Abstract
Oilseed plants such as cotton (Gossypium sp.) generate abundant biomass residues which contain significant levels of edible oil, crude proteins and other desirable biomolecules for the animal nutrition industry. The application of cottonseed cake in animal feed, a by-product of the cotton industry, is limited due to the natural presence of toxic free gossypol (FG), wherein efficient and cost-effective methods for FG detoxification are necessary. Herein, pretreatment methods for reducing FG in crushed whole cottonseed (CWCS) were compared, with residual FG quantified using a sensitive Ultra High-Performance Liquid Chromatography method for detection at trace levels in cottonseed materials. Physical treatment by autoclaving resulted in up to 96% detoxification of FG, without reduction in crude protein (CP) content. Chemical treatment with 1% and 2% Ca(OH)2 eliminated FG to as low as 0.04%, although a reduction in CP content was observed. Similarly, native fermentation, whilst reducing FG content by 99.66% after 6 days incubation, also reduced CP content. In combined physical and biological solid-state fermentation (SSF), basidiomycete fungi Ganoderma lucidum CC351, Panus lecomtei CC40, Pleurotus ostreatus CC389, Pleurotus sapidus CC28 and Pycnoporus sanguineus CC400 all degraded FG in autoclaved CWCS to trace levels often lower than obtained by individual treatments. A reduction in total lipids and increase in CP were also observed, improving nutritional quality. The most efficient fungi, P. ostreatus CC389 and P. lecomtei CC40, secreted considerable laccase and manganese peroxidase enzymes during SSF, potentially involved in FG detoxification. Cost effective, non-polluting, value-adding approaches for FG detoxification offer potential in animal feed industries.
Graphic Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Statement of Novelty
Toxic free gossypol in cottonseed cake limits application in feed industries. This work reports combined treatments for free gossypol detoxification, based on autoclaving and solid-state fermentation.
Introduction
The processing of oilseed plants such as cotton (Gossypium sp.) generates abundant biomass residue containing significant levels of edible oil, crude proteins and other desirable biomolecules for industrial sectors devoted to animal nutrition. The application of whole cottonseed (WC) or cottonseed cake (CSC), a by-product of the cotton fiber industry, in animal feed is limited due to the natural presence of toxic gossypol in the plant [1]. Gossypol is a terpenopolyphenol secondary metabolite produced in pigment glands in cotton plant leaves, flower buds, stems and seed, as a possible defense chemical against diverse pathogens and herbivore insects [2,3,4]. Gossypol exists in both free (FG) and bound (BG) forms, with the free form most common in intact whole seeds. Cottonseed processing has been reported to result in gossypol binding to proteins, specifically to the epsilon group of amino acids such as lysine, arginine and cysteine [5]. Once in the bound form, gossypol is considered to be non-toxic, as it is not absorbed in the digestive tract. However, a portion of the gossypol that is bound may subsequently be released as FG during digestion, as reported in processed WC [6] and cottonseed meal [7]. Greatest concentration of gossypol typically occurs in seed tissues in cotton, with values of up to 7000 mg/kg of FG documented [8, 9]. Officially considered an anti-nutritional factor, monogastric livestock are sensitive to this polyphenol, with the European Union directive 2002L00032 limiting FG in cottonseed cake feedstuffs to 20 ppm for laying hens and piglets, 100 ppm for poultry and calves, and 500 ppm for cattle, goats and sheep. Toxicity is associated with reactions of the aldehyde groups of this metabolite with amino acids by cross linking resulting in inhibition of enzyme activities. Such linking of this phenolic group with amino acids and minerals can also result in the accumulation of inert and indigestible complexes, which are detrimental to animal health. Decreased growth, reduced fertility and abnormalities in internal organs are all common symptoms in non-ruminant livestock [10,11,12].
A number of physical and chemical methods have been described for detoxification of FG in CSC to permissible levels for feed [13, 14]. Solvent-based chemical treatment, although able to significantly reduce FG content in CSC, is typically costly, and, requiring considerable volumes of water for neutralization, generates contaminated effluents. Solvents may also alter the organoleptic characteristics of feed products, as observed with anhydrous acetone [14, 15]. Additional methods of FG detoxification involving mineral application or extrusion processes have also been employed for different cotton co-products [16,17,18]. While offering varying efficiencies in FG degradation, such methods, however, can be unsuitable because of deleterious effects on CSC. Treatment with iron sulphate, for example, causes discoloration and reduces palatability of animal feed [17]. Similarly, Ca(OH)2, while reducing FG levels, also reduces protein and vitamin content in CSC.
Given such limitations, research has also examined the potential for microbial biodetoxification of FG in cottonseed residues through solid-state fermentation (SSF). A number of fungi have been reported to efficiently detoxify FG, including the ascomycetes Aspergillus niger, Aspergillus oryzae, Candida tropicalis and Saccharomyces cerevisiae [19,20,21]. Macro-basidiomycetes, such as the white rot fungi (WRF), are well known degraders of complex carbon sources such as lignocelluloses and xenobiotic compounds [22]. In this context, a number of species have also been shown to detoxify both pure gossypol and FG in cottonseed [23, 24].
The objective of this study was to develop and evaluate the efficiency of physical, chemical, biological or combined (physical and biological) methods for the detoxification of FG in crushed whole cottonseed (CWCS).
Materials and Methods
Cottonseed
Cottonseed was obtained from FARMOTEC®, Buritis, MG, Brazil. Material was crushed in a commercial forage crusher (Trapp TRF400 super, SC, Brazil) using a sieve for 5 mm particle size. CWCS samples were stored at room temperature prior to treatment.
