Abstract
Purpose
Agro-industrial waste, being biodegradable and environmentally-benign, is a sustainable resource for edible film production. Edible films were fabricated from by-products, prickly pear peel mucilage (PPM) and potato husk starch (PHS), and characterised for their physical–chemical properties.
Methods
Various films were prepared by varying the PPM, PHS and glycerine (plasticiser) while maintaining a constant amount of vinegar (acidifying agent).
Results
Results showed that the formulation composition influenced the properties of the films. High concentrations of PPM and glycerine led to films with higher thickness, opacity, moisture and water retention capacity (WRC), and the percentage of water solubility (% WS) was influenced by the PHS content. All edible films presented very low water permeability (WP), and thereby good barrier properties. The WS, WRC and WP were closely associated with the PPM and glycerine contents. Consequently, the FTIR and SEM analyses showed similarities between the spectra and images.
Conclusion
The preparation of edible films from agro-industrial wastes, along with their specific application in food packaging, especially for fresh fruits and vegetables, contributes to sustainable alternatives due to the recovery and reuse of the processing residues.
Graphic Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Statement of Novelty
This paper demonstrates the feasibility of obtaining edible films from polymers extracted from agro-industrial by-products, such as prickly pear peel mucilage and potato husk starch.
Introduction
In the forthcoming era of bio-economy, there has been a growing interest in the use of biodegradable, edible films, mainly for environmental reasons, as an alternative to conventional petroleum derivative plastics [1, 2]. Edible films act as a barrier against the transfer of moisture, oxygen, CO2, and the loss of lipids and flavour components to maintain quality and extended the shelf-life of food products [3,4,5]. Numerous studies have shown the effectiveness of edible films in the preservation of diverse products [6, 7]. Besides acting as a barrier, edible films can also be used as carriers of flavourings and functional ingredients, such as nutraceuticals, antimicrobials, antioxidants, and vitamins and minerals [2, 7,8,9].
Edible films are fabricated from natural biopolymers, mainly polysaccharides, carbohydrates, proteins, lipids or combinations and blends of these components [1, 3, 4, 10]. Among the most commonly used polysaccharides are cellulose derivatives, chitosan, starch, alginates, carrageenan and pectin due to their good film-forming properties.
For applications, it is necessary to characterise the films for their physical–chemical attributes, such as water solubility (WS), water absorption/desorption, thickness, microstructure, crystallinity, biopolymers compatibility, thermal behaviour, barrier properties [gas and water vapour permeability (WVP)], mechanical properties and optical properties [10].
As the attention towards renewable materials grows [11], there is great emphasis on fabricating edible films from unconventional sources of hydrocolloids [12,13,14,15,16,17,18,19]. Even biopolymers obtained from agro-industrial waste, like bagasse, fibre, cake, peels, husk, pits and seeds, are considered. This approach seeks to harness the full potential of residues and materials produced as by-products of the agro-industries and reduce environmental pollution [20]. Edible films have been prepared from banana peel [21], fruit and vegetable residues [11, 22], cranberry pomace extracts [23] prickly pear mucilage [24, 25], whey protein isolate [26], commercial fish skin gelatine [27], soybean cake, cassava bagasse, turmeric dye extracted from waste flour, among others [20].
In 2017, worldwide potato production was 388 million metric tons [28]. Potato husk is a zero-value waste from potato processing plants [29] that has been traditionally used in animal feed and as an organic soil fertiliser [30]. The husk represents between 15 to 40% of the tuber [29], and the maximum starch content [29, 31] is 52.14% on a dry weight basis [29]. Likewise, prickly pear production in 2018 was 500 thousand metric tons [32]. Opuntia fruit peel represents up to 69% of the fruit, and is a valuable source of natural biopolymers, particularly polysaccharides, like mucilage [33], obtaining a 7.3% yield based on the dry matter of prickly pear peels [34].
Thus, the valorisation of these agro-industrial by-products in the development of edible films motivated the proposed methodology of fabricating edible films from the biopolymers present in prickly pear peel mucilage (PPM) and potato husk starch (PHS). The films were characterised for their physical–chemical properties, including thickness, opacity, moisture, water retention capacity (WRC), WS, WVP, matrix interaction by Fourier-transform infrared red (FTIR) spectroscopy and morphology by scanning electron microscopy (SEM).
Methodology
Raw Material
Purple prickly pear (Opuntia ficus-indica L. Mill) and white potato (Solanum tuberosum) were purchased from a local market of Abancay, Apurímac, Perú, transported to the laboratory and stored under refrigeration at 4 °C until application.
Prickly Pear Peel Mucilage (PPM) Extraction
Mucilage from prickly pear (O. ficus-indica L. Mill) peel was extracted by adapting the method described by Allegra et al. [6] and Koubaa et al. [33], such that the peels of prickly pear were cut into 2 cm × 2 cm pieces. To extract the mucilage, peels were crushed in an Osterizer blender (Sunbeam. Miami, FL, USA) and homogenised with distilled water (1:1, w/v) at room temperature. The homogenate was filtered through medium-mesh and fine-mesh strainers to collect the filtrate. The fibre retained on the mesh was discarded, and the filtrate containing the mucilage was centrifuged in a C2 series (Centurion Scientific Ltda, West Sussex, UK) at 3000 rpm for 21 min. The supernatant was boiled in a water bath (WNB10, Memmert GmbH & Co. KG, Schwabach, Germany) at 75 °C for 30 min until the liquid mucilage reached 6°Brix. It was then cooled and maintained under refrigeration (4 °C) until use.
