Abstract
Pomegranate seed oil presents nutraceutical properties. Industrial processes for extraction of edible oils from seeds generally involve a solvent extraction step. Safety considerations on the use of organic solvents prompted attempts to develop aqueous extraction. However, aqueous extraction processes are usually characterized by low oil yields. The objective of this work was to overcome these low yields by using enzymes. The pomegranate seeds were treated with two enzymes—cellulase and Peclyve V. The extraction temperature, the time, the enzyme concentration, and the water/seeds ratio were varied between 35 and 55 °C, 2 and 8 h, 2 and 4 % w/w, and 2/1 and 6/1 mL/g, respectively. The optimum yield accomplished (15.33 g oil/100 g dry seeds at 2 h or 81 % oil recovery) was comparable to the yields obtained by other extraction methods, such as normal stirring, cold pressing, superheated fluid extraction, indirect ultrasound-assisted extraction and supercritical extraction, (4.29–25.11 g oil/100 g seeds) at similar or longer extraction times (10 min–72 h). In addition, this work studies the enhancement of aqueous enzymatic extraction of pomegranate seed oil by ultrasound probe. It was found that only 10 min in water are needed to recover oil with a yield of 18.15 g oil/100 g dry seeds, a value similar to or higher than those of the conventional procedures. Thus, the use of ultrasounds increased the yield of enzymatic extraction by 18.4 % and reduced the extraction time by 91.7 %.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The production and consumption of pomegranate have greatly increased throughout the world in recent years due to the health-promoting potential of different components of pomegranates [1]. Pomegranates are rich in aril, the percentage of which ranges from 50 to 70 % of total fruit and comprises of 78 % juice and 22 % seeds [2]. Pomegranate seeds show average contents of about 37–143 g/kg of fruit depending on variety, geographical location, growing conditions, maturity stage, etc. [3]. Oil content of seeds varies from 12 to 20 % of the seed on a dry weight basis [4–6]. Pomegranate seed oil consists of 65–80 % conjugated fatty acids, such as 9-trans, 11-cis, 13-trans, and octadecatrienoic acid (punicic acid) [7]. Besides punicic acid, pomegranate seed oil contains tocopherols and phytosterols.
Pomegranate seed oil has been well documented for its potential health benefits, such as antioxidant properties, lipoperoxidation, activity of antioxidant enzymes, immune function, lipid metabolism, estrogene content, skin photoaging inhibition effect, and protective effect against nephrotoxicity [8–14]. Due to these nutritional and medicinal properties, the seeds could have more applications instead of being used as animal feed or in cosmetic products. One way to utilize the seeds is to extract the oil and use it as a functional ingredient in the food industry.
Pomegranate seed oil can be extracted with various extraction methods. Kalamara et al. [15] extracted pomegranate seed oil using the direct ultrasound-assisted method with a yield varying between 15.66 and 18.16 g oil/100 g seeds at extraction time of 10 min, whereas Tian et al. [16], who extracted pomegranate seed oil using ultrasounds by the indirect method, obtained a higher efficiency of 25.11 g oil/100 g seeds at extraction time of 35 min. Several researchers extracted oil from pomegranate seeds with lower or similar yields using different extraction methods and reported much longer extraction times [15]. Goula and Adamopoulos [17] achieved an extraction yield of about 9.5 g oil/100 g seeds at 4 h using the conventional stirring method. Superheated hexane extraction showed higher extraction efficiency (22.18 g oil/100 g seeds at 2 h) [4] than Soxhlet extraction (17.94 g oil/100 g seeds at 24 h) [4, 7, 18], cold pressing (4.29 g oil/100 g seeds at 72 h), and supercritical carbon dioxide extraction (15.72 g oil/100 g seeds at 2 h) [19].
Generally, processes for extraction of seeds oil involve pressing and solvent extraction. Environmental problems, such as solvent loss and associated pollution problems, and safety points of view are the main concerns relating to the conventional solvent-based oil extraction. These considerations prompted attempts to develop aqueous extraction methods. In aqueous extraction, there is no chemical potential for oil dissolution and extraction is based on the insolubility of oil in water rather than on the dissolution of oil. According to Rosenthal et al. [20], the water soluble components of seeds diffuse in the water rather than in oil, thereby releasing the oil, which was previously bound in the original structure. On the contrary, the conventional solvent extraction is based on the oil capacity to dissolve.
However, aqueous extraction processes are usually characterized by low oil yields. The low extraction yields can be overcome by using enzymes, such as cellulases, hemicellulases, and pectinases, that hydrolyze the structural polysaccharides forming the cell wall of oilseeds or that hydrolyze the proteins, which form the cell and lipid body membranes [22], and facilitate oil release from the oil bodies. Thus, the soluble components diffuse into water and the released oil forms a separate liquid phase [21]. Researches have been done on aqueous enzyme-assisted oil extraction from various seeds such as soybean, Jatropha curcas, canola, grape, sunflower, Kalahari melon, peanut, sesame, bayberry (Myrica rubra) kernels, and pumpkin seeds [22–35]. However, it is very difficult to select the best enzyme for a given oilseed comparing the results reported in different studies, since these studies employ different extraction conditions, such as temperature, time, pH, and particle size [36].
