Abstract
This study reports on the evaluation of a two-stage pretreatment process for preparing cotton gin trash (CGT) for conversation to ethanol. During the first stage, CGT was mixed with acid at 12% H2SO4 on solids and heated to 180 °C for 15 min in a pressurised stirred reactor. Pressed first-stage pretreated fibres were heated to 200 °C for 5 min during the second stage. The two-stage process facilitated excellent sugar recovery from both cellulose (84%) and hemicellulose (78%) fractions of CGT. Recombinant yeast GSF335 propagated on first-stage liquors yielded 41.8 g kg−1 of dry CGT and was compared with the commercial yeast during separate hydrolysis and fermentation (SHF) and simultaneous saccharification and fermentations (SSF). SHF of pretreated CGT fibre yielded the equivalent of 66.2 and 64.0 kg ethanol per tonne of unprocessed CGT with GSF335 and the commercial yeast, respectively. SSF ethanol yields for the commercial yeast were significantly lower (50.3 kg) while GSF335 correspondingly produced 63.8 kg ethanol per tonne of raw CGT.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In Australia, cotton is a significant broad acre crop with more than half a million hectares currently under cultivation. It has been estimated that the ginning process in Australia generates somewhere between 25 and 60 kg of cotton gin trash (CGT) per bale of cotton; therefore, based on the 2017/2018 production statistics, up to 280,000 tonnes of CGT is projected [1, 2]. The high concentration of CGT residues poses a significant burden to the industry since the conventional practice of burning trash has ceased. The current practices for managing CGT varies greatly across the industry but for most, handling, storage, transport and disposal options add considerable cost to cotton-ginning processes [1]. Owing to increased disposal fees, stricter regulations on environmental applications and emissions and higher fuel prices, interest in converting this waste residue into bioenergy, particularly biofuels, is gaining attention.
CGT has been examined by several researchers as an exploitable biomass resource, particularly as a renewable feedstock in reinforcing commercial bioenergy purposes [3]. For instance, it has promising compositional attributes for effective conversion to biofuels and other valuable products relative to other candidate biomass feedstocks [4]. In biochemical conversion-based approaches, high yields of fermentable sugars have been generated from CGT and shown to support cellulosic ethanol production systems [5,6,7]. CGT is an ideal biorefinery feedstock because it is concentrated at processing sites, consequently minimising operational costs associated with harvesting and transportation. However, commercial production of ethanol from CGT requires development of protocols for cost-effective pretreatment and fermentation.
Converting lignocellulosic materials to fermentable sugars entails a pretreatment step to break up the lignocellulosic structure thereby allowing enzymes to access and hydrolyse cellulose to glucose. A number of approaches to pretreatment have been investigated, with acid pretreatment the most commonly reported [5,6,7,8,9,10]. By increasing the severity of acid pretreatment, cellulose digestibility improves but as a consequence xylose recovery declines, leading to an increase in degradation by-products that inhibit enzyme digestion and fermentation. This occurs because the pretreatment conditions that favour recovery of cellulose are generally more severe than pretreatment conditions that favour hydrolysis of hemicellulose. Thus, single-stage pretreatments are problematic for maximising recovery of glucose without generating inhibitory compounds.
To overcome this issue, two-stage pretreatments are applied. Sequential dilute acid pretreatments with steam explosion are often used for woody residues [11]. Mild conditions during the first stage of pretreatment remove the hemicellulose-derived pentose sugars, and cellulosic fibres amendable to enzyme digestion are produced during the second pretreatment stage. This approach requires sufficient residual acid on first-stage pretreated fibres for effective defibrillation during the second stage. Recently, a comparison of multiple combinations of conditions for two-stage dilute acid pretreatment of CGT to identify permutations that maximise recovery of both C5 and C6 sugars was reported [12]. A primary rationale for developing this approach was that production costs could be reduced by propagating recombinant Saccharomyces cerevisiae yeast on C5-enriched liquors pressed from first-stage pretreated CGT for use in subsequent C6 fermentations. Thus, sequential pretreatment of residual CGT fibre under optimal conditions may be undertaken without detrimentally affecting C5 prehydrolysate liquors. The criterion for this reasoning was based on the assertions that xylose metabolism in engineered S. cerevisiae favours respiration instead of fermentation [13] and that these yeast strains are unable to simultaneously co-ferment C5 and C6 hydrolysate sugars into ethanol in an economical manner [14, 15].
In the recent paper, Vancov and co-workers [12] proposed that propagating the recombinant yeast strain GSF335 [16] in xylose-enriched first-stage pretreatment liquors would improve efficiency by reducing processing steps and removing propagation media material costs. Although hydrolysates from first-stage pretreatment were suitable for propagating GSF335 and pretreated fibres from the successive stage were highly acquiescent to enzyme digestion, the two-stage process was only validated at a miniaturised scale. Further, the suitability of pretreated CGT fibres for ethanol fermentation was not evaluated. Thus, the purpose of this study was to (1) confirm that the optimal two-stage pretreatment process could be adapted to a pressurised stirred reactor scale, (2) demonstrate that two-stage pretreated CGT is suitable for ethanol fermentation and (3) confirm that the recombinant xylose-fermenting strain GSF335 propagated in first-stage liquors is comparatively proficient in producing ethanol from two-stage pretreated CGT under SHF and SSF conditions. This is a relatively novel approach that has not been previously reported.
Materials and Methods
Cotton Gin Trash and Materials
CGT was sourced from the Yarraman Gin from the 2017 harvest, New South Wales, Australia. The raw CGT biomass material was dried at 50 °C for 48 h prior to grinding in a rotary mill (Gelder & Co., New South Wales, Australia) fitted with a No. 5 American Standard Test Sieve (ASTM). Samples were pulverised for 60 s (Labtechnics Pulveriser, WA, Australia) to improve uniformity and reduce particle size (≤ 4.00 mm) of raw CGT. NREL methods [17] were applied to determine the composition of the milled CGT. The chemical composition of dry-milled CGT is presented in Table 1. Milled material was stored at room temperature in air-tight containers until use.