Potato Husk Starch (PHS) Extraction
Potato (S. tuberosum) husk starch was extracted according to Valcárcel-Yamani et al. [35]. Initially, potato husks of 2-, 3- and 5-mm thickness were cleaned, selected, disinfected with 2% sodium hypochlorite solution, manually cut into small pieces of 2 cm × 2 cm and ground in a blender with distilled water (1:2, w/v) for 3 min. The homogenate was filtered through a thin membrane of cotton and washed with distilled water (four times) to collect the filtrate. The residue retained on the membrane was discarded, and the filtrate containing the starch was resuspended in distilled water (1:4, w/v). Afterwards, the starch was separated from the supernatant and resuspended again in water until the starch settled. This washing procedure was repeated approximately four times to obtain a white starch and a translucent supernatant. The starch was collected and dried in a UN30 oven (Memmert GmbH & Co. KG) at 40 °C for 8 h. The dry starch was stored in bags at room temperature until use.
Edible Films Preparation
The edible films were prepared by the casting or plate cast method reported by Debeaufort et al. [1] and García et al. [10]. Formulations were prepared with varying PPM, PHS and glycerine (GLY; Table 1) contents, keeping the amount of vinegar (acidifying agent) constant. Initially, the PHS and PPM were mixed with GLY, immediately homogenised for 5 min, then commercial vinegar with 2.5% acidity was added to reduce the pH. Next, the solution was placed in a water bath at 82 °C and stirred for 1 min. Finally, was cast in a plate and oven-dried at 45 °C for 24 h. The dried films were carefully peeled from the plate, and placed in hermetical polyethylene bags before storing at room temperature until analysis.
Physical–Chemical Characterisation of Biofilms
Thickness
The edible films thickness was determined, according to Espino-Díaz et al. [36], using a digital stainless-steel micrometric device with 0.01-mm sensitivity (Truper en Edo., Jilotepec, Mexico). The thickness measurements were recorded in ten different sections of the film, and the average of the measurements was reported.
Density
The edible films apparent density was determined from the ratio between the weight and volume. The film samples were cut into 2 cm × 2 cm pieces and weighed on an analytical scale (Entris 224-IS, Sartorius Lab Instruments GmbH & Co. KG, Göttingen, Germany). The volumes of the samples were calculated from the area and thickness. The experiments were performed in triplicate, and mean values were reported according to Pelissari et al. [37].
Opacity
The edible films opacity or transparency was calculated using Eq. (1), as reported by Gómez-Estaca et al. [38]. The edible films were cut into rectangles and placed directly in the UV–Vis G10S spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA) test cell, using an empty test cell as the reference.
Moisture Content
The edible films moisture content (%) was determined according to the Association of Official Analytical Chemists method 934.01 [39]. The moisture content of the films is defined as the percentage of water removed from the initial mass sample. It was analysed gravimetrically by drying the samples at 105 °C for 24 h in a UN30 oven (Memmert GmbH & Co. KG) and then recording the weight using an analytical scale (Entris 224-IS, Sartorius Lab Instruments GmbH & Co.) in triplicate.
Water Solubility (WS)
The WS was evaluated as described by Romero-Bastida et al. [12] with modifications. For this, previously dried square films (2 cm × 2 cm) were weighed (initial dry weight) on an analytical scale (Entris 224-IS, Sartorius Lab Instruments GmbH & Co.) and then placed into test beakers with 80 mL of distilled water. The samples were maintained under constant agitation in H3 (Ingenieurbüro CAT, M. Zipperer GmbH, Dottingen, Germany) for 10 min at room temperature (approximately 25 °C). The remaining pieces of film were soaked, then dried again in an oven (UN30, Memmert GmbH & Co. KG) at 60 °C until constant weight. The WS percentage was calculated by weight difference, according to Eq. (2). All samples were analysed in triplicate.
Water Retention Capacity (WRC)
The WRC or swelling was evaluated as detailed by Nouraddini et al. [16] and Basiak et al. [40] with modifications. The film samples were cut into 2 cm × 2 cm pieces, weighed (initial weight) on a scale (Entris 224-IS, Sartorius Lab Instruments GmbH & Co.) and then immersed in a flask with distilled water for 10 min. After recovering the samples from the flask, excess water was removed by wiping them with filter paper, and each sample was weighed (final weight). The WRC was calculated by Eq. (3). All samples were analysed in triplicate.
Water Vapour Permeability (WVP)
The WVP was measured in accordance with the standard ASTM E96-95 [41] and Gennadios et al. [42] with some modifications. The previously-weighed sample was sealed in the open mouth of a test-tube containing 6 mL of distilled water and then the assembly placed under controlled conditions (24 °C for 24 h) in a desiccator. The test-tube was weighed every 8 h to obtain the slope of the variability of weight per unit of time (J), and thereby determine the rate of water vapour circulation from the water through the sample under the controlled conditions. To determine the WVP, Eqs. (4), (5) and (6) were used:
where WVTR is the water vapour transmission rate (g s−1 m−2); J is the slope of the weight loss in the linear region of the plot (g s−1); A is the effective area for water vapour transmission (m2); Pw1 is the water vapour partial pressure on the film surface (face oriented towards the inside of the tube; Pa); Pw2 is the water vapour partial pressure on the film surface (face oriented outward of the cell; Pa); L is the film thickness (m); im is the initial mass (g); fm is the final mass (g), and t is the time (s).
