˚C, pH 5.0 and enzyme dosage 15 U/mg, a maximum of 26.1 ± 0.4 g of XOS was obtained per 100 g of hemicellulose at the end of 24 h (Fig. 5). The total XOS consists of xylobiose and xylotriose which are known for their maximum prebiotic potential [23, 54]. The residual hemicellulose can further be converted into a wide range of value-added chemicals such as butanol, furfural derivatives, xylitol and others [55,56,57,58,59,60,61,62]. Furfurals further find potential application in the pharmaceutical, plastics and agro-chemicals industries [55]. Conversion of pentose sugars into ethanol is another widely studied approach for valorizing hemicellulose in the lignocellulosic biorefineries [63,64,65,66].
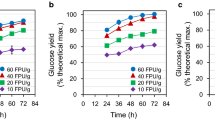
The pomace powder was analysed by scanning electron microscopy for its morphological changes after the extraction of hemicellulose. The SEM micrographs (Fig. 6) show that the structure of cellulosic pomace residue has opened up after the hydrothermal-assisted alkali treatment and is more prone to enzymatic degradation for its easy conversion into glucose which can further be valorized to obtain other value-added chemicals. The enzymatic hydrolysis of cellulose led to a recovery of 85.3 ± 1.7 g of glucose per 100 g of cellulose-rich residue at the end of 72 h (Fig. 7) which is at par with the recovery of glucose reported in literature. Singh et al. 2017, reported the production of 71.5 ± 1.9 g of glucose per 100 g of pre-treated arecanut husk biomass via enzymatic hydrolysis [67].
Conclusion
In this study, the valorization approach was focussed upon the generation of multiple products in an integrated biorefinery using fresh pineapple processing waste as the feedstock. Bromelain was obtained as a crude enzyme extract. Further purification was done by precipitating the enzyme using ethanol. The highest content of active bromelain was obtained from pineapple core waste (Smooth Cayenne variety) (~ 1.9 ± 0.1 CDU/mg) which is close to the activity of commercial pineapple stem bromelain (2.2 ± 0.1 CDU/mg, % purity of 95–96%). The core waste from other varieties of pineapple, i.e. Giant Kew (1.6 ± 0.1 CDU/mg) and MD2 (1.5 ± 0.1 CDU/mg), also produced bromelain with similar enzymatic activity. The residue left after extraction of bromelain was found to be rich in insoluble fibre content and was analysed for its functional properties. Among the different pineapple by-products, the fibre concentrates from pomace were found to possess the highest water-holding capacity i.e. 14.4 ± 1.5 g water/g sample followed by peels (11.2 ± 1.1 g water/g sample) and core (11.6 ± 1.3 g water/g sample). Thus, pomace can be blended with soluble dietary fibres such as apple pomace (rich in pectin) to meet the requirements for both soluble and insoluble fibres. Being low in lignin content (2.1 ± 0.6 g per 100 g of dried extractive-free sample), pomace was further fractionated into hemicellulose and cellulose-rich residues which were valorized into XOS (26.1 ± 0.4 g/100 g of hemicellulose) and glucose (85.3 ± 1.7 g/100 g of cellulose in pomace), respectively. The glucose can further be converted into potential biofuels and biochemicals in the biorefinery portfolio. The products (bromelain, insoluble dietary fibre, XOS and glucose) have an existing market demand in the food and therapeutic sector. Therefore, the biorefinery approach described in this work might help in the overall utilization of fresh pineapple processing waste. Methods for complete utilization of by-products resulting from pineapple processing should be developed on a large scale and at affordable levels. Life-cycle analysis and techno-economic feasibility studies must be conducted to evaluate the commercial viability of the proposed biorefinery.