Unless otherwise stated in the text, all chemicals including acid, bases, salts, solvents and analytical standards were of reagent grade or higher and purchased from Sigma Chemical Co. (St. Louis, MO). Yeast extract and peptone were purchased from AMYL Media (VIC, Australia).
Two-Stage Pretreatment
Pretreatment was carried out in two stages under conditions previously described by Vancov et al. [12]. These pretreatment conditions were identified to be ideal in generating prehydrolysate liquors (stage 1) for optimal propagation of recombinant yeast and CGT fibres (stage 2) highly acquiescent to enzyme hydrolysis.
For the first stage, a total of 1.9 kg of CGT was processed over 19 runs in a 2-L pressurised stirred reactor (Parr Instruments, USA). For each run, 100-g CGT was subjected to dilute acid (DA) pretreatment using conditions described by Vancov et al. [12]: CGT was mixed with liquids at a ratio of 1:6 w/w with 12 wt% H2SO4 and the mixture held at 180 °C for 15 min. The pressurised stirred reactor (Parr reactor) was heated by an external aluminium block heater and water cooled through the heating block internal cooling coil. The heating controller was set on maximum output and reaction material was stirred at 60 rpm. Water was pushed through the cooling coil using the maximum water valve opening to rapidly cool the system (about 15 min). Following pretreatment, the vessel was rapidly cooled to 90 °C and then the slurry was immediately decanted. The liquors and solids were separated by vacuum filtration using Whatman® glass microfibre GF/A filters mounted in a Büchner funnel.
For second-stage pretreatment, 400 g of first-stage pretreated solids—equivalent to 129.2 g dry wt.—was placed in a 2-L stirred pressurised reactor and mixed with 300 mL of type 1 (ultrapure) water to give a biomass-to-liquids ratio of 1:5.4 w/w. The slurry was mixed at 60 rpm in the reaction vessel and heated to 200 °C for 5 min. After cooling, liquors were pressed from a subsample of the wet solids for compositional analysis (‘Analytical Methods’ section). The moisture content of pretreated solids was determined by drying at 70 °C with compositional analysis carried out on dried samples. Pretreated solids and liquors were stored at − 20 °C until use. All acids and chemicals associated with pretreatment processing were of reagent or analytical grade (Sigma-Aldrich, USA).
Cellulase Hydrolysis
The activity of Cellic® CTec 2 cellulase (kindly supplied by Novozymes, Denmark and herein referred to as CTec 2) was determined as 103 filter paper units (FPU) per millilitre according to NREL laboratory procedure LAP006 [18]. Enzyme hydrolysis reactions were set up in 50 mM citrate buffer (pH 5.2) containing 0.02% sodium azide and 30 mg peg 6000 g/dry wt. added solids. Cellic® CTec 2 cellulase was added to provide 20 FPU g/glucan and solids added to provide a glucan loading of 2% w/v or 3.75% w/v. Unwashed and washed solids were compared during cellulase hydrolysis. Washing was carried out by passing distilled water through pretreated solids until the filtrate was pH 6.0. Hydrolysis mixtures were incubated at 50 °C on a rotating wheel at 50 rpm for up to 72 h with 1 mL of each sampled at 0 h, 12 h and 24 h and then at 24-h intervals to 96 h. Hydrolysate samples were centrifuged at 8000g for 5 min and stored at − 20 °C prior to analysis. Glucan digestibility after enzymatic hydrolysis was calculated as described by Vancov et al. [12].
Propagation of GSF335 Yeast
Recombinant S. cerevisiae GSF335 (VIB, Belgium) was propagated using first-stage pretreatment liquors as the exclusive source of carbon/sugars. A seed culture of GSF335 was initially grown in 500-mL baffled Erlenmeyer flasks containing 100 mL sterile YPD broth consisting of 10 g L−1 yeast extract, 20 g L−1 peptone and 20 g L−1 dextrose. Incubations were carried out overnight at 30 °C while agitated in an orbital shaker at 250 rpm. Following growth, the yeast culture was centrifuged and recovered cells were washed twice and resuspended in 0.9% sterile saline prior to measuring the optical density at 600 nm (OD600) of the cell suspension. The OD600 reading was then used to calculate the dry weight biomass against pre-determined growth curves.
Production of GSF335 yeast biomass for use in fermentations was carried out in 1-L baffled Erlenmeyer flasks containing a filter-sterilised medium consisting of 200 mL first-stage liquors with 10 g L−1 yeast extract, 20 g L−1 peptone and 10 mg L−1 tetracycline. Each flask was inoculated with the GSF335 seed culture added at a rate of 1 g yeast L−1 medium and incubated overnight at 30 °C in an orbital shaker with a rotation speed of 250 rpm. Following incubation, the biomass of the yeast cultures was estimated using a pre-determined OD600 curve. The amount required to achieve 4 g L−1 in the fermentations was transferred to sterile tubes and centrifuged at 2100 rpm for 5 min. The supernatant was removed and yeast cells were washed in 0.9% sterile saline and then centrifuged with the supernatant removed. The cells were resuspended in sterile 0.9% saline and used as inoculum.