The ASTM E96-95 method establishes that resistance to water transport, through the air space between the surface of the water and the film, is negligible (Pw0 = Pw1). However, for hydrophilic films, this is not true and can induce significant errors in the calculated permeability. In order to consider the effect of the air-tight layer on the WVP measurements, the Pw1 value was calculated by the following equations:
where Tp is the total system pressure (Pa); Pw0 is the water vapour saturated pressure at the working temperature (Pa); Nw is the water flow in the film (g mol cm−2 s−1); hi is the distance between the distilled water and film (m); C is the total molar concentration of air and water vapour (g mol cm−3); D is the water vapour diffusivity in the air (cm2 s−1); T is the working temperature (K), and R is the universal gas constant (Pa cm3 mol−1 K−1).
Fourier-Transform Infrared (FTIR) Spectroscopy
The functional groups present in the components and edible films were characterised by FTIR in attenuated total reflection (ATR) mode using a Nicolet IS10 spectrometer (Thermo Scientific, Madison, WI, USA) to supplement the microstructural characterisation of composite films, by providing insight into the interactions between different films components [10]. The spectra were recorded at a wavenumber range from 4000 to 400 cm−1, at room temperature, using 16 accumulated scans and 4-cm−1 resolution.
Scanning Electron Microscopy (SEM)
The film morphology was evaluated by SEM using a Jeol JSM-7600F (Jeol Ltd., Tokyo, Japan) field-emission scanning electron microscope. The dehydrated samples were first placed in a sample holder and then coated with palladium–gold in a Q150R ES rotary pump ion-jet coater (Quorum Technologies Ltd., USA) and then subjected to an electron beam reflecting surface topography at 5 kV. The images were captured at different magnifications to find the best resolution.
Statistical Analysis
Data were processed by analysis of variance, and the means were compared by Duncan’s test at p < 0.05, using InfoStat student version 2011, as per Di Rienzo et al. [43].
Results and Discussion
Polymers Extract and Film Formation
Extraction of the biopolymers from the by-products yielded 5 g of starch/100 g of potato husk, which was less than that reported by Arapoglou et al. [29], who indicated that the yield is influenced by several factors, such as the extraction conditions. The yield of 64 mL of purple liquid mucilage at 6°Brix/100 g of prickly pear peel was obtained, which, when dehydrated, yielded 0.18 g/100 g of prickly pear peel, and its less than that obtained from the aqueous extract [34].
The edible films were transparent, flexible in appearance, sparkly, with a slight purple hue due to the PPM influence (Fig. 2a).
Thickness
The films thickness varied from 0.09 to 0.22 mm (Table 2) and differed between each treatment (p < 0.05). Similar results were obtained in banana peel-based films, with a thickness of 0.11–0.17 mm [21], but slightly lower than native starch tapioca films of 0.20–0.44 mm [18]. Generally, the thickness of films is less than 0.33 mm [4].
The films thickness is related to the content of solids in the film-forming solution, which is mainly represented by starch and mucilage [17], and associated with the ratio of the polymers used to form the mixture, since in this study, the PPM and GLY contents were more influential than PHS because the formulations F6 and F1 had a greater thickness than F3 and F4, and this it could arise from differences in the space between biopolymer chains, favouring the interaction of hydrophilic groups between different polymers [44].
Density
The apparent density of the films varied from 1.00 to 2.06 g cm−3 (p < 0.05; Table 2). These data are similar to those made from salep glucomannan, with densities ranging from 1.12 to 1.27 g cm−3 [14], and eggplant flour films with corn starch that presented densities of 1.221–1.444 g cm−3 [16]. These results were also influenced by the formulation composition and the thickness of the films. Accordingly, treatments that produced films of greater density were also thinner films (e.g., F3), while films with lower density presented greater thickness, such as F6, and this film had more PPM and GLY than the other formulations.
Opacity
The opacity or transparency of the films varied from 0.089 to 0.541 (p < 0.05; Table 2). These results are close to obtained in others studied, like tuna gelatine films combined with antioxidants extract, showing opacities between 0.479 to 0.804 [27], and commercial fish gelatine films, which displayed opacities below 1.0 [38], This property is affected by the formulation composition, and mixtures with more than one component, especially lipids, reduce the films transparency [26]. Here, the results were influenced by the presence of PPM and GLY, and also, the thickness. As a result, films that presented higher opacity were thinner, as exemplified by formulation F4, in agreement with previous work [27] that higher values of opacity indicate a lower degree of transparency. Consequently, F4 film was the most transparent, and F1 was the opaquest. The transparency of food packaging is desirable because the consumer wants to observe the products through the packaging [45], but the opacity could be an excellent barrier to prevent UV light-induced lipid oxidation when applied in food systems [27], as in this research.
Moisture Content
The moisture content of the films was in a range of 10.079–12.462% (p < 0.05; Table 2). These results are consistent with other studies, such as non-conventional starch films obtained by thermal and cold gelatinisation, obtaining moisture contents of 5.86 and 21.34%, respectively [12], and tapioca starch–GLY films that presented moisture contents of 15.7–19.8% [9]. The composition again influenced the results because moisture contents were higher for films F1, F2 and F4 than the other films. Such differences might be attributed to their different chemical structures and hygroscopic properties. Hydrophilic plasticisers [14] can reduce water loss from the film by increasing the amount of bound water [46]. GLY is a highly hygroscopic plasticiser bearing many hydroxyl groups, which easily retain water in the film matrix during the drying process and storage at 50% relative humidity [25].