References
Banerjee, S., Ranganathan, V., Patti, A., Arora, A.: Valorisation of pineapple wastes for food and therapeutic applications. Trends Food Sci. Technol. 82, 60–70 (2018). https://doi.org/10.1016/j.tifs.2018.09.024
Patsalou, M., Chrysargyris, A., Tzortzakis, N., Koutinas, M.: A biorefinery for conversion of citrus peel waste into essential oils, pectin, fertilizer and succinic acid via different fermentation strategies. Waste Manag. 113, 469–477 (2020). https://doi.org/10.1016/j.wasman.2020.06.020
Goula, A.M., Lazarides, H.N.: Integrated processes can turn industrial food waste into valuable food by-products and/or ingredients: the cases of olive mill and pomegranate wastes. J. Food Eng. 167, 45–50 (2015)
Garcia-Garcia, G., Stone, J., Rahimifard, S.: Opportunities for waste valorisation in the food industry—a case study with four UK food manufacturers. J. Clean. Prod. 211, 1339–1356 (2019). https://doi.org/10.1016/j.jclepro.2018.11.269
del Pozo, C., Bartrolí, J., Alier, S., Puy, N., Fàbregas, E.: Production of antioxidants and other value-added compounds from coffee silverskin via pyrolysis under a biorefinery approach. Waste Manag. 109, 19–27 (2020). https://doi.org/10.1016/j.wasman.2020.04.044
Karmee, S.K.: A spent coffee grounds based biorefinery for the production of biofuels, biopolymers, antioxidants and biocomposites. Waste Manag. 72, 240–254 (2018). https://doi.org/10.1016/j.wasman.2017.10.042
Venkata Mohan, S., Nikhil, G.N., Chiranjeevi, P., Nagendranatha Reddy, C., Rohit, M.V., Kumar, A.N., et al.: Waste biorefinery models towards sustainable circular bioeconomy: critical review and future perspectives. Bioresour. Technol. 215, 2–12 (2016). https://doi.org/10.1016/j.biortech.2016.03.130
Lokesh, K., Ladu, L., Summerton, L.: Bridging the gaps for a “circular” bioeconomy: selection criteria, bio-based value chain and stakeholder mapping. Sustain 10, 1695 (2018). https://doi.org/10.3390/su10061695
Hennig, C., Brosowski, A., Majer, S.: Sustainable feedstock potential—a limitation for the bio-based economy? J. Clean. Prod. 123, 200–202 (2016). https://doi.org/10.1016/j.jclepro.2015.06.130
Pfaltzgraff, L.A., De Bruyn, M., Cooper, E.C., Budarin, V., Clark, J.H.: Food waste biomass: a resource for high-value chemicals. Green Chem. 15, 307–314 (2013). https://doi.org/10.1039/c2gc36978h
Roda, A., De Faveri, D.M., Giacosa, S., Dordori, R., Lambri, M.: Effect of pre-treatments on the saccharification of pineapple waste as a potential source for vinegar production. J. Clean. Prod. 112, 4477–4484 (2016)
Lamine, M., Gargouri, M., Rahali, F.Z., Mliki, A.: Recovering and characterizing phenolic compounds from citrus by-product: a way towards agriculture of subsistence and sustainable bioeconomy. Waste Biomass Valoriz (2020). https://doi.org/10.1007/s12649-020-01306-9
Goula, A.M., Papatheodorou, A., Karasavva, S., Kaderides, K.: Ultrasound-assisted aqueous enzymatic extraction of oil from pomegranate seeds. Waste Biomass Valoriz (2018). https://doi.org/10.1007/s12649-016-9740-9
Talekar, S., Patti, A.F., Vijayraghavan, R., Arora, A.: An integrated green biorefinery approach towards simultaneous recovery of pectin and polyphenols coupled with bioethanol production from waste pomegranate peels. Bioresour. Technol. 266, 322–334 (2018). https://doi.org/10.1016/j.biortech.2018.06.072