Physical Treatment
CWCS samples (20 g dry matter) were transferred to 250 mL Erlenmeyer flasks and moisture content adjusted to 60% using sterile distilled water. Samples were subjected to different autoclaving time periods at 121 °C: for 15, 30 or 60 min. Autoclaved samples were stored at − 20 °C until further analysis. Physical treatment experiments were performed in triplicate.
Chemical Treatment
CWCS samples (100 g dry matter) were mixed with 100 mL of 1% and 2% (m/v) solutions of Ca(OH)2 in 1000 mL Erlenmeyer flasks. Material was agitated at 120 rpm for 14 h at 30 °C, filtered using cotton gauze and filtrate stored at − 20 °C. Chemical treatment experiments were performed in triplicate.
Biological Native Fermentation Treatment
A total of 120 kg of cottonseed was crushed in a commercial forage crusher (Trapp TRF400 super, SC, Brazil) using a sieve for 5 mm particle size. Resultant CWCS was then transferred to a commercial mixer (Maqtron M-400, SC, Brazil), 60 L of water added and the material mixed for 15 min. The moistened CWCS was transferred to 0.6m3 metal bin composting structures. Triplicate native fermentation samples were disassembled at three time points (2, 4 and 6 days), with 6-day old samples of native fermentation also autoclaved at 121 °C for 60 min, to evaluate potential further detoxification of FG. For each sample, the temperature at the center of the pile was monitored. Sample material was dried in a hot air oven at 60 °C for 2 days, then stored at − 20 °C.
Combined Physical and Biological (SSF) Treatments
Macro-basidiomycete fungi were selected for evaluation in detoxification of FG by SSF. Five fungal isolates were selected as candidate biodetoxifiers from a local collection of microorganisms, based on previous investigations by the research group [25]. A total of 70 g (dry matter) of CWCS, previously moistened to 60% with sterile distilled water, was autoclaved at 121 °C for 15 min (physical treatment). After cooling, substrate was inoculated with 5 mm discs containing mycelial plugs of each fungal isolate (CC389, CC40, CC351, CC400, CC28), previously grown for 7 days on potato dextrose agar. SSF cultures were then incubated for 15 days at 28 °C. All experiments were conducted in triplicate.
Molecular Identification of Fungi
For molecular-based identification of each fungal isolate screened, genomic DNA was extracted from 150 mg of mycelium using an Ultra Clean Gel Spin DNA Extraction kit (MoBio Laboratories, CA, USA), according to the manufacturer’s recommendations. For accurate species level identification, sequence data was analyzed for the nuclear ribosomal DNA Internal Transcribed spacers (rDNA ITS), using the primers ITS1 and ITS4 [26], together with partial sequences of the Large Subunit (LSU) of the nuclear ribosomal RNA gene, amplified using the primers LROR and LR5 [27]. Where necessary, the RNA polymerase II gene (RPB2) was also employed for improved species resolution, following amplification with primers fRPB2-5F and bRPB2-7.12 [28, 29]. PCR products were enzymatically purified with ExoSAP-IT® (USB, Cleveland, Ohio, USA) and sequenced on a Genetic Analyzer 3500xL capillary sequencer (Thermo Fisher Scientific, Waltham, MA, USA), according to the manufacturer's guidelines. Sequences were compared against the NCBI nucleotide database (https://www.ncbi.nlm.nih.gov/) using the BLAST algorithm. Phylogenetic analysis was conducted by the Maximum Likelihood method using the program MEGA, version 6.06 [30].
Enzymatic Assays
Based upon FG biodetoxification data following combined physical (autoclaving) and SSF, fungal isolates CC40 and CC389 were selected for further evaluation of their enzymatic activities during SSF periods. Repeat cultures were prepared as described previously, with samples taken from colonized and control non-colonized substrate at 5, 10 and 15 days after inoculation. Crude extracts were obtained from homogenized samples (40 g) and 200 mL distilled water added (4 °C). Samples were vortexed at 100 rpm for 30 min, centrifuged for 10 min at 10,000×g and the supernatant filtered using Whatman filter paper no.1. The supernatant was denominated as crude extract (CE) and stored at 4 °C until evaluation of enzymatic activities. Laccase (Lac, EC 1.10.3.2) activity was determined based on oxidation of ABTS (2,2′-Azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) following mixing of 180 μL of crude extract in 180 μL of 0.2 M sodium acetate buffer (pH 5, 25 °C). The oxidation of ABTS was monitored for 90 s at 25 °C using a spectrophotometer at an absorbance of 420 nm (ε420:36,000 L/mol/cm) [31]. Mn-peroxidase (MnP EC.1.11.1.13) activities were determined by spectrophotometric measurement of the oxidation of phenol red at 610 nm (ε610 = 460 L/mol/cm) [32]. For this, 0.5 mL CE was mixed with 0.1 mL phenol red (1%), 0.05 mL MnSO4⋅(2 mM), 0.02 mL H2O2⋅(2 mM), 0.1 mL sodium lactate (250 mM), 0.2 mL serum albumin (0.5%) and sodium succinate buffer (pH 4.5) and incubated for 5 min at 30 °C. The reaction was interrupted by the addition of 40 μL 2 M NaOH. All enzymatic activities were expressed in international units as UI mL−1, defined as the amount of enzyme required to convert 1 μmol of substrate or to produce 1 μmol of product per minute of reaction (µmol min−1). All enzymatic assays were performed in triplicate.