Water Solubility (WS)
The films WS percentages varied in a range of 39.676–54.430% (p < 0.05; Table 2), similarly to babassu starch films of 22.5–41.1% [19], and slightly higher than banana peel films, with values from 7.21 to 27.57% [21]. The formulation, specifically the PPM, PHS and GLY ratios, influenced the results, as exemplified by the relatively higher solubility of F2, F4 and F6 treatments. The number of free hydroxyl groups present in the biopolymer matrix can directly influence the water solubility of the film, by facilitating the establishment of hydrogen bonds between the biopolymer and water molecules [17], as demonstrated in this work. The film solubility is an important attribute in the film characterisation. It enables understanding the behaviour that the film presents when it comes in contact with water. This parameter directly depends on the application and purpose of the material. Films should be of low solubility when used in a product with high moisture [17], such as fresh fruits and vegetables.
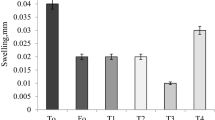
Water Retention Capacity (WRC)
The WRC or swelling index of films varied from 19.704 to 77.860% (p < 0.05; Table 2), consistent with chitosan films [47] and chitosan–starch–gallic acid films [48], which exhibited WRC values of 23.494–320.361% and 79–158%, respectively. The influence of the composition, specifically the PPM and GLY ratios was evident, as the treatments with comparatively higher WRC presented more mucilage and GLY (F2 and F6). The swelling index refers to the film’s ability to retain water in the matrix, which, in turn, is related to the presence of hydrophilic groups, such as carboxylic and hydroxyl groups, in its structure (as in the current formulations) because of the preferential interactions between these groups and water molecules [49]. This property is an important parameter to analyse, as it indicates the supposed behaviour of the material in different environments [17]. It also predicts the preservation quality of the packaging during the food products packaging and storage [50]. Sometimes, a high WRC may be desirable to absorb the excess water on the surface of food with a high moisture content [7], like minimally processed fruits and vegetables, and cheese.
Water Vapor Permeability (WVP)
The WVP varied from 1.2 × 10–15 to 5.7 × 10–16 g s−1 m−1 Pa−1 (p < 0.05; Table 2). In general, these results are lower than those reported in other studies, such as tapioca starch films, which showed a permeability of 5.8–11.0 (× 10–10) g s−1 m−1 Pa−1 [9], and babassu starch films, with permeabilities of 1.3–5.5 (× 10–10) g m−1 s−1 Pa−1 [19]. Among the films, those with greater permeability were F2 and F6, corresponding to treatments with more PPM and GLY and less PHS. The barrier properties of edible films are greatly affected by the film composition and structure, as well as the environmental conditions [14, 46]. The results possibly indicate a high interaction of the polymer chains in the starch with the polysaccharides present in the PPM and GLY, which makes the film more compact and more resistant [17]. It could also be related to structural modifications to the starch network produced by the plasticiser, and to the hydrophilic character of GLY, which favours the absorption and desorption of water molecules to promote permeability [10]. A low permeability may indicate that a film is resistant to the interactions with water molecules in the form of vapour and has structural uniformity, which hinders the vapour´s passage [17]. Films with low permeability can be used for food applications that resist small changes in water vapour in the environment during storage [45], like fresh fruits and vegetables. The lower the WVP, the greater the efficiency of the film as a barrier material [45]. The films WVP should be as low as possible to reduce the moisture transfer between food and the surrounding atmosphere [14].
Fourier-Transform Infrared (FTIR) Spectroscopy
Figure 1a displays the FTIR spectra of the film components, namely, PPM, PHS and GLY, presenting characteristic patterns of biopolymers [51,52,53]. Figure 1b provides the FTIR spectra of the six different edible films, highlighting the similarity between them, evidenced by the same number of peaks and peak positions, indicating that in all cases, there were the same interactions between the components underlying the films formation. All the spectra displayed a wide band between 3000 and 3500 cm−1, corresponding to the stretching vibration of O–H [24, 25] associated with inter- and intramolecular bonds of hydroxyl groups of nearby molecules that constitute the main conformation of starch [9, 54], and the binding of hydrogen bonds between the polysaccharide chains, GLY and water molecules [24]. The bands between 2920 and 2830 cm−1 correspond to the stretching vibration of C–H [55, 56] and C–H2 vibration [25] due to GLY addition [53].
The films band between 1700 and 1600 cm−1 also suggest the presence of water absorbed by PHS, PPM and GLY (plasticiser) molecules because of the observed modifications of this band relative to the component’s spectra (Fig. 1a). Such changes are due to the interactions of biopolymers with absorbed water molecules [14, 17, 53,54,55], leading to a decrease in the intensity of the mucilage peaks (1594 cm−1) and a shift to the left (1615 cm−1), likewise, the signal at 1407 cm−1, besides the shift to the right when compared with the starch spectrum (1641 cm−1; Fig. 1b). Coupling and disappearance of peaks indicate the involvement of the corresponding functional groups in some reactions or interactions [57]. There is a fingerprint region spanning 1200 to 800 cm−1, unique for each component [58]. Two peaks within that region, close to 1151 and 1024 cm−1, are related to stretching of C–O present in glycosidic bonds between monosaccharide units [17, 59]. Van Soest et al. [60] indicate the bands between 1100 to 900 cm−1 are considered characteristic of saccharides and are attributed to the stretching of C–C and C–O bonds, with some contribution from C–H bonds of the polymer components that shape the films. Most carbohydrates are neutral, while some gums are negatively-charged due to the large numbers of hydroxyl groups or other hydrophilic moieties in the neutral carbohydrate structure. Hydrogen bonds play the main role in film formation and characteristics [46].