Banerjee, J., Singh, R., Vijayaraghavan, R., MacFarlane, D., Patti, A.F., Arora, A.: A hydrocolloid based biorefinery approach to the valorisation of mango peel waste. Food Hydrocoll. 77, 142–151 (2018). https://doi.org/10.1016/j.foodhyd.2017.09.029
Boukroufa, M., Boutekedjiret, C., Petigny, L., Rakotomanomana, N., Chemat, F.: Bio-refinery of orange peels waste: a new concept based on integrated green and solvent free extraction processes using ultrasound and microwave techniques to obtain essential oil, polyphenols and pectin. Ultrason. Sonochem. 24, 72–79 (2015). https://doi.org/10.1016/j.ultsonch.2014.11.015
Dávila, J.A., Rosenberg, M., Castro, E., Cardona, C.A.: A model biorefinery for avocado (Persea americana mill.) processing. Bioresour. Technol. 243, 17–29 (2017). https://doi.org/10.1016/j.biortech.2017.06.063
FAO: Major Tropical Fruits Market Review 2018. FAO, Rome (2019)
APEDA.: Indian production of pineapple. National Horticulture Board (2018). http://agriexchange.apeda.gov.in/IndiaProduction/India_Productions.aspx?cat=fruit&hscode=1056. Accessed March 3, 2020
HortInnovation.: Australian Horticulture Satistics Handbook—Fruits (2018). https://www.horticulture.com.au/growers/help-your-business-grow/research-reports-publications-fact-sheets-and-more/HA18002/. Accessed March 3, 2020
Abdullah, M.B., Mat, H.: Characterisation of solid and liquid pineapple waste. Reaktor 12, 48–52 (2008)
Ketnawa, S., Chaiwut, P., Rawdkuen, S.: Pineapple wastes: a potential source for bromelain extraction. Food Bioprod. Process 90, 385–391 (2012). https://doi.org/10.1016/j.fbp.2011.12.006
Banerjee, S., Patti, A.F., Ranganathan, V., Arora, A.: Hemicellulose based biorefinery from pineapple peel waste: xylan extraction and its conversion into xylooligosaccharides. Food Bioprod. Process 117, 38–50 (2019). https://doi.org/10.1016/j.fbp.2019.06.012
Seguí, L., Fito Maupoey, P.: An integrated approach for pineapple waste valorisation. Bioethanol production and bromelain extraction from pineapple residues. J. Clean. Prod. 172, 1224–1231 (2018). https://doi.org/10.1016/j.jclepro.2017.10.284
Sepúlveda, L., Romaní, A., Aguilar, C.N., Teixeira, J.: Valorization of pineapple waste for the extraction of bioactive compounds and glycosides using autohydrolysis. Innov. Food Sci. Emerg. Technol. 47, 38–45 (2018). https://doi.org/10.1016/j.ifset.2018.01.012
Kongsuwan, A., Suthiluk, P., Theppakorn, T., Srilaong, P., Setha, V.: Bioactive compounds and antioxidant capacities of phulae and nanglae pineapple. Asian J. Food Agro-Ind. 2, S44–S50 (2009)
Roda, A., Lambri, M.: Food uses of pineapple waste and by-products: a review. Int. J. Food Sci. Technol. 54, 1009–1017 (2019). https://doi.org/10.1111/ijfs.14128
Campos, D.A., Ribeiro, T.B., Teixeira, J.A., Pastrana, L., Pintado, M.M.: Integral valorization of pineapple (Ananas comosus L.) by-products through a green chemistry approach towards added value ingredients.Foods (2020). https://doi.org/10.3390/foods9010060
Aruna, T.E.: Production of value-added product from pineapple peels using solid state fermentation. Innov. Food Sci. Emerg. Technol. (2019). https://doi.org/10.1016/j.ifset.2019.102193