To the best of our knowledge, there are no reports about the aqueous enzymatic extraction of oil from pomegranate seeds. Generally, the use of pectinases and cellulases has been reported to improve the yield of aqueous extractions. The aim of this study is to investigate the effect of various operational parameters on extraction yield of pomegranate seed oil, when cell-wall degrading enzymes (cellulase and pectinase) are added to aqueous extraction media. The conventional one-factor-at-a-time approach for an extraction optimization is time consuming and may ignore the interactions among various factors. In addition, evaluation of possible interaction effects arising between factors is difficult and misleading inferences may occur. Response surface methodology enables evaluation of several process parameters such as time, temperature, enzyme type, and concentration.
In addition, in recent years, ultrasound-assisted extraction of compounds has been widely applied. Ultrasound is transmitted through a medium as a pressure wave and causes an excitation in the form of enhanced molecular motion. According to Vilkhu et al. [37], the ultrasonically induced cavitation increases the permeability of the plant tissues. Microfractures and disruption of cell walls provide more evidence for the mechanical effects of ultrasound, thus, facilitating the release of their contents. According to our knowledge, only a few studies related to combined use of enzymes and ultrasonication have been reported until now [36–40]. Thus, another objective of the present work is to study the enhancement of aqueous enzymatic extraction of pomegranate seed oil by ultrasound treatment.
Materials and Methods
Pomegranate Seeds
Fresh, good quality pomegranates (Wonderful variety) procured from the local market were used. Pomegranate seeds were separated from the juice and washed. The seeds were dried at 60 °C for 48 h and kept at −30 °C until use. The seeds were ground in a laboratory mill (Type A10, Janke and Kunkel, IKA Labortechnik, Germany) prior to extraction. The seeds contained lipids, protein, crude fibers, pectin, sugars, and ash representing 27.2, 13.2, 35.3, 6.0, 4.7, and 2.0 %, respectively (on dry wt basis). The particle size distribution of the milled seeds showed a bimodal distribution, due to the two main anatomical parts (germ, seed coat) of the seeds. The small size (~0.32 mm) may be associated with seed coats, whereas the size of about 0.58 mm could be attributed to aggregated germ particles [15].
Enzymes
Cellulase, produced from Trichoderma reesei, was purchased from Sigma-Aldrich. The declared activity was 700 EGU/g (EGU, endoglucanase units). Peclyve V, a pectinase enzyme preparation that is concentrated in β-glucosidasic activities, was also bought from Lyven.
Soxhlet Extraction
10 g of ground pomegranate seeds were extracted with 100 mL of n-hexane in a Soxhlet extractor for 6 h, as described in the Soxhlet standard extraction method (AOAC, 1997). The n-hexane was removed at 50 °C under reduced pressure using a rotary evaporator and the oil was dried at 105 °C to a constant mass. This method gave 18.94 ± 0.59 g of oil per 100 g of seeds, which was set as 100 % oil recovery for comparison.
Aqueous Enzymatic Extraction
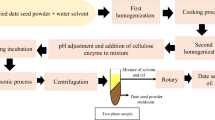
The milled seeds were commixed with distilled water at a designed ratio. The enzyme was added and the mixture was incubated using a magnetic stirrer Gallenhamp (model SWT-500-010L, England) at a specific temperature. The pH was adjusted to 5.0 with citric acid. The extraction process was performed under a range of conditions according to the experimental design. Following the incubation, the suspension was centrifuged at 6000 g for 20 min in a Karl Kolb (Labofuge III, Frankfurt, Germany) centrifuge. The layers of free oil and emulsion phase were collected separately. The oil was withdrawn using a micropipette and the emulsion was further centrifuged to obtain any residual oil. The oils were combined, weighed and the extraction yield, Y, was expressed as percent ratio (g oil/100 g dry seeds). In addition, Soxhlet extraction was used as the control method to determine the recovery obtained in the aqueous enzymatic extraction of the oil.
The seeds slurry was treated with two different enzymes—cellulase and Peclyve V. The extraction temperature (T), the extraction time (t), the enzyme concentration (EC), and the water/seeds ratio (L/S) were varied between 35 and 55 °C, 2 and 8 h, 2 and 4 % w/w, and 2/1 and 6/1 mL/g, respectively.
The factors were optimized using response surface methodology. A four-factor, five-level central composite rotatable design 24 + star was used to determine the optimum levels of these variables. This central composite design consisted of three groups of design points, including two-level factorial design points, axial or star points, and center points. Therefore, four selected independent variables (extraction temperature (X 1), extraction time (X 2), enzyme concentration (X 3), water/seeds ratio (X 4)) were studied at five different levels coded as −a, −1, 0, 1, and +a. The value for alpha (2.0) was chosen to fulfill the rotatability in the design. The actual level of each factor was calculated by Eq. (1). According to the central composite design matrix, a total of 31 experiments, including 16 factorial points, 8 axial points, and 7 center points for estimation of the pure error sum of squares, were required.
Ultrasound-Assisted Enzymatic Extraction
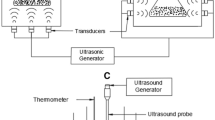
The extraction of pomegranate seed oil by enzymatic hydrolysis and simultaneous ultrasound treatment was carried out following the same conditions as for the enzymatic digestion. Ultrasonic irradiation was applied with a 130 W, 20 kHz VCX-130 Sonics and Materials (Danbury, CT, USA) sonicator equipped with a Ti–Al–V probe (13 mm). The pulse duration and pulse interval refer to “on” time and “off” time of the sonicator. The amplitude control of the processor allowed the ultrasonic vibrations at the probe to be set at any desired level in the 10–100 % range of the nominal power.