Scanning Electron Microscopy (SEM)
In Fig. 2b, the SEM images (×500 magnification) are presented in which it can be seen that in all treatments the films tended to present a smooth, regular, homogeneous and continuous surface in the polymeric matrix that could be attributed to polymer–plasticiser interactions by hydrogen bonds. One of the functions of the plasticiser is to reduce intermolecular forces between polymer chains, increasing their mobility and flexibility [61], which facilitates the integration of the components, without any microphase, and enhancing the mechanical properties of the film [24, 61]. Conversely, the appearance of tiny bubbles or small pores, mostly in F4 and F5, indicate incomplete dissolution/gelatinisation of starch granules linked to the solubilised–gelatinised fraction of starch [62]. No cracks or breaks are seen, which could indicate that the films would be useful for food protection when used as a coating for fresh fruits and vegetables.
Conclusions
Edible films were obtained from polymers extracted from two agro-industrial by-products, PPM and PHS. The different compositions of the formulations influenced the physical–chemical characteristics of the films, such as thickness, moisture, opacity, solubility and WRC, mainly due to the mucilage and GLY contents rather than the starch. Films were obtained with good barrier properties due to the low WVP observed in all treatments. As a result, these films would be particularly useful as packaging for fresh fruits and vegetables. Molecular interaction of the components through hydrogen bonds was evidenced by FTIR and SEM.
References
Debeaufort, F., Quezada-Gallo, J.A., Voilley, A.: Edible films and coatings: tomorrow's packagings: a review. Crit. Rev. Food Sci. Nutr. 38(4), 299–313 (1998). https://doi.org/10.1080/10408699891274219
Cha, D.S., Chinnan, M.S.: Biopolymer-based antimicrobial packaging: a review. Crit. Rev. Food Sci. Nutr. 44(4), 223–237 (2004). https://doi.org/10.1080/10408690490464276
Kester, J.J., Fennema, O.: An edible film of lipid and cellulose ethers: barriers properties to moisture vapor transmission and structure evaluation. J. Food Sci. 54(6), 1383–1389 (1989). https://doi.org/10.1111/j.1365-2621.1989.tb05118.x
Pavlath, A.E., Orts, W.: Edible films and coatings: why, what, and how?. In: Hurber, K.C., Embuscado, M. (eds.) Edible Films and Coatings for Food Applications, pp. 1–23. Springer, New York (2009). https://doi.org/10.1007/978-0-387-92824-1_1
Olivas, G.I., Barbosa-Cánovas, G.: Edible films and coatings for fruits and vegetables. In: Hurber, K.C., Embuscado, M. (eds.) Edible Films and Coatings for Food Applications, pp. 211–244. Springer, New York (2009). https://doi.org/10.1007/978-0-387-92824-1_7
Allegra, A., Inglese, P., Sortino, G., Settanni, L., Todaro, A., Liguori, G.: The influence of Opuntia ficus-indica mucilage edible coating on the quality of ‘Hayward’ kiwifruit slices. Postharvest Biol. Technol. 120, 45–51 (2016). https://doi.org/10.1016/j.postharvbio.2016.05.011
Moradi, M., Tajik, H., Rohani, S.M.R., Oromiehíe, A.R., Malekinejad, H., Aliakbarlu, J.: Hadian, M: Characterization of antioxidant chitosan film incorporated with Zataria multiflora Boiss essential oil and grape seed extract. LWT-Food Sci. Technol. 46(2), 477–484 (2012). https://doi.org/10.1016/j.lwt.2011.11.020
López-Mata, M.A., Ruiz-Cruz, S., Silva-Beltrán, N.P., De Jesús Ornelas-Paz, J., Zamudio-Flores, P.B., Burruel-Ibarra, S.E.: Physicochemical, antimicrobial and antioxidant properties of chitosan films incorporated with carvacrol. Molecules 18(11), 13735–13753 (2013). https://doi.org/10.3390/molecules181113735
Piñeros-Hernández, D., Medina-Jaramillo, C., López-Córdoba, A., Goyanes, S.: Edible cassava starch films carrying rosemary antioxidant extracts for potential use as active food packaging. Food Hydrocolloids 63, 488–495 (2017). https://doi.org/10.1016/j.foodhyd.2016.09.034
García, M.A., Pinotti, A., Martino, M.N., Zaritzky, N.E.: Characterization of starch and composite edible films and coatings. In: Hurber, K.C., Embuscado, M. (eds.) Edible Films and Coatings for Food Applications, pp. 169–209. Springer, New York (2009). https://doi.org/10.1007/978-0-387-92824-1_6
Andrade, R.M.S., Ferreira, S.L.M., Gonçalves, E.C.B.A.: Development and characterization of edible films based on fruit and vegetable residues. J. Food Sci. 81(2), E412–E418 (2016). https://doi.org/10.1111/1750-3841.13192