Casabar, J.T., Unpaprom, Y., Ramaraj, R.: Fermentation of pineapple fruit peel wastes for bioethanol production. Biomass Convers Biorefinery 9, 761–765 (2019). https://doi.org/10.1007/s13399-019-00436-y
de Ramos, R.M.Q., Siacor, F.D.C., Taboada, E.B.: Effect of maltodextrin content and inlet temperature on the powder qualities of spray-dried pineapple (Ananas comosus) waste extract. Waste Biomass Valoriz. 11, 3247–3255 (2020). https://doi.org/10.1007/s12649-019-00651-8
Teixeira, W.F.A., Batista, R.D., do Amaral Santos, C.C.A., Júnior, A.C.F., Terrasan, C.R.F., de Santana, M.W.P.R., et al.: Minimal enzymes cocktail development by filamentous fungi consortia in solid-state cultivation and valorization of pineapple crown waste by enzymatic saccharification. Waste Biomass Valoriz. 12, 2521–2539 (2021). https://doi.org/10.1007/s12649-020-01199-8
Banerjee, S., Ranganathan, V., Arora, A., Patti, A.F.: Green approach towards hydrolysing wheat gluten using waste ingredients from pineapple processing industries. Int. J. Food Sci. Technol. (2020). https://doi.org/10.1111/ijfs.14796
Murachi, T.: [39] Bromelain enzymes. Methods Enzymol 45, 475–485 (1976). https://doi.org/10.1016/S0076-6879(76)45042-5
AOAC.: Determination of moisture, ash, protein and fat. Off method. Anal. Assoc. Anal. Chem. (2005)
Lee, S.C., Prosky, L., DeVries, J.W.: Determination of total, soluble and insoluble dietary fiber in foods-enzymatic-gravimetric method, MES-tris buffer: collaborative study. J. AOAC Int. 75, 395–416 (1992)
Martínez, R., Torres, P., Meneses, M.A., Figueroa, J.G., Pérez-Álvarez, J.A., Viuda-Martos, M.: Chemical, technological and in vitro antioxidant properties of mango, guava, pineapple and passion fruit dietary fibre concentrate. Food Chem. 135, 1520–1526 (2012). https://doi.org/10.1016/j.foodchem.2012.05.057
Van Soest, P.J., Robertson, J.B., Lewis, B.A.: Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 74, 3583–3597 (1991). https://doi.org/10.3168/jds.S0022-0302(91)78551-2
Sluiter, A., Hames, B., Ruiz, R., Scarlata, C., Sluiter, J., Templeton, D., et al.: Determination of structural carbohydrates and lignin in Biomass. Laboratory Analytical Procedures NREL/TP-510-42618 (2012)
Sluiter, A., Ruiz, R., Scarlata, C., Sluiter, J., Templeton, D.: Determination of extractives in biomass: laboratory analytical procedure (LAP); Issue Date 7/17/2005 (2008).
Khedkar, M.A., Nimbalkar, P.R., Kamble, S.P., Gaikwad, S.G., Chavan, P.V., Bankar, S.B.: Process intensification strategies for enhanced holocellulose solubilization: beneficiation of pineapple peel waste for cleaner butanol production. J. Clean. Prod. 199, 937–947 (2018). https://doi.org/10.1016/j.jclepro.2018.07.205
Harde, S.M., Jadhav, S.B., Bankar, S.B., Ojamo, H., Granström, T., Singhal, R.S., et al.: Acetone-butanol-ethanol (ABE) fermentation using the root hydrolysate after extraction of forskolin from Coleus forskohlii. Renew. Energy 86, 594–601 (2016). https://doi.org/10.1016/j.renene.2015.08.042
Martins, B.C., Rescolino, R., Coelho, D.F., Zanchetta, B., Tambourgi, E.B., Silveira, E.: Characterization of bromelain from ananas comosus agroindustrial residues purified by ethanol factional precipitation. Chem. Eng. Trans. 37, 781–786 (2014). https://doi.org/10.3303/CET1437131