The milled seeds were commixed with distilled water at the optimum ratio in a 250-mL beaker and the appropriate enzyme was added to the suspension at the optimum concentration. The probe submerged about 4 cm under the surface of the mixture. During the extraction process, the sample container was held in a thermostat-controlled water bath and two T-type thermocouples were immersed into the extraction solution. The temperature was kept constant by adding ice to the water in the bath throughout the experiment.
The extraction process was performed under the optimum values of the investigated factors (enzyme type, enzyme concentration, extraction temperature, water/seeds ratio) at an amplitude level of 40 % and a pulse duration/pulse interval ratio of 7/6 [41]. The extracts were collected at 3, 5, 10, 20, 30, 40, 50, and 60 min and treated as in the enzymatic extraction.
Statistical Analyses
A joint statistical analysis of the two sets of experiments—(1) with cellulase (31 experiments) and (2) with Peclyve V (31 experiments)—was carried out to examine the effect of the enzyme type on the extraction [42]. In order to perform a joint statistical analysis of both experiments, a “dummy” factor was introduced. The dummy factor could assume two different levels: 1, for cellulase; and 2, for Peclyve V.
To determine the effect of a particular factor x (Ex), Eq. (2) was applied.
where \( \sum x\left( + \right) \) is the sum of the factors at their highest level (+1), \( \sum x\left( - \right) \) is the sum of the factors at their lowest level (−1), and n/2 is half of the number of measurements used in the calculation. Interactions between factors were also evaluated. To estimate an interaction between two factors, one has to estimate the effect of the first at the lowest level of the second, subtracting it from the effect of the first one at the highest level of the second one. To identify the significance of the effects and interactions between them, analysis of variance (ANOVA) was performed for each parameter. A p value less than 0.05 was considered to be statistically significant. Minitab™ v 13.32 software (Minitab Inc., Pennsylvania, USA) was used for analysis of the obtained experimental data.
The response (extraction yield) was modeled through the full second-order polynomial equation. The goodness of fit of the model was also evaluated by the analysis of variance and the graph of residuals, where the residuals are the differences between the values predicted by the models and the observed (experimental) values.
Scanning Electron Micrographs (SEM)
A Quanta-200 environmental scanning electron microscope system (FEI Company, USA) was used to study the structural changes in pomegranate seeds texture after the extraction process. The samples were fixed on a specimen holder with an aluminum tape and sputtered with a thin layer of gold prior to examination under high vacuum condition at an accelerating voltage of 12.5 kV at 1000 × magnification level.
Results and Discussion
Effect of Process Parameters on Extraction Yield
The highest yields obtained depending on the applied enzyme were 8.30 and 13.17 g oil/100 g of dry seeds using cellulase and Peclyve, respectively (Fig. 1). Soxhlet extraction was used as the control method to determine the recovery. Thus, the obtained recoveries were about 43.8 and 69.5 %, respectively. The higher yield obtained with Peclyve as compared to cellulase may be attributed to the fact that pectic substances are the prevalent cell-wall polysaccharides in pomegranate seeds [4]. Additionally, Peclyve contains partial cellulase and hemicellulase activities. A similar observation was reported by Zhang et al. [43] in the extraction of rapeseed oil. In this case, the application of pectolytic enzyme prior to pressing led to higher rapeseed oil yield (16.5 %) as compared to cellulolytic enzyme (15.5 %). According to Yusoff et al. [44], it is not possible to decide whether it is better to use enzymes individually or in combination. The optimum enzyme depends on the location of the oil within the cellular matrix and the nature of the components surrounding the oil and acting as a barrier against its release.
Figure 2 presents the effect of extraction temperature (T), extraction time (t), liquid/solid ratio (L/S), and enzyme concentration (EC) on extraction yield (Y) using cellulase and Peclyve, respectively. As far as the temperature is concerned, in general, the optimum temperature range for enzymatic extraction varies between 40 and 55 °C and researchers usually use the lowest possible temperature yielding adequate activity [26]. As it can be seen in Fig. 2, the yield increased with the increase of temperature using Peclyve. The same trend was observed with temperatures up to 38 °C using cellulase. In this case, the increase of temperature above the optimum temperature for enzymatic activity (about 38 °C) led to a gradual decrease in yield, due to the destruction of the tertiary structure. This observation is similar to those reported by many researchers, who concluded that oil yield increases up to certain temperature only, followed by steady or decreased rate afterwards [45]. According to Jiao et al. [35], at a fixed enzyme concentration, oil yield increased with rising temperature as the rate of enzyme-catalyzed reactions increased with increasing temperature. However, at higher temperatures (45–55 °C), the yield decreased significantly as enzymes became denatured. Sharma et al. [21] and Gros et al. [46], who extracted peanut and linseed oil, observed highest oil yield at 40 and 34 °C, respectively.