Estimation of FG
Residual FG in untreated and treated CWCS samples was accurately measured by reversed phase Ultra High-Performance Liquid Chromatography (UHPLC), according to [25]. Briefly, 1 g of dried and ground sample material was placed into 15 mL centrifuge tubes and extracted with 10 mL of 70% aqueous acetone. Extraction was followed by ultra-sonication and centrifugation at 9000 rpm for 5 min at 8 °C. Supernatant was evaporated by speed vacuum at room temperature and re-suspended with 200 μL of 70% aqueous acetone solution. FG detection was conducted using an ACQUITY UPLC H-Class system (Waters, Milford, USA) and photodiode array detector, with gradient elution performed using a Phenomenex Kinetex™ reversed phase C18 column. This method developed by our group enables analysis of trace levels of FG in cottonseed materials, with a limit of detection at 0.2 μg/mL.
Bromatological Analysis
Untreated and treated CWCS samples were dried for 48 h using a hot air circulation drying oven (± 25 °C) and ground with a Wiley mill with a 60-mesh sieve screen. The dry matter was determined following drying at 105 °C for 12 h, while the mineral matter (ash) was determined following heating in a muffle furnace at 600 °C for 3 h. Analysis of cell wall components was conducted according to [33], with ethereal extracts, total lipids and crude protein (CP) determined according to [34].
Statistical Analysis
Gossypol trace values, crude protein and enzymatic activities were all expressed as mean values ± standard error of the mean. Analysis of variance with multiple comparisons (ANOVA) was conducted, followed by the Tukey post-test. Differences at p < 0.05 were considered as significant.
Results and Discussion
Efficient methods for detoxification of FG are essential for application of CSC in animal feed industries. The objective of this study was to develop and compare the efficiency of different physical, chemical, biological and combined methods for FG detoxification. CWCS was selected as substrate for accurate evaluation of efficiency of FG detoxification treatments given the higher concentrations of gossypol at this stage, prior to oil extraction. In addition, although not appropriate for monogastric animal feed prior to detoxification, whole cotton seed is widely employed in ruminant feed in Brazil [35], comprising up to 15% of the daily diet.
Fungal Molecular Identification
Molecular identification of the evaluated macro-basidiomycete fungi was based upon sequence analysis of rDNA ITS regions, and where increased resolution was necessary, based on analysis of LSU regions and RPB2 gene sequences. Following comparison against the nucleotide database NCBI using BLASTn (data not shown), together with clustering in a consensus phylogenetic tree based on alignment of rDNA ITS sequences (Supplementary Fig. 1), the fungi screened in this study were identified as: Ganoderma lucidum CC351; Panus lecomtei CC40; Pleurotus ostreatus CC389; Pycnoporus sanguineus CC400 and Pleurotus sapidus CC28. For these respective fungi, sequences enabling molecular identification were deposited in GenBank (https://www.ncbi.nlm.nih.gov) under accession numbers MK603977, MK603978, MK603976, MK603979 and MK603975.
Physical and Chemical Treatments
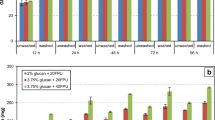
Physical treatment through autoclaving resulted in significant decreases in FG content according to exposure time. In comparison to non-autoclaved controls, a 60-min period of autoclaving reduced FG content by 96.35%, from 2446.93 to 89.26 μg/g (Fig. 1; Table 1). Although shorter autoclaving times also resulted in detoxification of FG, percentage reductions were lower, with 15- and 30-min reducing FG in CWCS by 86.64 and 91.33%, respectively. Such efficiency in autoclaving for FG detoxification has been described previously [36]. Heat treatment without autoclaving has also been widely employed in FG detoxification [37, 38] with efficiency increasing according to exposure time and temperature. Whether volatilization and/or transformation of gossypol are responsible for the reduction in FG has also been investigated [38, 39], with detection of molecules related to gossypol indicative of transformation after 1 h at 260 °C. Gossypol has a molecular weight of 518 Daltons and a boiling point of 707 °C. As such, volatilization under the conditions tested would not be possible. Transformation products detected have been reported to include mainly gossypolone, with other products also present, though less abundant. Other studies, however, have also reported that heat treatment can transform FG into bound gossypol, involving links between gossypol and amino acids, mainly lysine [11, 16]. Temperature-based treatment has also been reported to significantly negatively affect the quality of cottonseed, decreasing protein value and reducing amino acid content, mainly for lysine and arginine [37, 38, 40]. Although autoclaving did not significantly affect protein content in CWCS in our study, such reports highlight that reliance exclusively on heat treatment for FG elimination may not be appropriate for feed applications.
Free gossypol content in crushed whole cottonseed following physical and chemical treatments. Treatments: physical (autoclaving at 121 °C for 15, 30 or 60 min); chemical (1 and 2% solutions of Ca(OH)2); control: untreated CWCS 100%. Bars for FG values followed by the same letter do not differ statistically from each other (Tukey test, differences at p < 0.05 were considered significant)
Chemical treatment using Ca(OH)2 also resulted in efficient detoxification of FG in CWCS, with a 1% Ca(OH)2 solution reducing FG content by 98.9% and a 2% solution by 99.6% (Fig. 1). Phenolic oxidative coupling of hemigossypol is known to occur during the biosynthesis of gossypol. Chemical conversion of gossypol, by contrast, can result in decoupling and accumulation of hemigossypol, together with additional products such as gossypolone, apogossypol and apogossypolone [41,42,43]. In our study, chemical treatment with calcium hydroxide using concentrations up to 2.0% resulted in an almost total detoxification of FG. Previous reports using calcium hydroxide, by contrast, have achieved reductions of up to only 40% [17]. While a combination of 1.5% calcium hydroxide and 0.2% ferrous sulphate has also been shown to reduce FG levels to 0.04%, resultant meal can be inappropriate as feed [39, 44]. Previously, [44] reported that, although no benefits could be observed following incorporation of calcium hydroxide-treated CWCS into feed, no adverse effects were noted in blood parameters or animal performance. Such chemical treatment, however, can reduce both CP and total lipid (ethereal extract) content in meal, as observed in our study, with 2% Ca(OH)2 significantly reducing both protein and ethereal content in CWCS. Although potentially a negative effect, widespread availability of Ca(OH)2 at the farmer level in cotton growing countries, together with the absence of residue problems, continues to make this approach for FG detoxification an attractive alternative.