Soares, P.A.G., Vaz, A.F.M., Correia, M.T.S., Pessoa, A., Carneiro-Da-Cunha, M.G.: Purification of bromelain from pineapple wastes by ethanol precipitation. Sep. Purif. Technol. 98, 389–395 (2012). https://doi.org/10.1016/j.seppur.2012.06.042
Banerjee, S., Arora, A., Vijayaraghavan, R., Patti, A.F.: Extraction and crosslinking of bromelain aggregates for improved stability and reusability from pineapple processing waste. Int. J. Biol. Macromol. 158, 318–326 (2020). https://doi.org/10.1016/j.ijbiomac.2020.04.220
Abreu, D.C.A., De Figueiredo, K.C.S.: Bromelain separation and purification processes from pineapple extract. Brazilian J. Chem. Eng. 36, 1029–1039 (2019). https://doi.org/10.1590/0104-6632.20190362s20180417
Hu, H., Zhao, Q.: Optimization extraction and functional properties of soluble dietary fiber from pineapple pomace obtained by shear homogenization-assisted extraction. RSC Adv. 8, 41117–41130 (2018). https://doi.org/10.1039/C8RA06928J
Chareonthaikij, P., Uan-On, T., Prinyawiwatkul, W.: Effects of pineapple pomace fibre on physicochemical properties of composite flour and dough, and consumer acceptance of fibre-enriched wheat bread. Int. J. Food Sci. Technol. 51, 1120–1129 (2016). https://doi.org/10.1111/ijfs.13072
Devi, L.K., Karoulia, S., Chaudhary, N.: Preparation of high dietary fibre cookies from pineapple (Ananas comosus) pomace. Int. J. Sci. Res. 5, 1368–1372 (2016). https://doi.org/10.21275/v5i5.nov163610
Selani, M.M., Brazaca, S.G.C., Dos Santos Dias, C.T., Ratnayake, W.S., Flores, R.A., Bianchini, A.: Characterisation and potential application of pineapple pomace in an extruded product for fibre enhancement. Food Chem. 163, 23–30 (2014). https://doi.org/10.1016/j.foodchem.2014.04.076
Prakongpan, T., Nitithamyong, A., Luangpituksa, P.: Extraction and application of dietary fiber and cellulose from pineapple cores. J. Food Sci. 67, 1308–1313 (2002). https://doi.org/10.1111/j.1365-2621.2002.tb10279.x
Figuerola, F., Hurtado, M.L., Estévez, A.M., Chiffelle, I., Asenjo, F.: Fibre concentrates from apple pomace and citrus peel as potential fibre sources for food enrichment. Food Chem. 91, 395–401 (2005). https://doi.org/10.1016/j.foodchem.2004.04.036
Raghavendra, S.N., Ramachandra Swamy, S.R., Rastogi, N.K., Raghavarao, K.S.M.S., Kumar, S., Tharanathan, R.N.: Grinding characteristics and hydration properties of coconut residue: a source of dietary fiber. J. Food Eng. 72, 281–286 (2006). https://doi.org/10.1016/j.jfoodeng.2004.12.008
Aachary, A.A., Prapulla, S.G.: Value addition to corncob: production and characterization of xylooligosaccharides from alkali pretreated lignin-saccharide complex using Aspergillus oryzae MTCC 5154. Bioresour. Technol. 100, 991–995 (2009). https://doi.org/10.1016/j.biortech.2008.06.050
Takkellapati, S., Li, T., Gonzalez, M.A.: An overview of biorefinery-derived platform chemicals from a cellulose and hemicellulose biorefinery. Clean Technol. Environ. Policy 20, 1615–1630 (2018). https://doi.org/10.1007/s10098-018-1568-5
Xin, F., Chen, T., Jiang, Y., Dong, W., Zhang, W., Zhang, M., et al.: Strategies for improved isopropanol-butanol production by a clostridium strain from glucose and hemicellulose through consolidated bioprocessing. Biotechnol. Biofuels (2017). https://doi.org/10.1186/s13068-017-0805-1