In general, extraction yield enhances increasing extraction time (t). Oil extractability was markedly affected by hydrolysis time during the first 6.5 h for cellulose and 3.5 h for Peclyve V, thereafter reaching a plateau. An increase in incubation time up to 8 h did not provide any significantly higher oil yield, which may be due to the depletion of the substrates and/or product inhibition of enzymes. Contact period between enzyme and substrate influences the extent of the reaction as long as the enzyme is still active. However, degradation of cell wall components can be enhanced by prolonging the incubation time [44]. Time of hydrolysis used in previous works varies from 10 min up till 24 h; specifically 10 min [47], 1 h [48], 1.5 h [49], 2 h [50], 3 h [51], 3.2 h [52], 18 h [53], and 24 h [54]. These differences might be due to difference in other processing parameters that directly influence time of hydrolysis [55]. Passos et al. [26] reported that the use of an enzyme mixture for 120 h resulted in 3.8 % higher yield as compared to 24 h of time. However, this time duration (120 h) is far too long to be acceptable in practice. Heo et al. [56], who studied the effect of time on degree of hydrolysis and radical scavenging ability of cellulast hydrolyzates of E. cava, found that both parameters increased sharply within the first 2 min of and steadily increased until it reached the peak at 12 h, followed by reduction afterwards.
Concentration of enzyme (EC) affects the rate of hydrolysis and the extraction yield. Generally, the more enzyme used, the faster the extraction and the higher the yield (Fig. 2). A similar observation was reported by Sirwadhana et al. [57], who found that extraction yield obtained from Hizikia fusiformis increased as enzyme concentration increased to 5 %. Likewise, an increase in concentration of enzymes reportedly increased the yield of hydrolysates obtained from E. cava [37]. However, a high enzyme concentration also increases processing costs and may result in bitterness and off flavors [30]. According to Hammed et al. [55], the required amount of enzyme should be used within the active phase of the particular enzyme in order to achieve efficient hydrolysis of biopolymers barriers.
As far as the water-to-seeds ratio (L/S) is concerned, the extraction yield increased with increasing the ratio up to values around 5 mL/g. This may be due to the fact that thick suspensions prevent the effective penetration of the enzymes. According to Yusoff et al. [44], the water in aqueous enzymatic extraction not only serves as an extraction medium, but also enters the oil-bearing material and the resulting moisture content assists hydrolytic reaction, diffusion, and mobility of the enzymes and products. A similar observation was reported by Sineiro et al. [58], who found that only certain areas in sunflower kernels were degraded by enzymes at low moisture content. However, solvent/solid ratios higher than 5 mL/g resulted in lower yields, since the chance of an interaction between the enzyme and substrate molecules is low in very dilute suspensions [59]. Hammed et al. [55] also found that a ratio of 5 mL/g gives the highest yield of oil in aqueous enzymatic extraction. According to Teixeira et al. [60], at high levels of dilution (up to 4), the enzyme concentration had little effect on the oil yield.
Optimization of Enzymatic Extraction
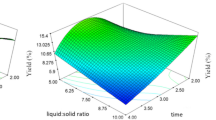
In order to determine the optimal levels of each variable for maximum yield, three-dimensional response surface plots were constructed by plotting the response on the Z-axis against any two independent variables while maintaining other variables at their optimal levels (Fig. 3).
The analysis of variance of oil extraction is given in Table 1. The p value of the model was less than 0.0001, indicating that the model was significant. Regression analysis showed that the coefficient of determination (R 2 = 0.909) were satisfactory to validate the significance of the model. The plot of actual and predicted values for oil extraction yield also showed the consistency of the model. Furthermore, results of the error analysis indicated that the lack of fit was not significant with p-value of 0.074 (>0.05). In addition, it was clear that errors were normally distributed and insignificant from the normal (%) probability plot of the residuals. From Table 1, it was also seen that the factor with the largest effect on the oil yield was the interactions between enzyme concentration and water-to-seeds ratio (p = 0.001) and extraction temperature and enzyme type (p = 0.032).
A mathematical model was developed to describe the relationship between operating variables and the response variable Y. The regression coefficients were calculated and the data was fitted to a second-order polynomial equation. F values for all reduced second order polynomials and linear models with an R 2 ≥ 0.70 were calculated to determine if the models could be used in place of the full second order polynomial to predict the response of the extraction yield to the independent variables. The best model was found the following:
For cellulase:
For Peclyve V:
The optimum operating conditions were found to be: enzyme type, Peclyve V; extraction temperature (T), 55 °C; liquid/solid ratio (L/S), 6/1 mL/g; enzyme concentration (EC), 2 % w/w; extraction time (t), 2 h. Under these optimized conditions, the predicted value for extraction yield was 15.87 g oil/100 g dry seeds, whereas the observed experimental value was found 15.33 ± 0.78 g oil/100 g dry seeds, confirming the validity of the model.
Abbasi et al. [7, 18], Liu et al. [19], Eikani et al. [4], Goula and Adamopoulos [17], Tian et al. [16], Kalamara et al. [15] extracted pomegranate seed oil using different extraction methods (Soxhlet, normal stirring, supercritical fluid extraction, cold pressing, ultrasounds) (Fig. 4). The optimum yield accomplished by aqueous enzymatic extraction (15.33 g oil/100 g dry seeds at extraction time of 2 h) was comparable to the yields obtained by other extraction methods (4.29–25.11 g oil/100 g seeds) at similar or longer extraction times (10 min–72 h) [4, 7, 15–19].
Soxhlet extraction was used as the control method to determine the recovery obtained in the aqueous enzymatic extraction of the oil. Thus, the optimum oil recovery was about 81 %, a value similar to that obtained by other researchers. Bocevska et al. [61] evaluated a group of commercial enzymes for aqueous extraction of corn germ oil and concluded that cellulase from Trichoderma reesei was most effective; it released 84.7 % of the total oil. Zhang et al. [59] reported recovery values of 73–76 % for the extraction of rapeseed oil using pectinase, cellulase, and β-glucanase, whereas a maximum of 77.8 % oil recovery was achieved extracting oil from Iranian wild almond using protease and cellulase [62].