Separate Hydrolysis and Fermentation
For SHF the enzyme hydrolysis step was carried out in 1-L glass stirred reactor vessels (Duran GLS80©) fitted with an overhead stirrer (RZR 2020 Heidolph, Germany). Reactor vessels contained a total mass of 700 g consisting of 10% dry wt. unwashed pretreated solids corresponding to a 3.75% glucan loading, 462.6 g of dissolved reagents added to give final concentrations of 30 mg peg 6000 g/dry wt. of added solids and 20 FPU CTec 2 cellulase per gram of added glucan with the balance made up with 20 mM citrate buffer (pH 5.0). Enzymatic hydrolysis was carried out over 72 h at 50 °C with the stirring speed maintained at 100 rpm. The resulting hydrolysate was recovered by filtering through a 1.2-μm glass micro-fibre filter. The pH was adjusted to pH 5.0 with KOH and then the hydrolysate was refrigerated for 12 h to allow fine particles to settle. The hydrolysate was then vacuum-filtered through a 0.45-μm cellulose nitrate membrane filter. To prepare the hydrolysate for fermentation, the following was added: 10 g L−1 yeast extract, 20 g L−1 peptone, 860 mg L−1 MgSO4, 52 mg L−1 ZnSO4 and 294 mg L−1 CaCl2. The SHF medium was then filter-sterilised and stored in a sterile container at 4 °C for a short period prior to use.
SHFs were performed in 120-mL serum glass bottles with silicone crimp top closures, containing 60-mL filter-sterilised medium. The medium consisted of above CGT digested hydrolysate plus 10 g L−1 yeast extract, 20 g L−1 peptone, 0.86 g L−1 MgSO4, 0.052 g L−1 ZnSO4, 0.294 g L−1 CaCl2, 300 μL 0.1% antifoam 204 and 10 μg mL−1 tetracycline. SHFs were inoculated at a cell range of approx. 4.0 g L−1 (dry weight equivalent), sparged with nitrogen gas for 10 min to ensure total anaerobic conditions and performed in batch mode equipped with a water trap to release CO2 and exclude oxygen. The temperature was set to 30 °C and agitation to 50 rpm. Prior to inoculation and filtration, the pH of all cultivation media was adjusted to 5.0 with 10 M KOH. GSF335 yeast inoculum was prepared as described in the ‘Propagation of GSF335 Yeast’ section. The commercial ethanol S. cerevisiae strain Fali (herein referred to as Fali) which was supplied as an active dry preparation (AB Mauri, Australia) is well suited for use in industrial SSF processes. Fali was prepared by transferring 4 g dry into 4 mL 0.9% saline. Time series sampling—1-mL volume on each occasion—was carried out at 2-h intervals up to 12 h and then at 24, 30 and 48 h. These samples were used to determine OD600 values at each time point and for compositional analysis by HPLC. Incubations were carried out in triplicate and the experiment was repeated twice.
Simultaneous Saccharification and Fermentation
SSFs were carried out in 1-L glass stirred reactor vessels (Duran GLS80©) fitted with an overhead stirrer (RZR 2020 Heidolph, Germany). Moist pretreated solids were added to the vessel at a ratio of 10%—corresponding to a 3.75% glucan loading—relative to the total reaction mass of 700 g. The SSF medium also contained yeast extract, peptone, MgSO4, ZnSO4, CaCl2, PEG 6000 and tetracycline at concentrations described for SHF media. The balance of the 700-g reaction mass was made up with sterile type 1 water, with the amount adjusted depending on the moisture content of the pretreated solids. The slurry was adjusted to pH 5.0 with KOH prior to addition of 10 mg L−1 tetracycline and 20 FPU CTec 2 cellulase per gram of added glucan.
GSF335 yeast biomass was prepared as described in the ‘Propagation of GSF335 Yeast’ section and Fali yeast inoculum was prepared as described in the ‘Separate Hydrolysis and Fermentation’ section. Immediately after adding yeast inoculum, the reactor vessel was sparged with N2 for 10 min and then incubation was carried out at 30 °C with the stirring speed maintained at 100 rpm. A 1-mL sample of the hydrolysate was taken at 4-h intervals up to 12 h, followed by 12-h intervals up to 48 h and then 24-h intervals through to 120 h. The composition of these samples was determined by HPLC as described in the ‘Analytical Methods’ section. SSFs were performed in duplicate and repeated twice.
Calculations
The maximum specific growth rate of yeast (μmax) and the maximum specific glucose uptake rate (qsmax,glucose) were calculated as described by Jeon et al. [19].
The maximum specific ethanol production rates (qpmax) were calculated over the exponential phase of growth and based on the following formulae:
where Δs and Δp are the changes in the glucose and ethanol concentrations, respectively, over the time period Δt, and xav is the average biomass concentration over Δt.
In the xylose utilisation phase, the formulae for maximum specific xylose uptake rate is defined as follows:
where Δs is the change in the xylose concentration over the time period Δt, and xav is the average biomass concentration over Δt.
Ethanol product yield is based on total sugar consumption and is accordingly determined by:
where Eint and Ef are the initial and final concentrations of ethanol, and Sint and Sf are the initial and final concentrations of total fermentable sugars (glucose + xylose).
The theoretical ethanol yields for SHF and SSF were calculated according to the following equations, respectively:
where [E] is the final ethanol concentration and [G] and [Xyl] are the theoretical glucose and xylose content in the substrate.
Analytical Methods
Raw and pretreated biomass and fermentation liquids were prepared for compositional analysis according to standardised NREL methods, and the composition of pretreatment liquors was determined before and after dilute acid hydrolysis (DAH) [17]. Analysis of monosaccharide sugars, xylitol, glycerol, furfural, hydroxymethylfurfural (HMF), organic acids and ethanol content was determined by HPLC. The HPLC system consisted of a solvent delivery system (Controller 600, Waters, USA) equipped with an autosampler (Model 717, Waters) and a refractive index detector (Model 412, Waters) managed by the Waters Empower® software program. Sugars were determined using Phenomenex Rezex™ RHM-Monosaccharide column (7.8 mm × 300 mm) fitted with a Carbo-Pb guard column. The column was maintained at 60 °C and compounds were eluted with 0.005 M H2SO4 at a flow rate of 0.5 mL min−1. The refractive index detector was maintained at 50 °C for all applications. Peaks detected by the refractive index detector were identified by matching retention times and quantified by comparison, with analytical standards (Sigma-Aldrich, USA) included within each run.