Biological Treatment: Native Fermentation
Biological treatment of CWCS by means of native fermentation was evaluated after 2, 4- and 6-days of incubation. Prior to native fermentation (control), FG was detected at a concentration of 2446.93 μg/g, whereas after 6 days incubation only 8.26 μg/g of FG remained, representing detoxification in excess of 99%. Following the six-day native fermentation period, samples were autoclaved for 60 min at 121 °C, resulting in a slight further increase in FG degradation, to 99.88% (Fig. 2; Table 1). Native fermentation of biological material involves the addition of water to organic substrate, followed by the appropriate stacking of material to favor growth of naturally present mesophilic and thermophilic microorganisms. Although the process does not require the addition of specific microorganisms, water content, aeration and temperature must be optimized for microbial growth [45]. Aerobic composting of cotton residue biomass has been employed in the detoxification of chemical toxins and for the control of phytopathogens [45,46,47]. To date, however, this approach has not been tested for detoxification of FG in CWSC or CSC. Whilst recognizing that complete composting will only be achieved after C/N ratios become constant, native fermentation over a shorter time period may theoretically be sufficient for FG degradation. Here, detoxification of FG by more than 99.6% was achieved after only 6 days of native fermentation. It is likely that FG detoxification occurs as a result of the combined effect of the elevated temperature and the ecological succession of the thermophilic microbiota, secreting extracellular enzymes capable of gossypol conversion and degradation of components of the plant cell wall. The action of fungal enzymes in the detoxification of gossypol has been reported previously, for example in Pleurotus florida [23], with laccase production correlating with gossypol degradation. Further investigation into the potential in native fermentation for FG detoxification is necessary, involving analysis of the influence of physical factors on FG degradation, such as pH, temperature, and CO2 and O2 concentrations. Analysis of the microbial ecology during native fermentation of CWCS is also warranted, as described on non-autoclaved cottonseed meal [40]. Although these authors observed a variable bacterial community, mostly comprising Acinotobacter sp., Enterobacter sp. and Bacillus sp., there have been no reports on the presence of fungi in such composting, despite their widely documented ability to degrade FG [21, 23, 48,49,50,51]. The employment of promising fungi or their enzymes in native fermentation offers commercial potential in FG detoxification associated with gains in the degradability of organic matter for animal nutrition.
Free gossypol content in crushed whole cottonseed following native fermentation and following combined native fermentation and physical treatment. Bars for FG values followed by the same letter do not differ statistically from each other (Tukey test, differences at p < 0.05 were considered significant)
Combined Physical and Biological (SSF) Treatments
Certain macro-basidiomycete fungi are able to colonize diverse agro-industrial residues [52,53,54,55]. G. lucidum, P. sanguineus and Pleurotus sp. for example, have been employed in the bioremediation of toxic heavy metals and other xenobiotic compounds in effluents and soils [22, 56,57,58]. Such fungi secrete ligninolytic extracellular enzymes and other oxidative enzymes, capable of degrading complex aromatic pollutants through catalytic oxidation–reduction reactions [59, 60]. A number of macro-basidiomycetes have also been applied in the detoxification of toxic and/or anti-nutritional factors in plant biomass, such as phorbol esters in jatropha cake, with detoxification occurring during lignocellulose decomposition [57, 61, 62]. Here, combined physical (autoclaving for 15 min) and biological (15-day axenic SSF cultures) treatment with the tested macro-basidiomycete fungi reduced FG in CWCS to levels lower than those obtained exclusively through autoclaving. The five fungi were able to further reduce FG residue levels remaining after autoclaving for 15 min (Fig. 3). P. lecomtei CC40 and P. ostreatus CC389 were the most efficient in FG detoxification through SSF, reducing concentrations by 99.47 and 99.43%, respectively. Ongoing toxicological analysis of the biomass after each of these combined treatments on arthropods (Artemia salina) and rats (data not presented) has also confirmed detoxification to levels safe for application in monogastric nutrition.
Free gossypol content in crushed whole cottonseed following combined physical and biological solid-state fermentation treatment with macro-basidiomycete fungi. Bars for FG values followed by the same letter do not differ statistically from each other (Tukey test, differences at p < 0.05 were considered significant)
Bromatological Analysis
Bromatological analysis revealed an increase in dry matter (with the exception of calcium hydroxide treatment) and ash following all treatments, which can be considered as an indicator of mineralization. A significant increase in CP content was also apparent after autoclaving and SSF for P. lecomtei, G. lucidum, P. sanguineus and P. sapidus (Table 2). This is likely due to an increase in proteinaceous microbial biomass following fungal growth. Together with the deconstruction of plant biomass, liberation of soluble proteins and metabolites, species-specific SSF can be an effective approach for increasing nutritional value and digestibility of lignocellulosic material [63,64,65,66]. Single-cell protein derived from such fungal biomass has also been applied as animal feed [67, 68].