However, the main advantage of the aqueous enzymatic extraction technologies is the devoid of harmful chemicals. In addition, aqueous processes can potentially be more cost effective since cost relating to solvent recovery, process safety, and solvent loss control systems will be much lower. The effect of enzymes on the economics of the aqueous process will depend on the balance of costs and benefits resulting. According to Rosenthal et al. [20], the economic comparison between aqueous processes with conventional solvent-based extractions depends on details relating to the aqueous processes such as the separation method and water and enzyme recycle. In addition, it is now well established that aqueous enzymatic extraction of oil results in oil with better qualitative characteristics (higher contents of phenolics, tocopherols, trans-2-hexanal and other volatile aromatics, enhanced oxidative stability, lower turbidity values, and higher ratios of 1, 2 diglycerides/l, 3 diglycerides, campesteroll stigmasterol and trans-2-hexanal/total aroma) [63].
SEM Observation
Changes in pomegranate seeds texture before and after extraction were investigated in order to examine the mechanism for the aqueous enzymatic extraction of oil from pomegranate seeds. As shown in Fig. 5a, before extraction, the intact oil cells were presented on the surface of seed tissues. According to Fig. 5b, after Soxhlet extraction, these oil cells disappeared. However, the surface of seed tissues was still smooth. In the case of aqueous enzymatic extraction, a partial destruction of seed tissues was observed using cellulase (Fig. 5c), whereas after extraction with Peclyve V, most cells were crimped and broken (Fig. 5d).
In Soxhlet extraction, the mass transfer was affected by the chemical affinity between solvents and oils. In contrast, aqueous enzymatic extraction of oils is based on the insolubility of oils in water. Therefore, the destruction of cell walls and oleosin-based membranes with the help of hydrolytic enzymes was beneficial for releasing oils, which were previously bound in the plant cell structure [35].
Combination of Enzymatic Extraction with Ultrasounds Treatment
The extraction yield increased with ultrasonic time from 3 to 10 min, whereas the further increase of time (from 10 to 60 min) led to a decrease in yield (Fig. 6). Thus, the efficient extraction time for achieving maximum yield of pomegranate seed oil was about 10 min. This can be attributed to the fact that extraction presents two stages; the first stage, which involves the penetration of the solvent into the cellular structure, whereas the second one involves the external diffusion of oil and its transfer from the solution in contact with the particles to the bulk of the solution [64]. The ultrasonic waves increases the mass transfer rate mainly in the first stage [42], whereas during the second stage, impurities suspended in the extract are absorbed into the ruptured tissue particles lowering the solvent’s permeability into cell structures [16, 19, 65].
The combined use of enzymes and ultrasonication had a maximum yield of 18.15 g oil/100 g of dry seeds or 95.8 % oil recovery at extraction time of 10 min using as enzyme Peclyve V at extraction temperature of 55 °C, liquid/solid ratio of 6/1 mL/g, and enzyme concentration of 2 % w/w. Thus, the extraction of pomegranate seed oil by enzymatic hydrolysis and simultaneous ultrasound treatment increased extraction yield by 18.4 %, but mainly shortened the treatment time by 91.7 %, as it can be seen in Fig. 4. Only 10 min in water (a green environmental solvent) are needed to recover oil from pomegranate seed with yields similar or higher than those of the conventional extraction procedures. This observation is similar to that obtained by Siwek et al. [40], who studied the enzymatic extraction of selenium organic compounds from Antarctic krill by ultrasound treatment, and reported that the particle size reduction achieved by the enzymatic extraction becomes more obvious when a combined treatment with enzyme and ultrasounds is applied. According to the researchers, this could be partly due to the mixing, but does not definitely exclude direct effect of ultrasounds on the enzyme–substrate interaction and on the intrinsic rate constants of the biocatalytic reaction. However, in this work the percentage of yield enhancement was lower. This mild effect could be linked to the level of ultrasonic power applied, since these authors carried out the experiments in an ultrasonic bath, which actually supplies lower ultrasonic intensities than probe systems like the one used in the current study. Capelo et al. [38] also described the dramatic activity enhancement of two proteolytic enzymes when treated with an ultrasonic probe and their application to total Se determination and Se speciation in biological samples.
Conclusions
This work examined the feasibility of aqueous enzymatic extraction as a potential extraction method for oil from a pomegranate by-product; pomegranate seeds, which represent a low-cost source of valuable bioactive compounds. The highest yield obtained in aqueous enzymatic extraction (15.33 g oil/100 g dry seeds at 2 h or 81 % oil recovery) was comparable to the yields obtained by other extraction methods (4.29–25.11 g oil/100 g seeds) at similar or longer extraction times (10 min–72 h). This higher yield is of major interest from an industrial point of view due to the use of non-organic solvents. Extraction yield increased with an increase in temperature, time, enzyme concentration, and water-to-seeds ratio up to values around 5 mL/g. The optimum levels of each variable were determined to be as follows: enzyme type, Peclyve V; extraction temperature, 55 °C; liquid/solid ratio, 6/1 mL/g; enzyme concentration, 2 % w/w; extraction time, 2 h.