Residual acid on second-stage pretreated fibres was determined by titration. Preparation of samples for titration was carried out by mixing 7-g dry solids in 60 mL type 1 water (ultrapure deionised) for 2 h with a further 1-h mixing after an additional 60 mL type 1 water was added. Liquids were separated from solids by vacuum filtration with titrations carried out on the liquid fraction against 0.025 M CaCO3 using an automatic titrator (Titralab TIM 870, Radiometer, Australia).
Statistical Analysis
Enzyme digestibility data was analysed using analysis of variance (ANOVA) to determine significant differences between the means. A Tukey HSD post hoc analysis (α = 0.05) was conducted using the real statistics data analysis tool in Microsoft Excel (2016) to reveal where the differences between the groups stand. A t test (Microsoft Excel) was used to determine the significant difference between the yeast’s fermentation parameter means.
Results and Discussion
Characteristics of Feedstock, Pretreatment Liquors and Solids
CGT is uniquely different in composition to most other agricultural residues in that it consists of a heterogeneous mixture of cotton stems, leaf motes, burrs, lint and seeds. The proportion of these varies substantially and primarily depends on the ginning operation and time of cotton harvest. The CGT used in this study comprised of high acid-insoluble lignin and ash (Table 1). The latter presumably arises from the manner in which the CGT was stockpiled—stored directly on gravel-strewn earth pads. Better stowage (paved/concrete pads) of CGT should minimise grit, dust and gravel contamination, ensuring fewer downstream processing concerns and its part in the biomass feedstock mix. On an extractive and ash-free basis, the lignin content approaches 46%, which is markedly higher than most reported crop residues, and is more akin to hardwoods, while the carbohydrate content is significantly lower [20,21,22]. Seasonal variation and geographical location accounts for discrepancies between samples used in this study—low sugar to lignin ratio (1.18)—and previously reported values between 1.82 and 3.8 for CGT [6, 8, 12, 23]. This profoundly impacts the hydrolysis (lignin recalcitrance) and ethanol yields.
The dry weight of solids recovered following first-stage DA pretreatment was 67.7% of the initial CGT biomass. The chemical composition of recovered fibres is listed in Table 1. Liquors pressed from first-stage pretreated solids contained low concentrations of degradation products with 1.43 g L−1 acetic acid, 0.48 g L−1 HMF and 0.82 g L−1 furfural. Xylose release into the liquor fraction corresponded to 58.4% of its original content in the CGT, which was slightly higher than the amount (56.1%) recovered during the mini-scale trials under similar pretreatment conditions [12]. The ratio of total xylose released during first-stage pretreatment relative to the theoretical maximum release of xylose—as determined by DAH—was 78% and relatively lower than the theoretical hemicellulose dissolution attained at miniaturised scale (87%). Free and total glucose release into prehydrolysate liquors was consistent with miniaturised scale (7 and 12%, respectively) and other studies [5, 7, 12]. Depolymerisation and solubilisation of glucan from CGT is attributed to the unique composition of the biomass and corresponds to the amount of exposed naked cotton fibre [7]. It thus renders the cellulose more susceptible to depolymerisation than other lignocellulosic feedstock. Overall, the conditions applied during the first-stage DA pretreatment achieved the goal of maximising recovery of xylose in liquors with minimal loss of fibre, glucan and production of inhibitory compounds.
Following the second-stage pretreatment, the dry weight of recovered solids was 72% of added first-stage pretreated solids. Besides greater fibre recovery, post second-stage pretreated fibres were notably more enriched in glucan and xylan compared to those arising from the previously reported mini-scale study [12]. This finding was somewhat expected because the pretreatment settings (temperature) during the pressurised stirred reactor treatment were intrinsically more accurately regulated. The composition of recovered second-stage pretreated fibres is presented in Table 1 and represents recoveries of 84.8% glucan, 10.4% xylan, 91.4% lignin and 21.8% ash. Glucan recovery of 84.4% using the two-stage process described herein is comparable to recoveries of 86 and 91% with steam exploded and alkali pretreatment of cotton gin waste (CGW) [7, 23]. Moreover, the results presented in this work surpasses reported studies on CGW exploiting dilute sulphuric acid single-step pretreatment regimes with theoretical glucan recoveries ranging from 62 to 71% [5, 8].
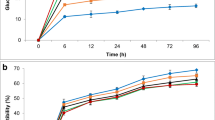
Approximately half of the residual xylose in the first-stage fibre, 3.3 g L−1—equivalent to 11.7% in the original dry matter (ODM)—was recovered in second-stage liquors. Loss of glucose from the solids fraction was minimal within second-stage liquors containing 1.32 g L−1 (corresponding to 1.8% in the ODM). Compounds that inhibit enzyme digestion or interfere with yeast fermentation were also minimal with liquors pressed from second-stage solids containing 0.96 g L−1 acetic acid, 0.44 g L−1 HMF and 0.85 g L−1 furfural. Effective second-stage pretreatment requires sufficient residual acid on solids to maximise enzyme digestibility [5, 24]. In this instance, 0.02 g g−1 residual acid on first-stage pretreated solids appears to have been adequate, as indicated by the results from cellulase saccharification of second-stage pretreated fibres (Fig. 1).