Romero-Bastida, C.A., Bello-Pérez, L.A., García, M.A., Martino, M.N., Solorza-Feria, J., Zaritzky, N.E.: Physicochemical and microstructural characterization of films prepared by thermal and cold gelatinization from non-conventional sources of starches. Carbohydr. Polym. 60(2), 235–244 (2005). https://doi.org/10.1016/j.carbpol.2005.01.004
Zahedi, Y., Ghanbarzadeh, B., Sedaghat, N.: Physical properties of edible emulsified films based on pistachio globulin protein and fatty acids. J. Food Eng. 100(1), 102–108 (2010). https://doi.org/10.1016/j.jfoodeng.2010.03.033
Kurt, A., Kahyaoglu, T.: Characterization of a new biodegradable edible film made from salep glucomannan. Carbohydr. Polym. 104, 50–58 (2014). https://doi.org/10.1016/j.carbpol.2014.01.003
Saberi, B., Chockchaisawasdee, S., Golding, J.B., Scarlett, C.J., Stathopoulos, C.E.: Physical and mechanical properties of a new edible film made of pea starch and guar gum as affected by glycols, sugars and polyols. Int. J. Biol. Macromol. 104, 345–359 (2017). https://doi.org/10.1016/j.ijbiomac.2017.06.051
Nouraddini, M., Esmaiili, M., Mohtarami, F.: Development and characterization of edible films based on eggplant flour and corn starch. Int. J. Biol. Macromol. 120, 1639–1645 (2018). https://doi.org/10.1016/j.ijbiomac.2018.09.126
Araújo, A., Galvão, A., Silva Filho, C., Mendes, F., Oliveira, M., Barbosa, F., Sousa Filho, M., Bastos, M.: Okra mucilage and corn starch bio-based film to be applied in food. Polym. Test. 71, 352–361 (2018). https://doi.org/10.1016/j.polymertesting.2018.09.010
Silva, O.A., Pellá, M.G., Pellá, M.G., Caetano, J., Simões, M.R., Bittencourt, P.R.S., Dragunski, D.C.: Synthesis and characterization of a low solubility edible film based on native cassava starch. Int. J. Biol. Macromol. 128, 290–296 (2019). https://doi.org/10.1016/j.ijbiomac.2019.01.132
Maniglia, B.C., Tessaro, L., Ramos, A.P., Tapia-Blácido, D.R.: Which plasticizer is suitable for films based on babassu starch isolated by different methods? Food Hydrocolloids 89, 143–152 (2019). https://doi.org/10.1016/j.foodhyd.2018.10.038
Tapia-Blácido, D.R., Maniglia, B.C., Martelli-Tosi, M., Passos, V.F.: Agroindustrial biomass: potential materials for biopolymeric film production. In: Masuelli, M., (ed.) Biopackaging, 1st ed. pp. 226–245, CRC Press, Boca Raton (2017). https://doi.org/10.1201/9781315152349-6
Anchundia, K., Santacruz, S., Coloma, J.: Physical characterization of edible films based on banana peel (Musa paradisiaca). Rev. Chil. Nutr. 43(4), 394–399 (2016). https://doi.org/10.4067/S0717-75182016000400009 (in Spanish)
Cavalcante Fai, A.E., de Souza Alves, M.R., Vinhoso Bruno, N., de Andrade Gonçalves, B.: Production of edible coating based on fruit and vegetable residues: application on minimally processed carrot (Daucus carota L.). Sci. Agropecu. 6(1), 59–68 (2015). https://doi.org/10.17268/sci.agropecu.2015.01.06
Park, S., Zhao, Y.: Development and characterization of edible films from cranberry pomace extracts. J. Food Sci. 71(2), E95–E101 (2006). https://doi.org/10.1111/j.1365-2621.2006.tb08902.x
Guadarrama-Lezama, A.Y., Castaño, J., Velázquez, G., Carrillo-Navas, H., Alvarez-Ramírez, J.: Effect of nopal mucilage addition on physical, barrier and mechanical properties of citric pectin-based films. J. Food Sci. Technol. 55(9), 3739–3748 (2018). https://doi.org/10.1007/s13197-018-3304-x
Gheribi, R., Puchot, L., Verge, P., Jaoued-Grayaa, N., Mezni, M., Habibi, Y., Khwaldia, K.: Development of plasticized edible films from Opuntia ficus-indica mucilage: a comparative study of various polyol plasticizers. Carbohydr. Polym. 190, 204–211 (2018). https://doi.org/10.1016/j.carbpol.2018.02.085
Pérez-Gago, M.B., Krochta, J.M.: Lipid particle size effect on water vapor permeability and mechanical properties of whey protein/beeswax emulsion films. J. Agric. Food Chem. 49(2), 996–1002 (2001). https://doi.org/10.1021/jf000615f
Gómez-Guillén, M.C., Ihl, M., Bifani, V., Silva, A., Montero, P.: Edible films made from tuna-fish gelatin with antioxidant extracts of two different murta ecotypes leaves (Ugni molinae Turcz). Food Hydrocolloids 21(7), 1133–1143 (2007). https://doi.org/10.1016/j.foodhyd.2006.08.006
FAOSTAT. Food and Agriculture Organization of the United Nations. Statistics Division. https://www.fao.org/faostat/es/#data/QC (2007). Accessed 22 August 2019
Arapoglou, D., Varzakas, Th, Vlyssides, A., Israilides, C.: Ethanol production from potato peel waste (PPW). Waste Manag. 30(10), 1898–1902 (2010). https://doi.org/10.1016/j.wasman.2010.04.017
Wu, D.: Recycle technology for potato peel waste processing: a review. Proced. Environ. Sci. 31, 103–107 (2016). https://doi.org/10.1016/j.proenv.2016.02.014