In addition, the aqueous enzymatic extraction of pomegranate seed oil was enhanced by ultrasound treatment by 18.4 %, whereas the extraction time was shortened by 91.7 %. It was found that only 10 min in water (a green environmental solvent) are needed to recover oil from pomegranate seed with a yield (18.15 g oil/100 g of dry seeds or 95.8 % oil recovery) similar or higher than those of the conventional extraction procedures. Thus, the combination of the emerging technology of ultrasounds and the use of enzymes for extraction purposes is an economical alternative to traditional extraction methods according to industry demands and a sustainable development.
References
Türkyılmaz, M., Tagi, S., Dereli, U., Özkan, M.: Effects of various pressing programs and yields on the antioxidant activity, antimicrobial activity, phenolic content and colour of pomegranate juices. Food Chem. 138, 1810–1818 (2013)
Mohagheghi, M., Rezaei, K., Labbafi, M., Mousavi, S.M.E.: Pomegranate seed oil as a functional ingredient in beverages. Eur. J. Lipid Sci. Tech. 113, 730–736 (2011)
Fernandes, L., Pereira, J.A., Lopéz-Cortés, I., Salazar, D.M., Ramalhosa, E., Casal, S.: Fatty acid, vitamin E and sterols composition of seed oils from nine different pomegranate (Punica granatum L.) cultivars grown in Spain. J. Food Compos. Anal. 39, 13–22 (2015)
Eikani, M.H., Golmohammad, F., Homami, S.S.: Extraction of pomegranate (Punica granatum L.) seed oil using superheated hexane. Food Bioprod. Process. 90, 32–36 (2012)
Al-Maiman, S.A., Ahmad, D.: Changes in physical and chemical properties during pomegranate (Punica granatum L.) fruit maturation. Food Chem. 76, 437–441 (2002)
Lansky, E.P., Newman, R.A.: Punica granatum (pomegranate) and its potential for prevention and treatment of inflammation and cancer. J. Ethnopharmacol 109, 177–206 (2007)
Abbasi, H., Rezaei, K., Rashidi, L.: Extraction of essential oils form the seeds of pomegranate using organic solvents and supercritical CO2. J. Am. Oil Chem. Soc. 85, 83–89 (2008)
Tong, P., Kasuga, Y., Khoo, C.S.: Liquid chromatographic mass spectrometric method for detection of estrogen in commercial oils and in fruit seed oils. J. Food Compos. Anal. 19, 150–156 (2006)
Yamasaki, M., Kitagawa, T., Koyanagi, N., Chujo, H., Maeda, H., KohnoMurase, J., Imamura, J., Tachibana, H., Yamada, K.: Dietary effect of pomegranate seed oil on immune function and lipid metabolism in mice. Nutrition 22, 54–59 (2006)
Meerts, I.A.T.M., Verspeek-Rip, C.M., Buskens, C.A.F., Keizer, H.G., Bassaganya-Riera, J., Jouni, Z.E., van Huygevoort, A.H.B.M., van Otterdijk, F.M., van de Wart, E.J.: Toxicological evaluation of pomegranate seed oil. Food Chem. Toxicol. 47, 1085–1092 (2009)
Asadpour, E., Boroushaki, M.T., Sadeghnia, H.: Protective effect of pomegranate seed oil against gentamicin induced nephrotoxicity in rat. Toxicol. Lett. 196, 232 (2010)
Melo, I.L.P., Carvalho, E.B.T., Silva, A.M.O., ManciniFilho, J.: Effects of pomegranate seed oil on lipoperoxidation and activity of antioxidant enzymes in liver and brain of rats. Free Radic. Biol. Med. 49, 189 (2010)
Park, H.M., Moon, E., Kim, A.J., Kim, M.H., Lee, S., Lee, J.B., Park, Y.K., Jung, H., Kim, Y.B., Kim, S.Y.: Extract of Punica granatum inhibits skin photoaging induced by UVB irradiation. Int. J. Dermatol. 49, 276–282 (2010)
Qu, W., Pan, Z., Ma, H.: Extraction modeling and activities of antioxidants from pomegranate marc. J. Food Eng. 99, 16–23 (2010)
Kalamara, E., Goula, A.M., Adamopoulos, K.G.: An integrated process for utilization of pomegranate wastes-Seeds. Innov. Food Sci. Emerg Technol. 27, 144–153 (2015)
Tian, Y., Xu, Z., Zheng, B., Lo, Y.M.: Optimization of ultrasonic-assisted extraction of pomegranate (Punica granatum L.) seed oil. Ultrason. Sonochem. 20, 202–208 (2013)
Goula, A.M., Adamopoulos, K.G.: A method for pomegranate seed application in food industries: seed oil encapsulation. Food Bioprod. Process. 90, 639–652 (2012)
Abbasi, H., Rezaei, K., Emamdjomeh, Z., Mousavi, S.M.E.: Effect of various extraction conditions on the phenolic contents of pomegranate seed oil. Eur. J. Lipid Sci. Technol. 110, 435–440 (2008)
Liu, G., Xu, X., Hao, Q., Gao, Y.: Supercritical CO2 extraction optimization of pomegranate (Punica granatum L.) seed oil using response surface methodology. Lebensm. Wiss. Technol. 42, 1491–1495 (2009)