Glucan conversion rates (a) and total glucose release (mg) (b) of washed and unwashed recovered solids following second-stage pretreatment in the pressurised stirred reactor. Enzyme hydrolysis conducted at 50 °C with specified glucan load and Ctec 2 enzyme. Error bars represent the standard error. n = 6
Cellulase Digestibility of Recovered Second-Stage Fibre
In determining the enzymatic digestibility of second-stage pretreated solids, glucan loadings of 2% w/v (equivalent to a 5.7% w/v solids loading) and 3.75% w/v (equivalent to a 10% w/v solids loading) with 20 and 40 FPU Ctec 2 cellulase were compared. Unwashed pretreated solids—which is atypical of industrial practices—with washed pretreated solids were also compared. The results obtained after 92 h of hydrolysis at nominate time points are presented in Fig. 1 and correspond to conversion (%) in relation to the substrate glucan content (Fig. 1a) and as the corresponding total glucose release (mg) in the CGT fibre digest (Fig. 1b). Since most of these values were statistically different (p < 0.05 in the ANOVA), Tukey’s test was used to identify which of the individual responses were different from one another.
The results in Fig. 1a reveal that for both unwashed and washed solids, the conversion of glucan to glucose peaked at 72 h with statistically insignificant (p < 0.05) increases in glucan digestibilities thereafter. At a glucan loading of 3.75% w/v (10% solid loading), the digestibility of unwashed solids was significantly better (p < 0.05) at a 40 FPU Ctec 2 loading than at 20 FPUs for all time points except for the first 12 h. After the initial 12 h, digestibility of unwashed 2% w/v glucan loading was significantly better than its 3.75% w/v glucan counterpart at enzyme concentrations of 20 FPUs. Increasing the enzyme load to 40 FPUs notably improved enzyme hydrolysis in 3.75% w/v glucan digests. Similar trends were noted amongst the glucan digestibilities of washed samples (Fig. 1a), except for the initial 12 h of digestion. Enzyme hydrolysis significantly increased with glucan and enzyme load during this period. Washing pretreated solids also significantly improved digestibility of 3.75% w/v glucan load peaking at 58% with 20 FPU Ctec 2 and 68% with 40 FPU Ctec 2. Similarly, at the lower glucan ratio of 2% w/v, the digestibility of washed solids with 20 FPU Ctec 2 reached a maximum of 70%. These results are consistently better than most reported pretreatment and digestion studies of cotton gin and cropping residues, for example 18% digestibility for microbially treated cotton stalks [25], 23% for ultrasonication with hot water and enzyme pretreatment of CGT [26], 24% for sulphuric acid–pretreated cotton stalks [10], 39% for alkali pretreated CGW [6] and 67% for steam exploded CGW [7] and a two-step organic acid and alkali treatment of CGW [8]. Although significantly lower than the 89% theoretical conversion reported with single-step DA pretreatment of CGT [5], the cellulase load in latter was 8.7-fold higher (174 FPU g−1 glucan or 80FPU g−1 of fibre) than that in this study. However, at comparable fibre and enzyme loads (viz. 10% solids with 40 FPU g−1 glucan), glucan hydrolysis of two-stage pretreated solids was greater than that produced from single DA pretreatment (68.0 vs 60.6% conversion, respectively).
The glucose yield for a 2% w/v glucan loading was significantly less (p < 0.05) than that for a 3.75% w/v loading (Fig. 1b) irrespective of washing. For unwashed solids, the glucose yield for a 2% w/v glucan loading was 137 mg and 156 mg for washed solids after 72 h of hydrolysis. For a 3.75% w/v loading, the glucose yield was 219 mg with 20 FPU Ctec 2 and 240 mg with 40 FPU Ctec 2 for unwashed solids. In comparison to other treatments, glucose yield was significantly (p < 0.05) higher for washed solids at a 3.75% glucan loading which yielded 282 mg glucose with 40 FPU Ctec 2 and 240 mg with 20 FPU Ctec 2. The improved digestibility of washed solids likely resulted from the washing process leading to a reduction in organic acid and salts which are recognised cellulase inhibitors [27, 28]. Increasing the dry fibre load to 10% significantly reduced digestibility at the lower enzyme load (20 FPUs)—presumably owing to the fibre’s higher lignin content. It is generally acknowledged that lignin acts as a substrate-specific barrier and limits cellulase access via unproductive binding and or steric hindrance [29]. During this study, increasing the enzyme load appears to have reduced lignin-associated inhibition, leading to a higher rate of glucan conversion. This was particularly evident with the washed 10% fibre digestions (Fig. 1a), which matched the 5.7 wt% fibre’s rates of glucan conversion up to time point 72 h. This result indicates that added cellulase was not instantaneously rendered inactive by higher lignin substrates, but rather that its activity slowed as the lignin to accessible cellulose ratio increased [30].
While these results indicate that washing pretreated solids improves enzyme digestibility of pretreated fibres, the process introduces additional processing costs and improvements in glucose yield need to be weighed against these. Similarly, the highest glucose yields were attained using a 3.75% w/v glucan loading with 40 FPU Ctec 2. However, for unwashed fibres with a 3.75% w/v glucan loading, doubling the enzyme rate from 20 FPU Ctec 2 to 40 FPU Ctec 2 led to only a 10% gain in glucose yield. This indicates that a 3.75% w/v glucan loading with 20 FPU Ctec 2 is an efficient combination if using unwashed solids. This combination was thus used for the fermentation experiments.
Ethanol Fermentation of Second-Stage Pretreated CGT
Two approaches for producing ethanol by fermentation of second-stage pretreated CGT were investigated. These were separate hydrolysis and fermentation (SHF) and simultaneous saccharification and fermentation (SSF). For both SHFs and SSFs, the performance of the recombinant xylose-fermenting yeast GSF335 was compared with that of Fali. Unwashed second-stage pretreated solids were used as the primary substrate in SSFs and enzyme hydrolysates for SHFs from the 3.75% glucan (10% fibre) loading with 20 FPU Ctec 2 treatment—as described in the ‘Characteristics of Feedstock, Pretreatment Liquors and Solids’ section. The glucan and xylan conversion efficiencies for SHF hydrolysate production were 71.7 and 100.0%, respectively.