Ncobela, C.N., Kanengoni, A.T., Hlatini, V.A., Thomas, R.S., Chimonyo, M.: A review of the utility of potato by-products as a feed resource for smallholder pig production. Anim. Feed Sci. Technol. 227, 107–117 (2017). https://doi.org/10.1016/j.anifeedsci.2017.02.008
Mora, M.: Mercado, estrategias y limitaciones de comunicación. In: Inglese, P., Jacobo, C.M., Nefzaoui, A., Sáenz, C. (eds.) Ecología del Cultivo, Manejo y Usos del Nopal, pp. 199–206. FAO-ICARDA. Rome, Italy (2018). ISBN:978-92-5-130494-5
Koubaa, M., Ktata, A., Barba, F.J., Grimi, N., Mhemdi, H., Bouaziz, F., Driss, D., Chaabouni, S.E.: Water-soluble polysaccharides from Opuntia stricta Haw. fruit peels: recovery, identification and evaluation of their antioxidant activities. Int. Agrophys. 29(3), 299–306 (2015). https://doi.org/10.1515/intag-2015-0035
Majdoub, H., Roudesli, S., Deratani, A.: Polysaccharides from prickly pear peel and nopals of Opuntia focus-indica: extraction, characterization and polyelectrolyte behaviour. Polym. Int. 50(5), 552–560 (2001). https://doi.org/10.1002/pi.665
Valcárcel-Yamani, B., Rondán-Sanabria, G.G., Finardi-Filho, F.: The physical, chemical and functional characterization of starches from Andean tubers: oca (Oxalis tuberosa Molina), olluco (Ullucus tuberosus Caldas) and mashua (Tropaeolum tuberosum Ruiz & Pavón). Braz. J. Pharm. Sci. 49(3), 453–464 (2013). https://doi.org/10.1590/S1984-82502013000300007
Espino-Díaz, M., De Jesús Ornelas-Paz, J., Martínez-Téllez, M.A., Santillán, C., Barbosa-Cánovas, G.V. Zamudio-Flores, P.B., Olivas G.I.: Development and characterization of edible films based on mucilage of Opuntia ficus-indica (L.). J. Food Sci. 75(6), E347–E352 (2010). https://doi.org/10.1111/j.1750-3841.2010.01661.x
Pelissari, F.M., Andrade-Mahecha, M.M., do Amaral Sobral, P.J., Menegalli, F.C.: Comparative study on the properties of flour and starch films of plantain bananas (Musa paradisiaca). Food Hydrocolloids. 30(2), 681–690 (2013). https://doi.org/10.1016/j.foodhyd.2012.08.007
Gómez-Estaca, J., Giménez, B., Montero, P., Gómez-Guillén, M.C.: Incorporation of antioxidant borage extract into edible films based on sole skin gelatin or a commercial fish gelatin. J. Food Eng. 92(1), 78–85 (2009). https://doi.org/10.1016/j.jfoodeng.2008.10.024
AOAC: Official Methods of Analysis, 18th edn. Association of Official Analytical Chemists, Washington (2005)
Basiak, E., Galus, S., Lenart, A.: Characterization of composite edible films based on wheat starch and whey-protein isolate. Int. J. Food Sci. Technol. 50(2), 372–380 (2015). https://doi.org/10.1111/ijfs.12628
ASTM: ASTM E96-95 Standard Test Methods for Water Vapor Transmission of Materials, pp. 1–8. American Society for Testing and Materials, West Conshohocken (1995)
Gennadios, A., Weller, C.L., Gooding, C.H.: Measurement errors in water vapor permeability of highly permeable, hydrophilic edible films. J. Food Eng. 21(4), 395–409 (1994). https://doi.org/10.1016/0260-8774(94)90062-0
Di Rienzo, J.A., Casanoves, F., Balzarini, M.G., González, L., Tablada, M., Robledo, C.W.: InfoStat version 2011. Universidad Nacional de Córdoba, Argentina, Grupo InfoStat FCA (2011)
Al-Hassan, A.A., Norziah, M.H.: Starch–gelatin edible films: water vapor permeability and mechanical properties as affected by plasticizers. Food Hydrocolloids 26(1), 108–117 (2012). https://doi.org/10.1016/j.foodhyd.2011.04.015
Bastos, M.D.S.R., da Silva Laurentino, L., Canuto, K.M., Mendes, L.G., Martins, C.M., Frota Silva, S.M., Furtado, R.F., Kim, S., Biswas, A., Cheng, H.N.: Physical and mechanical testing of essential oil-embedded cellulose ester films. Polym. Test. 49, 156–161 (2016). https://doi.org/10.1016/j.polymertesting.2015.11.006
Han, J.H., Gennadios, A.: Edible films and coatings: a review. In: Han, J.H. (ed.) Innovations in Food Packaging, pp. 213–255. Academy Press, Oxford (2005). https://doi.org/10.1016/B978-012311632-1/50047-4
Cao, W., Cheng, M., Ao, Q., Gong, Y., Zhao, N., Zhang, X.: Physical, mechanical and degradation properties, and Schwann cell affinity of cross-linked chitosan films. J. Biomater. Sci. 16(6), 791–807 (2005). https://doi.org/10.1163/1568562053992496
Pacheco, N., Naal-Ek, M.G., Ayora-Talavera, T., Shirai, K., Román-Guerrero, A., Fabela-Morón, M.F., Cuevas-Bernardino, J.C.: Effect of bio-chemical chitosan and gallic acid into rheology and physicochemical properties of ternary edible films. Int. J. Biol. Macromol. 125, 149–158 (2019). https://doi.org/10.1016/j.ijbiomac.2018.12.060