Rosenthal, A., Pyle, D.L., Niranja, K.: Aqueous and enzymatic processes for edible oil extraction. Enz. Microbial. Technol. 19, 402–420 (1996)
Sharma, A., Khare, S.K., Gupta, M.N.: Enzyme-assisted aqueous extraction of oil from peanut seeds. J. Am. Oil Chem. Soc. 78, 949–951 (2001)
Rosenthal, A., Pyle, D.L., Niranjan, K., Gilmour, S., Trinca, L.: Combined effect of operational variables and enzyme activity on aqueous enzymatic extraction of oil and protein from soybean. Enz. Microbial Technol 28, 499–509 (2001)
Towa, L.T., Kapchie, V.N., Hauck, C., Murphy, P.A.: Enzyme-assisted aqueous extraction of oil from isolated oleosomes of soybean flour. J. Am. Oil Chem. Soc. 87, 347–354 (2010)
Shah, S., Sharma, A., Gupta, M.N.: Extraction of oil from Jatropha curcas L. seed kernels by combination of ultrasonication and aqueous enzymatic oil extraction. Bioresour. Technol. 96, 121–123 (2005)
Latif, S., Diosady, L.L., Anwar, F.: Enzyme-assisted aqueous extraction of oil and protein from canola (Brassica napus L.) seeds. Eur. J. Lipid Sci. Technol. 110, 887–892 (2008)
Passos, C.P., Yilmaz, S., Silva, C.M., Coimbra, M.A.: Enhancement of grape seed oil extraction using a cell wall degrading enzyme cocktail. Food Chem. 115, 48–53 (2009)
Latif, S., Anwar, F.: Effect of aqueous enzymatic processes on sunflower oil quality. J. Am. Oil Chem. Soc. 86, 393–400 (2009)
Nyam, K.L., Tan, C.P., Lai, O.M., Long, K., Man, Y.B.C.: Enzyme-assisted aqueous extraction of kalahari melon seed oil: optimization using response surface methodology. J. Am. Oil Chem. Soc. 86, 1235–1240 (2009)
Jiang, L., Hua, D., Wang, Z., Xu, S.: Aqueous enzymatic extraction of peanut oil and protein hydrolysates. Food Bioprod. Process. 88, 233–238 (2010)
Latif, S., Diosady, L.L., Anwar, F.: Aqueous enzymatic sesame oil and protein extraction. Food Chem. 125, 679–684 (2011)
Zhang, Y., Li, S., Yin, C., Jiang, D., Yan, F., Xu, T.: Response surface optimisation of aqueous enzymatic oil extraction from bayberry (Myrica rubra) kernels. Food Chem. 135, 304–308 (2012)
Qi, Q.: Study on extraction of pumpkin seed oil by aqueous enzymatic method. J. Anhui Agric. Sci. 40, 7410–7413 (2012)
Jiao, J., Li, Z.G., Gai, Q.Y., Li, X.J., Wei, F.Y., Fu, Y.J., Ma, W.: Microwave-assisted aqueous enzymatic extraction of oil from pumpkin seeds and evaluation of its physicochemical propertiesm fatty acid compositions and antioxidant activities. Food Chem. 147, 17–24 (2014)
Kalia, V.C., Sadhana, Rashmi Lal, Gupta, M.N.: Using enzymes for oil recovery from edible seeds. J. Sci. Ind. Res. 60, 298–310 (2001)
Vilkhu, K., Mawson, R., Simins, L., Bates, D.: Applications and opportunities for ultrasound assisted extraction in the food industry—a review. Innov. Food Sci. Emerg. Technol. 9, 161–169 (2008)
Wu, H., Zhu, J., Diao, W., Wang, C.: Ultrasound-assisted enzymatic extraction and antioxidant activity of polysaccharides from pumpkin (Cucurbita moschata). Carbohyd. Polym. 113(26), 314–324 (2014)
Liu, Y., Gong, G., Zhang, J., Jia, S., Li, F., Wang, Y., Wu, S.: Response surface optimization of ultrasound-assisted enzymatic extraction polysaccharides from Lycium barbarum. Carbohyd. Polym. 110(22), 278–284 (2014)
Capelo, J.L., Ximénez-Embún, P., Madrid-Albarrán, Y., Cámara, C.: Enzymatic probe sonication: enhancement of protease-catalyzed hydrolysis of selenium bound to proteins in yeast. Anal. Chem. 76, 233–237 (2004)
Cabanero, A.I., Madrid, Y., Camara, C.: Enzymatic probe sonication extraction of Se in animal-based food samples: a new perspective on sample preparation for total and Se speciation analysis. Anal. Bioanal. Chem. 381, 373–379 (2005)
Siwek, M., Noubar, A.B., Bergmann, J., Niemeyer, B., Galunsky, B.: Enhancement of enzymatic digestion of Antarctic krill and successive extraction of selenium organic compounds by ultrasound treatment. Anal. Bioanal. Chem. 384, 244–249 (2006)
Kaderides, K., Goula, A.M., Adamopoulos, K.G.: A process for turning pomegranate peels into a valuable food ingredient using ultrasound-assisted extraction and encapsulation. Innov. Food Sci. Emerg. Technol. 31, 204–215 (2015)
Cochran, W.G., Cox, G.M.: Some methods for the study of response surfaces. Experimental designs, pp. 335–375. Wiley, New York (1992)