Enzymatic Activities
Laccase and manganese peroxidase enzyme activities were evaluated over the 15-day time course of SSF of CWCS with the two most efficient species observed for FG detoxification. P. lecomtei CC40 and P. ostreatus CC389 both secreted these oxidases throughout the time period during which FG concentrations declined, highlighting their potential involvement in detoxification. (Fig. 4). Previous studies have highlighted the potential of Pleurotus spp. and employment of crude enzymatic extracts for efficient biodetoxification of FG in rice straw [23]. Whilst manganese peroxidase activities were similar in both fungi, peaking at 10 days, laccase activities were greater in P. lecomtei CC40, with highest activity at 2.222,21 IU/mL after five days SSF (Fig. 4). Such phenol oxidases, which are involved in the ligninolytic system of white rot fungi, offer potential in the treatment of industry residues and in soil bioremediation. To date, the characterization of enzyme activities for species within the genus Panus has been limited [60]. reported an ability of Panus tigrinus to discolor textile dyes, with [63] reporting this species for detoxification of a mixture of 2,4-dichlorophenol, 2,4,6-TCP, and pentachlorophenol, attributed to manganese peroxidase activity. Although mechanisms responsible for gossypol biodetoxification are poorly understood, analysis of upregulation and silencing of genes encoding cytochrome P450 monooxygenases in the Lepidopteran pests of cotton, Helicoverpa armigera and Heliothis virescens, has clearly linked this family of monooxygenases with detoxification of gossypol [69, 70]. Considering their recognized role in metabolism of xenobiotic compounds, including in macro-basidiomycete fungi such as Phanerochaete sp.[71], the potential involvement of this superfamily of monooxygenases in gossypol biodetoxification in the fungi tested here warrants further investigation. Similarly, [72] reported laccase activities in mixed ascomycete and basidiomycete fungal cultures responsible for biotransformation and biodegradation of gossypol into non-toxic metabolites. The observed secretion of such oxidases in P. lecomtei CC40 and P. ostreatus CC389 in SSF, together with FG content reduction, provides further support for their role in detoxification of FG.
Free gossypol content in crushed whole cottonseed over a 15-day time period of solid-state fermentation (SSF) with Pleurotus ostreatus CC389 (a) and Panus lecomtei CC40 (b), together with fungal laccase and manganese peroxidase enzyme activities. Bars for FG values followed by the same letter do not differ statistically from each other (Tukey test, differences at p < 0.05 were considered significant)
Conclusions
Although different physical and chemical methods are appropriate for detoxification of FG, the reduction in final crude protein content in treated cottonseed residues limits the applicability of these approaches for animal feed industries. The results of this study clearly showed that combined physical and SSF approaches can enable both efficient FG biodetoxification and concomitant gains in organic matter degradability for animal feedstuff. Given their potential in detoxification of xenobiotic compounds, supported by ongoing toxicological analysis, further screening of candidate macro-basidiomycete species is recommended, together with investigation of FG and BG levels remaining after each combined treatment. Future transcriptomic and proteomic approaches for elucidation of genes and cellular mechanisms controlling the fungal enzymatic detoxification of FG will also permit downstream targeted genetic modification in microorganisms for efficient FG detoxification in this important residue for animal feed industries.
Data Availability
Sequences enabling molecular identification were deposited in GenBank (https://www.ncbi.nlm.nih.gov) under accession numbers MK603977, MK603978, MK603976, MK603979 and MK603975.
References
Mena, H., Santos, J.E.P., Huber, J.T., Tarazon, M., Calhoun, M.C.: The effects of varying gossypol intake from whole cottonseed and cottonseed meal on lactation and blood parameters in lactating dairy cows. J. Dairy Sci. 87(8), 2506–2518 (2004)
Stipanovic, R.D., Lopez, J.D., Dowd, M.K., Puckhaber, L.S., Duke, S.E.: Effect of racemic and (+)-and (−)-gossypol on the survival and development of Helicoverpa zea larvae. J. Chem. Ecol. 32(5), 959–968 (2006)
Dalle Zotte, A., Brand, T.S., Hoffman, L.C., Schoon, K., Cullere, M., Swart, R.: Effect of cottonseed oilcake inclusion on ostrich growth performance and meat chemical composition. Meat Sci. 93(2), 194–200 (2013)
Puckhaber, L.S., Zheng, X., Bell, A.A., Stipanovic, R.D., Nichols, R.L., Liu, J., Duke, S.E.: Differences in active defense responses of two gossypium barbadense L. cultivars resistant to Fusarium oxysporum f. sp. vasinfectum Race 4. J. Agric. Food Chem. 66(49), 12961–12966 (2018)