Prior to setting up fermentations, GSF335 inoculum was propagated in C5 sugar-enriched liquors pressed from first-stage pretreated solids. As presented in Fig. 2, the yeast GSF335 preferentially grows on glucose and once depleted (4 h) shifts to xylose. Between 12 and 24 h, GSF335 biomass continues to increase as xylose and acetic acid levels decline. Extending growth beyond 24 h resulted in marginal biomass gains. GSF335 did not consume any arabinose. Interestingly, GSF335 failed to consume all of the xylose implying either a bottleneck in the xylose pathway or the presence of other poorly metabolisable sugars co-migrating on the HPLC column (e.g. galactose). Nevertheless, the prehydrolysate liquor yielded up to 7.75 g yeast L−1 corresponding to 41.8 g kg−1 of dry CGT. Propagation of recombinant yeast in unmodified xylose laden prehydrolysate liquors has yet to be reported.
Separate Hydrolysis and Fermentation
During SHFs, GSF335 and Fali yeast strains demonstrated similar fermentation kinetics (Table 2; Fig. 3). GSF335 consumed the glucose fraction within the first 10 h and consumed the xylose fraction within 24 h, to yield a maximum of 15.2 g L−1 ethanol after 10 h with an MEVP (maximum ethanol volumetric productivity) of 2.4 g L−1 h−1. This compares with Fali yeast which consumed all glucose within the first 8 h to yield a maximum of 14.7 g L−1 ethanol after 10 h with an equivalent MEVP. The rapid conversion of sugars to ethanol by both yeasts indicates that any inhibitors present in the hydrolysates had little impact on ethanol production. Ethanol yields for GSF335 and Fali were 96 and 99%, respectively, of the theoretical yields based on sugars present in the hydrolysate. The MEVP and theoretical yields reported in this study are comparatively higher than those reported for CGT enzyme hydrolysate fermentations derived from acid-pretreated and/or alkali-delignified fibre [5, 8, 23]. However, if fibre hydrolysis efficiency during sugar hydrolysate preparation is taken into account, the actual ethanol yields per pretreated wet fibre (w/w) are c.a. 60 and 62% for Fali and GSF335, respectively. The cited studies above did not disclose the hydrolysis efficiency of their fermentation liquor, consequently making it difficult to draw firm conclusions.
The kinetic parameters presented in Table 2 reveal that both strains behaved similarly during the fermentation. Significant differences were noted in each strain’s maximum specific growth rate (μmax), and yield of cell mass and glycerol per substrate consumed. GSF335 had a μmax of 0.12 h−1, which was significantly (p < 0.05) higher than Fali’s (μmax of 0.08 h−1). During rapid growth, GSF335 increases production of glycerol to redress NAD+/NADH redox balances caused during synthesis of biomass and ethanol [31]. Thus, the faster growth rate and cell mass of GSF335 likely explains why glycerol production was higher in GSF335 fermentations than in Fali SHFs. The consequence of higher glycerol production during fermentation is that the substrate is diverted away from ethanol production, which likely explains why ethanol titres for GSF335 were slightly less than anticipated. During fermentations with Fali, xylose concentrations declined during the initial 8 h. This was unexpected as Fali does not have the metabolic capacity to ferment xylose to ethanol and there was no indication that xylitol was produced during fermentations either. Thus, this result is perplexing but may be explained by other fermentable sugars (e.g. fructose) co-migrating on the HPLC column to give a false reading for xylose at the start of the fermentation. The presence of residual xylose (1 g L−1) at the end of the Fali fermentation (Fig. 3) and its relatively high qsmax,xylose value indicates that this may have been the case (Table 2).
If considered exclusively on an ethanol yield efficiency basis, minor gains were achieved with GSF335 which produced an equivalent of 66.2 kg tonne−1 of raw CGT compared with 64.0 kg tonne−1 for Fali. Although the performances of both yeast strains during the SHF process are similar, the relatively modest ethanol yields for the entire process are slightly vexing. Partitioning of up to 35% of CGT’s sugar components (equivalent 80.5 and 31.5 kg t−1 CGT of xylose and glucose, respectively) into the prehydrolysate liquors partly accounts for the modest yields. Although this portion equates to an additional 57 kg of ethanol, the large diluted volumes of liquor makes it commercially impractical. Moreover, the ability to propagate GSF335 yeast inoculum in C5-rich liquors and generate up to 41.8 kg of yeast inoculum per tonne of raw CGT potentially offers a better option for industrial scale-up. This is more than sufficient inoculum for succeeding C6-fibre fermentations. Notwithstanding, the modest estimated ethanol yields are directly attributed to the uncharacteristically low carbohydrate and high lignin content of the CGT sample provided by the cotton gin.
Simultaneous Saccharification and Fermentation
During SSFs, GSF335 rapidly metabolised residual glucose and xylose contained within second-stage pretreated solids to yield 7.5 g L−1 ethanol within 12 h (Fig. 4). Thereafter, uptake of glucose and xylose by GSF335 maintained sugar levels below cellulase inhibition thresholds, with ethanol production continuing up to 120 h and a maximum yield of 12.8 g L−1. The rate of ethanol production in fermentations inoculated with Fali was slightly greater than that in fermentation with GSF335 during the initial 24 h, but ethanol production was negligible thereafter, reaching a maximum of 10.1 g L−1. As occurred in SHFs (‘Separate Hydrolysis and Fermentation’ section), GSF335 channelled its carbon flux toward production of glycerol, which is indicative of active growth. GSF335 produced up to 2.7 times more glycerol than Fali and similar quantities of acetic acid to Fali but its activity was less affected by these. Thus, GSF335’s robust proliferation under SSF conditions confirms that it is better suited to lignocellulosic ethanol production than Fali yeast. It is also likely that GSF335’s performance was enhanced by preconditioning during cultivation in C5 laden first-stage pretreatment liquors.