Zhang, Z.S., Wang, L.J., Li, D., Jiao, S.S., Chen, X.D., Mao, Z.H.: Ultrasound-assisted extraction of oil from flaxseed. Sep. Purif. Technol. 62, 192–198 (2008)
Yusoff, M.M., Gordon, M.H., Niranjan, K.: Aqueous enzyme assisted oil extraction from oilseeds and emulsion de-emulsifying methods: a review. Trends Food Sci. Technol. 41, 60–82 (2015)
Zuñiga, M.E., Soto, C., Mora, A., Chamy, R., Lema, J.M.: Enzymic pre-treatment of Guevina avellana mol oil extraction by pressing. Process. Biochem. 39, 51–57 (2003)
Gros, C., Lanoiselle, J.L., Vorobiev, E.: Towards an alternative extraction process for linseed oil. Chem. Eng. Res. Des. 81, 1059–1065 (2003)
Koponen, J.M., Happonen, A.M., Auriola, S., Kontkanen, H., Buchert, J., Poutanen, K.S., Torronen, A.R.: Characterization and fate of black currant and bilberry flavonols in enzyme-aided processing. J. Agric. Food Chem. 56, 3136–3144 (2008)
Hanmoungjai, D.L., Pyle, D.E., Niranjan, K.: Enzymatic process for extraction oil and protein from rice bran. J. Am. Oil Chem. Soc. 78, 817–821 (2001)
Zheng, H., Hwang, I., Kim, S., Lee, S., Chung, S.: Optimization of carbohydrate-hydrolyzing enzyme aided polyphenol extraction from unripe apples. J. Korean Soc. Appl. Biol. Chem. 53, 342–350 (2010)
Landbo, A.K., Meyer, A.S.: Enzyme-assisted extraction of antioxidative phenols from black current juice press residues (Ribes nigrum). J. Agric. Food Chem. 49, 3169–3177 (2001)
Costoya, N., Sineiro, J., Pinelo, M., Rubilar, M., Nuñez, M.J.: Enzyme-aided extraction of polyphenols from grape pomace. Electron. J. Environ. Agric. Food Chem. 9, 696–705 (2010)
Yin, X.L., You, Q.H., Jiang, Z.H.: Optimization of enzyme assisted extraction of polysaccharides from Tricholoma matsutake by response surface methodology. Carbohydr. Polym. 86, 1358–1364 (2011)
Fu, Y.-J., Liu, W., Zu, Y.-G., Tong, M.-H., Li, S.-M., Yan, M.-M., Efferth, T., Luo, H.: Enzyme assisted extraction of luteolin and apigenin from pigeonpea leaves. Food Chem. 111, 508–512 (2008)
Santala, O., Lehtinen, P., Nordlund, E., Suortti, T., Poutanen, K.: Impact of water content on the solubilisation of arabinoxylan during xylanase treatment of wheat bran. J. Cerealic. Sci. 54, 187–194 (2011)
Hammed, A.M., Jaswir, I., Amid, A., Alam, Z., Asiyanbi-H, T.T., Ramli, N.: Enzymatic hydrolysis of plants and algae for extraction of bioactive compounds. Food Rev. Int. 29, 352–370 (2013)
Heo, S.J., Lee, K.W., Song, C.B., Jeon, Y.J.: Antioxidant activity of enzymatic extracts from brown seaweeds. Algae 18, 71–81 (2003)
Siriwardhana, N., Jeon, Y.-J., Kim, S.-H., Ha, J.-H., Heo, S.-J., Lee, K.-W.: Enzymatic hydrolysis for effective extraction of antioxidative compounds from Hizikia fusiformis. Algae 19, 59–68 (2004)
Sineiro, J., Domínguez, H., Núñez, M.J., Lema, J.M.: Optimization of the enzymatic treatment during aqueous oil extraction from sunflower seeds. Food Chem. 61, 467–474 (1998)
Zhang, S.B., Wang, Z., Xu, S.Y.: Optimization of the aqueous enzymatic extraction of rapeseed oil and protein hydrolysates. J. Am. Oil Chem. Soc. 84, 97–105 (2007)
Teixeira, C.B., Macedo, G.A., Macedo, J.A., da Silva, L.H.M., Rodrigues, A.M.D.C.: Simultaneous extraction of oil and antioxidant compounds from oil palm fruit (Elaeis guineensis) by an aqueous enzymatic process. Bioresour. Technol. 129, 575–581 (2013)
Bocevska, M., Karlović, D., Turkulov, J., Pericin, D.: Quality of corn germ oil obtained by aqueous enzymatic extraction. J. Am. Oil Chem. Soc. 70, 1273–1277 (1993)
Balvardi, M., Rezaei, K., Mendiola, J.A., Ibáñez, E.: Optimization of the aqueous enzymatic extraction of oil from Iranian wild almond. J. Am. Oil Chem. Soc. 92, 985–992 (2015)
Kalia, V.C., Rashmi, S.L.: Using enzymes for oil recovery from edible seeds. J. Sci. Ind. Res. 60, 298–310 (2001)
Goula, A.M.: Ultrasound-assisted extraction of pomegranate seed oil–Kinetic modeling. J. Food Eng. 117, 492–498 (2013)
Dong, J., Liu, Y., Liang, Z., Wang, W.: Investigation on ultrasound-assisted extraction of salvianolic acid B from Salvia miltiorrhiza root. Ultrason. Sonochem. 17, 61–65 (2010)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Goula, A.M., Papatheodorou, A., Karasavva, S. et al. Ultrasound-Assisted Aqueous Enzymatic Extraction of Oil from Pomegranate Seeds. Waste Biomass Valor 9, 1–11 (2018). https://doi.org/10.1007/s12649-016-9740-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-016-9740-9