Calhoun, M.C., Kuhlmann, S.W., Baldwin. B.C.: Assessing the gossypol status of cattle fed cottonseed products. In: Proceedings of the Pacific Northwest Animal Nutrition Conference, pp. 147A–157A, Portland, OR (1995)
Noftsger, S.M., Hopkins, B.A., Diaz, D.E., Brownie, C., Whitlow, L.W.: Effect of whole and expanded-expelled cottonseed on milk yield and blood gossypol. J. Dairy Sci. 83, 2539–2547 (2000)
Blackwelder, J.T., Hopkins, B.A., Diaz, D.E., Whitlow, L.W., Brownie, C.: Milk production and plasma gossypol of cows fed cottonseed and oilseed meals with or without rumen-undegradable protein. J. Dairy Sci. 81, 2934–2941 (1998)
Alexander, J., Andersson, H.C., Bernhoft, A., Brimer, L., et al.: Gossypol as undesirable substance in animal feed. EFSA J. 908, 1–55 (2008)
Knutsen, H.K., Barregård, L., Bignami, M., Brüschweiler, B., et al.: Presence of free gossypol in whole cottonseed. Scientific opinion of the panel on contaminants in the food chain (CONTAN). EFSA J. 15, e048501 (2017)
Gadelha, I.C.N., Fonseca, N.B.S., Oloris, S.C.S., Melo, M.M., Soto-Blanco, B.: Gossypol toxicity from cottonseed products. Sci. World J. 2014, 231635 (2014)
Atia, A.I., Abdel-Rahim, G.A.: Detoxification treatments of free gossypol in cottonseed meal by microbial treatment of mixed cultures and biochemical evaluation on rabbits. J. Rad. Res. Appl. Sci. 2(2), 397–415 (2009)
Zhang, Y., Zhang, Z., Dai, L., Liu, Y., Cheng, M., Chen, L.: Isolation and characterization of a novel gossypol-degrading bacteria Bacillus subtilis strain Rumen Bacillus subtilis. Asian-Australas. J. Anim. Sci. 31(1), 63 (2018)
Nomeir, A., Abou-Donia, M.: Photodecomposition of gossypol by ultraviolet irradiation. J. Am. Oil Chem. Soc. 62, 87–89 (1985)
Gerasimidis, K., Fillou, D.T., Babatzimcpoulou, M., Tassou, K., Katsikas, H.: Preparation of an edible cottonseed protein concentrate and evaluation of its functional properties. Int. J. Food Sci. Nutr. 58(6), 486–490 (2007)
Wang, X., Howell, C.P., Chen, F., Yin, J., Jiang, Y.: Gossypol-a polyphenolic compound from cotton plant. Adv. Food Nutr. Res. 58, 215–263 (2009)
Nagalakshmi, D., Sastry, V.R.B., Agrawal, D.K.: Detoxification of undecorticated cottonseed meal by various physical and chemical methods. Anim. Nutr. Feed Technol. 2(2), 117–126 (2002)
Nagalakshmi, D., Sastry, V.R.B., Pawde, A.: Rumen fermentation patterns and nutrient digestion in lambs fed cottonseed meal supplemental diets. Anim. Feed Sci. Technol. 103(1), 1–14 (2003)
Buser, M.D., Abbas, H.K.: Mechanically processing cottonseed to reduce gossypol and aflatoxin levels. J. Toxicol. Toxin Rev. 20(3–4), 179–208 (2001)
Zhang, W.J., Xu, Z.R., Sun, J.Y., Yang, X.: Effect of selected fungi on the reduction of gossypol levels and nutritional value during solid substrate fermentation of cottonseed meal. J. Zhejiang Univ. Sci. B 7(9), 690–695 (2006)
Zhang, W.J., Xu, Z.R., Zhao, S.H., Jiang, J.F., Wang, Y.B., Yan, X.H.: Optimization of process parameters for reduction of gossypol levels in cottonseed meal by Candida tropicalis ZD-3 during solid substrate fermentation. Toxicon 48(2), 221–226 (2006)
Lim, S.J., Lee, K.J.: A microbial fermentation of soybean and cottonseed meal increases antioxidant activity and gossypol detoxification in diets for Nile tilapia Oreochromis niloticus. J. World Aquac. Soc. 42(4), 494–503 (2011)
Kulikova, N.A., Klein, O.I., Stepanova, E.V., Koroleva, O.V.: Use of basidiomycetes in industrial waste processing and utilization technologies: fundamental and applied aspects. Appl. Biochem. Microbiol. 47(6), 565 (2011)
Rajarathnam, S., Shashirekha, M.N., Bano, Z.: Biodegradation of gossypol by the white oyster mushroom, Pleurotus florida, during culturing on rice straw growth substrate, supplemented with cottonseed powder. World J. Microb. Biotechnol. 17(3), 221–227 (2001)
Fackler, K., Gradinger, C., Hinterstoisser, B., Messner, K., Schwanninger, M.: Lignin degradation by white rot fungi on spruce wood shavings during short-time solid-state fermentations monitored by near infrared spectroscopy. Enzyme Microb. Technol. 39(7), 1476–1483 (2006)
Conceição, A.A., Soares Neto, C.B., Ribeiro, J.A., Siqueira, F.G., Miller, R.N., Mendonça, S.: Development of an RP-UHPLC-PDA method for quantification of free gossypol in cottonseed cake and fungal-treated cottonseed cake. PLoS ONE 13(5), e0196164 (2018)
Gardes, M., Bruns, T.D.: ITS primers with enhanced specificity for basidiomycetes-application to the identification of mycorrhizae and rusts. Mol. Ecol. 2(2), 113–118 (1993)
Menolli Junior, N., Asai, T., Capelari, M., Paccola-Meirelles, L.D.: Morphological and molecular identification of four Brazilian commercial isolates of Pleurotus spp. and cultivation on corncob. Braz. Arch. Biol. Technol. 53(2), 397–408 (2010)
Liu, Y.J., Whelen, S., Hall, B.D.: Phylogenetic relationships among ascomycetes: evidence from an RNA polymerse II subunit. Mol. Biol. Evol. 16(12), 1799–1808 (1999)