Ethanol yield for Fali SSFs was significantly lower than GSF355 but similar to its counterpart SHFs (‘Separate Hydrolysis and Fermentation’ section), owing in part to GSF335’s capacity to ferment xylose (albeit xylose content in the fibre was minor) and its prior exposure and growth in prehydrolysate liquors. Based on mass data, ethanol yields were calculated at 50.3 and 63.75 kg dry tonne−1 of raw CGT of for Fali and GSF335 respectively. The final ethanol yield efficiency of 50.6% and 60.1% for Fali and GSF335, respectively, was below anticipated levels. The low conversion rate and yield during SSFs is mainly attributed to the change in the substrate’s composition and in part to the temperature compromise at 35 °C—being suboptimal for cellulase activity and yeast fermentation. As noted above, cellulose hydrolysis and consequently ethanol production were presumably hampered by increasing lignin to accessible cellulose ratio as hydrolysis progressed. Fermentation temperatures at 35 °C sustained over prolonged periods may have also impacted yeast cell viability [32,33,34]. At temperatures beyond their growth optimum (28 °C), S. cerevisiae is more susceptible to stresses such as ethanol accrual and can result in stuck fermentations [34]. Ethanol accumulation has also been noted to diminish cellulase activity over time [35, 36]. Post-treatment strategies leading to CGT fibre with reduced lignin content and fed-batch SSF processing—whereby yeast, enzyme and possibly substrate are incrementally added—are currently under investigation to overcome these challenges.
Nonetheless, ethanol yields from these SSFs were notably higher than or comparable with reported studies for other highly lignified DA-pretreated biomass. For example, Manfredi et al. [37] achieved 42.6 kg ethanol per tonne of corn stover, which was 45% of the theoretical yield. Cuevas et al. [38] conducted SSFs with a thermotolerant S. cerevisiae strain using 80 FPU cellulase incubated at 40 °C to achieve 53% of the theoretical ethanol yield or 81 kg t−1 of olive stones. Although ethanol yields from SSFs during the present study were relatively lower than previous observation of 110.7 kg t−1 CGT for SSF of single-stage pretreated CGT [5], the latter study used CGT with higher carbohydrate content, four times the quantity of cellulase compared to the present and commercially supplied yeast inoculum. This adds significant cost to processing and thus negates gains in ethanol yield.
Conclusion
This study confirms that the sequential two-stage pretreatment process proposed by Vancov et al. [12] is repeatable at the pressurised stirred reactor bench scale-up and demonstrates good prospects for valorisation of C5 and C6 sugars in CGT and other biomass resources. The two-stage pretreatment delivered xylose laden liquors fitting for yeast propagation (42 kg t−1 dry CGT) intended and underpins subsequent fermentations and highly digestible glucan-enriched CGT fibres.
The recombinant GSF335 performed marginally better under SHF conditions delivering 66.2 kg ethanol tonne−1 of raw CGT. The source of feedstock used and its progressive recalcitrance—especially under SSF conditions—account for the modest conversion efficiencies and yields. Future research should address problems affecting enzymatic hydrolysis and/or yeast performance during SSF prior to process validation and demonstration at pilot.
References
Hassall and Associates (2005) Value of research investment relating to the waste classification of cotton gin trash. Cotton Research and Development Corporation
Anon (2018) 2018 Cotton Annual - Statics booklet. Avaiable from http://cottonaustralia.com.au/cotton-library/publications/brochures. http://cottonaustralia.com.au/cotton-library/statistics. Accessed 12 Jun 2018
Hamawand I, Sandell G, Pittaway P, Chakrabarty S, Yusaf T, Chen GN, Seneweera S, Al-Lwayzy S, Bennett J, Hopf J (2016) Bioenergy from cotton industry wastes: a review and potential. Renew Sust Energ Rev 66:435–448
Egbuta MA, McIntosh S, Waters DLE, Vancov T, Liu L (2017) Biological importance of cotton by-products relative to chemical constituents of the cotton plant. Molecules 22(1):25
McIntosh S, Vancov T, Palmer J, Morris S (2014) Ethanol production from cotton gin trash using optimised dilute acid pretreatment and whole slurry fermentation processes. Bioresour Technol 173:42–51
Agblevor FA, Batz S, Trumbo J (2003) Composition and ethanol production potential of cotton gin residues. Appl Biochem Biotechnol 105(1–3):219–230
Jeoh T, Agblevor FA (2001) Characterization and fermentation of steam exploded cotton gin waste. Biomass Bioenergy 21(2):109–120
Sahu S, Pramanik K (2018) Evaluation and optimization of organic acid pretreatment of cotton gin waste for enzymatic hydrolysis and bioethanol production. Appl Biochem Biotechnol 186:1047–1060. https://doi.org/10.1007/s12010-018-2790-7
Shen J, Agblevor FA (2008) Kinetics of enzymatic hydrolysis of steam-exploded cotton gin waste. Chem Eng Commun 195(9):1107–1121
Silverstein RA, Chen Y, Sharma-Shivappa RR, Boyette MD, Osborne J (2007) A comparison of chemical pretreatment methods for improving saccharification of cotton stalks. Bioresour Technol 98(16):3000–3011
Inoue H, Fujimoto S, Sakaki T (2016) Two-step hot-compressed water treatment of Douglas fir for efficient total sugar recovery by enzymatic hydrolysis. BioResources 11(2):5124–5137
Vancov T, Palmer J, Keen B (2018) A two stage pretreatment process to maximise recovery of sugars from cotton gin trash. Bioresour Technol Rep 4:114–122