Gravitis, J., Vedernikov, N., Zandersons, J., Kokorevics, A.: Furfural and levoglucosan production from deciduous wood and agricultural wastes. ACS Symp. Ser. 784, 110–122 (2001). https://doi.org/10.1021/bk-2001-0784.ch009
Machado, G., Leon, S., Santos, F., Lourega, R., Dullius, J., Mollmann, M.E., et al.: Literature review on furfural production from lignocellulosic biomass. Nat. Resour. 07, 115–129 (2016). https://doi.org/10.4236/nr.2016.73012
Verma, S., Baig, R.B.N., Nadagouda, M.N., Len, C., Varma, R.S.: Sustainable pathway to furanics from biomass: via heterogeneous organo-catalysis. Green Chem. 19, 164–168 (2017). https://doi.org/10.1039/c6gc02551j
Yan, K., Wu, G., Lafleur, T., Jarvis, C.: Production, properties and catalytic hydrogenation of furfural to fuel additives and value-added chemicals. Renew. Sustain. Energy Rev. 38, 663–676 (2014). https://doi.org/10.1016/j.rser.2014.07.003
Mariscal, R., Maireles-Torres, P., Ojeda, M., Sádaba, I., López, G.M.: Furfural: a renewable and versatile platform molecule for the synthesis of chemicals and fuels. Energy Environ Sci 9, 1144–1189 (2016). https://doi.org/10.1039/c5ee02666k
Rafiqul, I.S.M., Sakinah, A.M.M.: Processes for the production of Xylitol-A review. Food Rev Int 29, 127–156 (2013). https://doi.org/10.1080/87559129.2012.714434
Chandel, A.K., Antunes, F.A.F., Terán-Hilares, R., Cota, J., Ellilä, S., Silveira, M.H.L., et al.: Bioconversion of hemicellulose into ethanol and value-added products: commercialization, trends, and future opportunities. Commercialization, trends, and future opportunities. Adv. Sugarcane Biorefinery Technol. Commer. Policy Issues Paradig. Shift Bioethanol. By-Products (2018). https://doi.org/10.1016/B978-0-12-804534-3.00005-7
Avanthi, A., Kumar, S., Sherpa, K.C., Banerjee, R.: Bioconversion of hemicelluloses of lignocellulosic biomass to ethanol: an attempt to utilize pentose sugars. Biofuels 8, 431–444 (2017). https://doi.org/10.1080/17597269.2016.1249738
Njoku, S., Iversen, J., Uellendahl, H., Ahring, B.: Production of ethanol from hemicellulose fraction of cocksfoot grass using pichia stipitis. Sustain Chem Process 1, 13 (2013). https://doi.org/10.1186/2043-7129-1-13
Chandel, A.K., Chandrasekhar, G., Radhika, K., Ravinder, R., Ravindra, P.: Bioconversion of pentose sugars into ethanol: a review and future directions. Biotechnol Mol Biol Rev 6, 8–20 (2011)
Singh, R.D., Bhuyan, K., Banerjee, J., Muir, J., Arora, A.: Hydrothermal and microwave assisted alkali pretreatment for fractionation of arecanut husk. Ind Crops Prod 102, 65–74 (2017). https://doi.org/10.1016/j.indcrop.2017.03.017
Acknowledgements
The authors would like to thank the IITB-Monash Research Academy to provide financial support (IMURA0453) to SB for her doctoral study. The authors acknowledge the use of the facilities and the assistance of Dr. Xiya Fang at the Monash Centre for Electron Microscopy.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Banerjee, S., Vijayaraghavan, R., Patti, A.F. et al. Integrated Biorefinery Strategy for Valorization of Pineapple Processing Waste into High-Value Products. Waste Biomass Valor 13, 631–643 (2022). https://doi.org/10.1007/s12649-021-01542-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-021-01542-7