Matheny, P.B., Wang, Z., Binder, M., Curtis, J.M., Lim, Y.W., Nilsson, R.H., Langer, E.: Contributions of rpb2 and tef1 to the phylogeny of mushrooms and allies (Basidiomycota, Fungi). Mol. Phylogenet. Evol. 43(2), 430–451 (2007)
Kumar, S., Stecher, G., Tamura, K.: MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 33(7), 1870–1874 (2016)
Wolfenden, B.S., Willson, R.L.: Radical-cations as reference chromogens in kinetic studies of ono-electron transfer reactions: pulse radiolysis studies of 2, 2′-azinobis-(3-ethylbenzthiazoline-6-sulphonate). J. Chem. Soc. Perkin Trans. 2, 805–812 (1982)
Kuwahara, M., Glenn, J.K., Morgan, M.A., Gold, M.H.: Separation and characterization of two extracelluar H2O2-dependent oxidases from ligninolytic cultures of Phanerochaete chrysosporium. FEBS Lett. 169(2), 247–250 (1984)
Van-Soest, P.J.: Nutritional Ecology of the Ruminant, 2nd edn. Comstock Publishing Assoc, Ithaca (1994)
Cunniff, P.: Official Methods of Analysis of AOAC International, 16th edn. AOAC International, Arlington, Washington, DC (1995)
Lima, E.S., Valente, T.N.P., Roça, R.O., Cezário, A.S., Santos, W.B.R., Deminicis, B.B., Ribeiro, J.C.: Effect of whole cottonseed or protected fat dietary additives on carcass characteristics and meat quality of beef cattle: a review. J. Agric. Sci. 9(5), 175–189 (2017)
Gallup, W.D.: Heat and moisture as factors in the destruction of gossypol in cottonseed products1. Ind. Eng. Chem. 19(6), 726–728 (1927)
Yu, F., McNabb, W.C., Barry, T.N., Moughan, P.J.: Effect of heat treatment upon the chemical composition of cottonseed meal and upon the reactivity of cottonseed condensed tannins. J. Sci. Food Agric. 72(2), 263–272 (1996)
Chai, X., Bi, Y., Sun, S.: Free fatty acids increase gossypol losses in soybean oil during heating. Eur. J. Lipid Sci. Technol. 118(4), 584–591 (2016)
Aslam, M., Arshad, M., Ali, S.M.: Biochemical and nutritional studies on indigenous cottonseeds for the production of detoxified cottonseed flour. Pak. J. Sci. Ind. 13, 271–275 (1970)
Wang, X., Tang, J.W., Yao, X.H., Wu, Y.F., Sun, H., Xu, Y.X.: Effect of Bacillus cereus Br on bacterial community and gossypol content during fermentation in cottonseed meal. Afr. J. Microbiol. Res. 6(36), 6537–6544 (2012)
Sun, Y., Wu, J., Aboukameel, A., Banerjee, S., Arnold, A.A., Chen, J., Wang, S.: Apogossypolone, a nonpeptidic small molecule inhibitor targeting Bcl-2 family proteins, effectively inhibits growth of diffuse large cell lymphoma cells in vitro and in vivo. Cancer Biol. Ther. 7(9), 1418–1426 (2008)
Mellon, J.E., Zelaya, C.A., Dowd, M.K., Beltz, S.B., Klich, M.A.: Inhibitory effects of gossypol, gossypolone, and apogossypolone on a collection of economically important filamentous fungi. J. Agric. Food Chem. 60(10), 2740–2745 (2012)
Lu, Y., Li, J., Dong, C.E., Huang, J., Zhou, H.B., Wang, W.: Recent advances in gossypol derivatives and analogs: a chemistry and biology view. Future Med. Chem. 9(11), 1243–1275 (2017)
Kannan, A., Sastry, V.R.B., Agrawal, D.K., Kumar, A.: Effect of feeding of calcium hydroxide-treated or vitamin E-supplemented cottonseed meal on plasma gossypol levels, blood parameters, and performance of Bikaneri lambs. Trop. Anim. Health Prod. 45(6), 1289–1295 (2013)
Knox, O., Rochester, I., Vadakattu, G., Lawrence, L.: Composting in Australian cotton production. Aust. Cotton Grow. 2006, 46–48 (2006)
Hills, D.J., Curley, R.G., Knutson, J.K., Seiber, J.N., Winterlin, W.L., Rauschkolb, R.S., Elmore, C.L.: Composting treatment for cotton gin trash fines. Trans. ASAE 24(1), 14–19 (1981)
Li, L., Frey, M., Browning, K.J.: Biodegradability study on cotton and polyester fabrics. J. Eng. Fiber Fabr. 5(4), 42–52 (2010)
Khalaf, M.A., Meleigy, S.A.: Reduction of free gossypol levels in cottonseed meal by microbial treatment. Int. J. Agric. Biol. 10(10), 185–190 (2008)
Yang, X., Guo, J., Sun, J.: Biodegradation of free-gossypol by a new fungus isolated from cotton planted soil. Afr. J. Microbiol. Res. 5(19), 3066–3072 (2011)
Nie, C., Zhang, W., Ge, W., Wang, Y., Liu, Y., Liu, J.: Effects of fermented cottonseed meal on the growth performance, apparent digestibility, carcass traits, and meat composition in yellow-feathered broilers. Turk. Vet. Anim. Sci. 39(3), 350–356 (2015)
Mageshwaran, V., Parvez, N.: Gossypol detoxification and lysine enrichment in cottonseed cake by solid state fermentation. J. Pure Appl. Microbiol. 10(2), 1333–1339 (2016)
Sainos, E., Díaz-Godínez, G., Loera, O., Montiel-González, A.M., Sánchez, C.: Growth of Pleurotus ostreatus on wheat straw and wheat-grain-based media: biochemical aspects and preparation of mushroom inoculum. Appl. Microbiol. Biotechnol. 72(4), 812–815 (2006)
Hoa, H.T., Wang, C.L., Wang, C.H.: The effects of different substrates on the growth, yield, and nutritional composition of two oyster mushrooms (Pleurotus ostreatus and Pleurotus cystidiosus). Mycobiology 43(4), 423–434 (2015)