Kwak S, Jin YS (2017) Production of fuels and chemicals from xylose by engineered Saccharomyces cerevisiae: a review and perspective. Microb Cell Factories 16:15
Jansen MLA, Bracher JM, Papapetridis I, Verhoeven MD, de Bruijn H, de Waal PP, van Maris AJA, Klaassen P, Pronk JT (2017) Saccharomyces cerevisiae strains for second-generation ethanol production: from academic exploration to industrial implementation. FEMS Yeast Res 17(5):20
Hou J, Qiu CX, Shen Y, Li HX, Bao XM (2017) Engineering of Saccharomyces cerevisiae for the efficient co-utilization of glucose and xylose. FEMS Yeast Res 17(4):11
Demeke MM, Dumortier F, Li Y, Broeckx T, Foulquie-Moreno MR, Thevelein JM (2013) Combining inhibitor tolerance and D-xylose fermentation in industrial Saccharomyces cerevisiae for efficient lignocellulose-based bioethanol production. Biotechnol Biofuels 6(1):120
Sluiter A, Hames B, Ruiz R, Scarlata C, Sluiter J, Templeton D, Crocker D (2008) Determination of structural carbohydrates and lignin in biomass. National Renewable Energy Laboratory
Adney B, Baker J (2008) Measurement of cellulase activity: NREL technical report NREL/TP-510-42628
Jeon Y, Xun Z, Rogers P (2011) Comparative evaluations of cellulosic raw materials for second generation bioethanol production. Lett Appl Microbiol 51:518–524
Gupta A, Verma JP (2015) Sustainable bio-ethanol production from agro-residues: a review. Renew Sust Energ Rev 41:550–567
Vancov T, McIntosh S (2011) Alkali pretreatment of cereal crop residues for second-generation biofuels. Energy Fuel 25(7):2754–2763
McIntosh S, Vancov T, Palmer J, Spain M (2012) Ethanol production from eucalyptus plantation thinnings. Bioresour Technol 110:264–272
Fockink DH, Maceno MAC, Ramos LP (2015) Production of cellulosic ethanol from cotton processing residues after pretreatment with dilute sodium hydroxide and enzymatic hydrolysis. Bioresour Technol 187:91–96
Vancov T, McIntosh S (2011) Effects of dilute acid pretreatment on enzyme saccharification of wheat stubble. J Chem Technol Biotechnol 86(6):818–825
Shi J, Chinn MS, Sharma-Shivappa RR (2008) Microbial pretreatment of cotton stalks by solid state cultivation of Phanerochaete chrysosporium. Bioresour Technol 99(14):6556–6564
Plácido J, Imam T, Capareda S (2013) Evaluation of ligninolytic enzymes, ultrasonication and liquid hot water as pretreatments for bioethanol production from cotton gin trash. Bioresour Technol 139:203–208
Ko JK, Um Y, Park YC, Seo JH, Kim KH (2015) Compounds inhibiting the bioconversion of hydrothermally pretreated lignocellulose. Appl Microbiol Biotechnol 99(10):4201–4212
Duarte G, Moreira L, Jaramillo P, Filho E (2012) Biomass-derived inhibitors of holocellulases. BioEnergy Res 5(3):768–777
Chang VS, Holtzapple MT (2000) Fundamental factors affecting biomass enzymatic reactivity. Appl Biochem Biotechnol 84-86:5–37
Jian S, Dong W, Libing Z, SB A, Seema S, Bin Y, WC E (2017) Dynamic changes of substrate reactivity and enzyme adsorption on partially hydrolyzed cellulose. Biotechnol Bioeng 114(3):503–515
Bakker BM, Overkamp KM, van Maris AJA, Kötter P, Luttik MAH, van Dijken JP, Pronk JT (2001) Stoichiometry and compartmentation of NADH metabolism in Saccharomyces cerevisiae. FEMS Microbiol Rev 25(1):15–37
D'Amore T, Panchal CJ, Russell I, Stewart GG (1990) A study of ethanol tolerance in yeast. Crit Rev Biotechnol 9(4):287–304
Casey GP, Ingledew WM (1986) Ethanol tolerance in yeasts. Crit Rev Microbiol 13(3):219–280
Ingledew WM (2009) Yeasts: physiology, nutrition and ethanol production. In: Ingledew WM, Kelsall DR, Austin GD, Kluhspies C (eds) The alcohol textbook : a reference for the beverage, fuel and industrial alcohol industries, 5th edn. Nottingham University Press, Nottingham, pp 101–113
Bezerra RM, Dias AA (2005) Enzymatic kinetic of cellulose hydrolysis: inhibition by ethanol and cellobiose. Appl Biochem Biotechnol 126(1):49–59
Wu Z, Lee YY (1997) Inhibition of the enzymatic hydrolysis of cellulose by ethanol. Biotechnol Lett 19(10):977–979
Manfredi AP, Ballesteros I, Sáez F, Perotti NI, Martínez MA, Negro MJ (2018) Integral process assessment of sugarcane agricultural crop residues conversion to ethanol. Bioresour Technol 260:241–247
Cuevas M, Sánchez S, García JF, Baeza J, Parra C, Freer J (2015) Enhanced ethanol production by simultaneous saccharification and fermentation of pretreated olive stones. Renew Energy 74:839–847
Acknowledgements
The work was undertaken as part of the Biorefineries for Profit project which was funded by the Sugar Research Australia (SRA) and the Australian Government Department of Agriculture and Water Resources through the Rural R&D for Profit Program and Queensland Government Department of Agriculture and Fisheries, Cotton Research and Development Corporation, Forest and Wood Products Australia, Australian Pork Limited, Southern Oil Refining, Queensland University of Technology and NSW Department of Primary Industries, Australia. We express our gratitude to Yarraman Gin (Namoi Cotton Co-operative Ltd., NSW Australia) for supplying cotton-ginning residues.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Vancov, T., Palmer, J. & Keen, B. Two-Stage Pretreatment Process Validation for Production of Ethanol from Cotton Gin Trash. Bioenerg. Res. 12, 593–604 (2019). https://doi.org/10.1007/s12155-019-09989-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12155-019-09989-2