Homez-Jara, A., Daza, L.D., Aguirre, D.M., Muñoz, J.A., Solanilla, J.F., Váquiro, H.A.: Characterization of chitosan edible films obtained with various polymer concentrations and drying temperatures. Int. J. Biol. Macromol. 113, 1233–1240 (2018). https://doi.org/10.1016/j.ijbiomac.2018.03.057
Srinivasa, P.C., Ramesh, M.N., Tharanathan, R.N.: Effect of plasticizers and fatty acids on mechanical and permeability characteristics of chitosan films. Food Hydrocolloids 21(7), 1113–1122 (2007). https://doi.org/10.1016/j.foodhyd.2006.08.005
Kowsik, P.V., Mazumder, N.: Structural and chemical characterization of rice and potato starch granules using microscopy and spectroscopy. Microsc. Res. Tech. 81(12), 1533–1540 (2018). https://doi.org/10.1002/jemt.23160
Madera-Santana, T.J., Vargas-Rodríguez, L., Núñez-Colín, C.A., González-García, G., Peña-Caballero, V., Núñez-Gastélum, J.A., Gallegos-Vázquez, C., Rodríguez-Núñez, J.R.: Mucilage from cladodes of Opuntia spinulifera Salm-Dyck: chemical, morphological, structural and thermal characterization. CYTA-J. Food. 16(1), 650–657 (2018). https://doi.org/10.1080/19476337.2018.1454988
Tee, Y.B., Tee, L.T., Daengprok, W., Talib, R.A.: Chemical, physical, and barrier properties of edible film from flaxseed mucilage. BioResources 12(3), 6656–6664 (2017). https://doi.org/10.15376/biores.12.3.6656-6664
Zhang, Y., Han, J.H.: Plasticization of pea starch films with monosaccharides and polyols. J. Food Sci. 71(6), E253–E261 (2006). https://doi.org/10.1111/j.1750-3841.2006.00075.x
Park, J.W., Im, S.S., Kim, S.H., Kim, Y.H.: Biodegradable polymer blends of poly(L-lactic acid) and gelatinized starch. Polym. Eng. Sci. 40(12), 2539–2550 (2000). https://doi.org/10.1002/pen.11384
Cerqueira, M.A., Souza, B.W.S., Teixeira, J.A., Vicente, A.A.: Effect of glycerol and corn oil on physicochemical properties of polysaccharide films—a comparative study. Food Hydrocolloids 27(1), 175–184 (2012). https://doi.org/10.1016/j.foodhyd.2011.07.007
Fox, D.I., Pichler, T., Yeh, D.H., Alcantar, N.A.: Removing heavy metals in water: the interaction of cactus mucilage and arsenate (As (V)). Environ. Sci. Technol. 46(8), 4553–4559 (2012). https://doi.org/10.1021/es2021999
Chen, Y., Zhang, J.G., Sun, H.J., Wei, Z.J.: Pectin from Abelmoschus esculentus: optimization of extraction and rheological properties. Int. J. Biol. Macromol. 70, 498–505 (2014). https://doi.org/10.1016/j.ijbiomac.2014.07.024
Kozarski, M., Klaus, A., Niksic, M., Jakovljevic, D., Helsper, J.P.F.G., Van Griensven, L.J.L.D.: Antioxidative and immunomodulating activities of polysaccharide extracts of the medicinal mushrooms Agaricus bisporus, Agaricus brasiliensis, Ganoderma lucidum and Phellinus linteus. Food Chem. 129(4), 1667–1675 (2011). https://doi.org/10.1016/j.foodchem.2011.06.029
van Soest, J.J.G., Tournois, H., de Wit, D., Vliegenthart, J.F.G.: Short-range structure in (partially) crystalline potato starch determined with attenuated total reflectance Fourier-transform IR spectroscopy. Carbohydr. Res. 279, 201–214 (1995). https://doi.org/10.1016/0008-6215(95)00270-7
Lin, S.Y., Chen, K.S., Run-Chu, L.: Organic esters of plasticizers affecting the water absorption, adhesive property, glass transition temperature and plasticizer permanence of Eudragit acrylic films. J. Controll. Release 68(3), 343–350 (2000). https://doi.org/10.1016/S0168-3659(00)00259-5
Bonilla, J., Atarés, L., Vargas, M., Chiralt, A.: Properties of wheat starch film-forming dispersions and films as affected by chitosan addition. J. Food Eng. 114(3), 303–312 (2013). https://doi.org/10.1016/j.jfoodeng.2012.08.005
Acknowledgements
The authors acknowledge the Vicerrectorado de Investigación of the Universidad Nacional Micaela Bastidas de Apurímac (UNAMBA) for financing the research and diffusion support; and the Escuela Académico Profesional de Ingeniería Agroindustrial of UNAMBA for access to equipment; the participation of the Centro de Investigación y de Estudios Avanzados del IPN CINVESTAV-IPN Unidad Mérida Yucatán, México, Laboratorio Nacional de Nano y Biomateriales (LANNBIO) projets FOMIX-YUCATAN 2008-108160; and CONACYT LAB-2009-01-123913, 188345, 204822. Special thanks to M.C. Dora A. Huerta Quintanilla for technical assistance in the SEM analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ayquipa-Cuellar, E., Salcedo-Sucasaca, L., Azamar-Barrios, J.A. et al. Assessment of Prickly Pear Peel Mucilage and Potato Husk Starch for Edible Films Production for Food Packaging Industries. Waste Biomass Valor 12, 321–331 (2021). https://doi.org/10.1007/s12649-020-00981-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-020-00981-y