Mkhize, S.S., Cloete, J., Basson, A.K., Zharare, G.E.: Performance of Pleurotus ostreatus mushroom grown on maize stalk residues supplemented with various levels of maize flour and wheat bran. Food Sci. Technol. 36(4), 598–605 (2016)
Oyetayo, V.O., Ariyo, O.: Antimicrobial and antioxidant properties of Pleurotus ostreatus (Jacq: Fries) cultivated on different tropical woody substrates. J. Waste Conserv. Bioprod. Biotechnol. 1(2), 28–32 (2013)
Ajith, T.A., Janardhanan, K.K.: Indian medicinal mushrooms as a source of antioxidant and antitumor agents. J. Clin. Biochem. Nutr. 40(3), 157–162 (2007)
Bose, A., Keharia, H.: Phorbol ester detoxificationin Jatropha seedcake using white rot fungi. 3 Biotech 4(4), 447–450 (2014)
Silambarasan, S., Abraham, J.: Efficacy of Ganoderma sp. JAS4 in bioremediation of chlorpyrifos and its hydrolyzing metabolite TCP from agricultural soil. J. Basic Microbiol. 54(1), 44–55 (2014)
Kulshreshtha, S., Mathur, N., Bhatnagar, P.: Mushroom as a product and their role in mycoremediation. AMB Express 4, 29 (2014)
Mustafa, M.M., Jamal, P., Alkhatib, M.A.F., Mahmod, S.S., Jimat, D.N., Ilyas, N.N.: Panus tigrinus as a potential biomass source for reactive blue decolorization: isotherm and kinetic study. Electron. J. Biotechnol. 26, 7–11 (2017)
da Luz, J.M.R., Paes, S.A., Nunes, M.D., da Silva, M.D.C.S., Kasuya, M.C.M.: Detoxificationof oxo-biodegradable plastic by Pleurotus ostreatus. PLoS ONE 8(8), e69386 (2013)
Gomes, T.G., Hadi, S.I., Costa Alves, G.S., Mendonça, S., De Siqueira, F.G., Miller, R.N.: Current strategies for the detoxification of Jatropha curcas seed cake: a review. J. Agric. Food Chem. 66(11), 2510–2522 (2018)
Nagpal, R., Shrivastava, B., Kumar, N., Dhewa, T., Sahay, H.: Microbial feed additives. In: Puniya, A.K., Singh, R., Kamra, D.N. (eds.) Rumen Microbiology: From Evolution to Revolution, pp. 161–175. Springer, India (2015)
Nayan, N., Sonnenberg, A.S., Hendriks, W.H., Cone, J.W.: Screening of white-rot fungi for bioprocessing of wheat straw into ruminant feed. J. Appl. Microbiol. 125(2), 468–479 (2018)
Wang, J., Cao, F., Su, E., Zhao, L., Qin, W.: Improvement of animal feed additives of Ginkgo leaves through solid-state fermentation using Aspergillus niger. Int. J. Biol. Sci. 14(7), 736 (2018)
Gunturu, D.R., Yegireddy, M., Mannem, S., Mekapogu, A.R., Tollamadugu, N.P.: Effective role of microorganisms in livestock development. In: Buddolla, V. (ed.) Recent Developments in Applied Microbiology and Biochemistry, pp. 185–194. Elsevier, Amsterdam (2019)
Villas-Bôas, S.G., Esposito, E., Mitchell, D.A.: Microbial conversion of lignocellulosic residues for production of animal feeds. Anim. Feed Sci. Technol. 98(1–2), 1–12 (2002)
Sousa, D., Venâncio, A., Belo, I., Salgado, J.M.: Mediterranean agro-industrial wastes as valuable substrates for lignocellulolytic enzymes and protein production by solid-state fermentation. J. Sci. Food Agric. 98(14), 5248–5256 (2018)
Leontievsky, A., Myasoedova, N., Golovleva, L., Sedarati, M., Evans, C.: Adaptation of the white-rot basidiomycete Panus tigrinus for transformation of high concentrations of chlorophenols. Appl. Microbiol. Biotechnol. 59(4–5), 599–604 (2002)
Mao, Y.B., Cai, W.J., Wang, J.W., Hong, G.J., Tao, X.Y., Wang, L.J., Chen, X.Y.: Silencing a cotton bollworm P450 monooxygenase gene by plant-mediated RNAi impairs larval tolerance of gossypol. Nat. Biotechnol. 25(11), 1307–1313 (2007)
Hirosue, S., Tazaki, M., Hiratsuka, N., Yanai, S., Kabumoto, H., Shinkyo, R., Ichinose, H.: Insight into functional diversity of cytochrome P450 in the white-rot basidiomycete Phanerochaete chrysosporium: involvement of versatile monooxygenase. Biochem. Biophys. Res. Commun. 407(1), 118–123 (2011)
Mageshwaran, V., Sharma, V., Chinnkar, M., Parvez, N., Krishnan, V.: Biodegradation of gossypol by mixed fungal cultures in minimal medium. Appl. Biochem. Microbiol. 54(3), 301–308 (2018)
Felsenstein, J.: Confidence limits on phylogenies: an approach using the bootstrap. Evolution 39(4), 783–791 (1985)
Jukes, T.H., Cantor, C.R., Munro, H.N.: Evolution of protein molecules. In: Munro, H.N. (ed.) Mammalian Protein Metabolism, pp. 21–132. Academic Press, New York (1969)
Funding
This work was financially supported by CAPES (project finance code 001) and CNPq/Embrapa (Project Number 404786/2013-8). RNGM was supported by a fellowship from CNPq (Project Number 305418/2017-3).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest, or competing financial or scientific interest.
Informed Consent
The authors declare consent to participate. The authors declare consent for publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Soares Neto, C.B., Conceição, A.A., Gomes, T.G. et al. A Comparison of Physical, Chemical, Biological and Combined Treatments for Detoxification of Free Gossypol in Crushed Whole Cottonseed. Waste Biomass Valor 12, 3965–3975 (2021). https://doi.org/10.1007/s12649-020-01290-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-020-01290-0