Abstract
Grape pomace is an abundant winery by-product produced worldwide, which contains a high concentration of polyphenols trapped in cell wall fibers. The fungus tannase enzyme finds many applications in the industry, but its use is currently limited. This is due to its high production cost derived from tannic acid, which is the typical inductor of tannase enzyme by Aspergillus species. Therefore, assessment of natural tannin sources as inductors is a strategy to overcome this limitation. We propose here to employ the red grape pomace, which is a rich source of tannins and polyphenols. We found that, although grape pomace is not able to induce tannase by itself, it is a useful complement for tannic acid induction, reducing the concentration of tannic acid necessary to achieve maximum levels of tannase induction, which ranged between 3.0 and 4.5 U/mL. We also explored the potential usage of this biomass to induce other relevant industrial enzymes and quantified the recovery of gallic acid from grape pomace by the fungus fermentation; finding new routes for this by-product valorisation.
Graphic Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Statement of Novelty
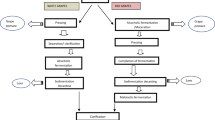
The novelty of the present study consists on the exploration of new routes for grape pomace valorisation that results in the recovery of valuable bioactives. Firstly, the employment of grape pomace as a natural tannin source is proposed in the production of tannase enzyme by Aspergillus niger. In the same process, we found that gallic acid, a potent antioxidant that has many applications in the food and pharmaceutical industries, can be recovered from grape pomace in quantitative amounts. Therefore, and integrated process is proposed where tannase enzyme and gallic acid can be obtained by the action of the fungus on the grape pomace biomass.
Introduction
Grape pomace (GP) is an abundant by-product generated during winemaking. At present, this by-product is derived to applications such as tartaric acid extraction, ethanol production, distillation processes and for fertilization and animal feeding purposes. Anyway, a high proportion is still disposed of as waste, representing a cost and managing problem [1]. This concern expands to the main wine producers placed in Europe (Spain, Italy, and France), the USA, and Latin America (Chile and Argentina). Novel procedures to treat and reuse the grape pomace are therefore being demanded, with the bioconversions at the centre of interest [1].
Grape pomace is a fibre rich material, which has a high proportion of polyphenols that remain trapped on it. A variety of methods have been tested to recover these polyphenols, which are valuable products that can be employed in the food industry [2]. In the present study, we propose to take advantage of the components contained in this biomass to produce valuable biomolecules, including tannase and other relevant industrial enzymes and phenolic acids.
Tannase or tannin acyl hydrolase enzyme (EC 3.1.1.20) catalyses the hydrolysis of ester and depside bonds of hydrolysable tannins and gallic acid esters [3]. At present, one of the principal uses is in the food industry, particularly in the elaboration of instant tea [4]. It also has applications as a clarifying agent in wine, fruit juices and beer elaboration [5, 6]. In addition, this enzyme is employed in the production of gallic acid [7], which is as an intermediate in the antimalarial drug trimethoprim synthesis; and in the food industry serves as substrate for the chemical or enzymatic synthesis of propyl gallate, a potent antioxidant.[6]. Moreover, the tannase enzyme has potential uses in the treatment of tannery effluents [8] and to increment the digestibility of tannin-containing animal feed [9].
Despite the variety of applications described, only a few of them are being exploited commercially due to the high cost of available tannase preparations and scarcity of suppliers [3]. Tannase is produced by certain filamentous fungi, mainly by the following species: Aspergillus, Penicillium, Fusarium, and Trichoderma [6], being the most common inductor tannic acid (TA). Employing natural sources of tannins has been proposed as a strategy to reduce the production cost [10]. Leaves, seeds, peels, and fruits of diverse plants have been tested [10,11,12,13], and also tea residues [14]. Most of these studies were aimed to test these substrates as the carbon source in fungus solid-state fermentation. Though solid-state fermentation is a promising strategy, industrial application of this method is not widespread. Fewer studies are available dealing with the employment of natural sources of tannin in the most common employed submerged fermentation cultures [10]. Therefore, we propose to employ grape pomace as the carbon source and inducer in submerged fermentation cultures of Aspergillus niger. We analyse tannase production, gallic acid liberation and production of other enzymatic activities, proposing new routes for grape pomace valorisation.
Materials and Methods
Materials
Grape pomace of red wine grapes (Vitis vinifera L.) was provided by BordeRío Bodega & Viñedos, Victoria, Entre Ríos, Argentina (Syrah), AER INTA-Victoria, Entre Ríos, Argentina (Marselan) and Bodegas Salentein, M-P-Wines, Los Árboles, Tunuyán, Mendoza, Argentina (Cabernet Sauvignon, Malbec and Pinot-Noir).
Aspergillus niger NRRL3 (NRRL culture collection, United State Department of Agriculture) was employed for tannase production. All the reagents employed were from Sigma-Aldrich, St. Louis, Missouri, USA.
The reagents used were of the best quality available, from Sigma Chemical Co, St. Louis, Missouri, USA.
Grape Pomace Preparation
Grape pomace, consisting in residual grape seeds, pulp, and skins, was collected after winemaking fermentation in wineries, and stored frozen at − 20 °C until processing in the laboratory. Grape pomace was dried in a drying oven (San-Jor, SL60SDB, San Andrés, Buenos Aires, Argentina) to reach < 6% humidity and milled in coffee grinders to a particle size of 0.25–2.38 mm (sieved by mesh No.8 and No.60) before extraction. When referring to “g of GP”, we refer to the dried and milled grape pomace.
Grape Pomace Characterization
Grape pomace was prepared 3% w/v in modified Czapex Dox medium (NaNO3 0.25% w/v, KH2PO4 0.1% w/v de, MgSO4·7H2O 0.05% w/v and KCl 0.05% w/v), adjusted at pH 5.50 and sterilized by autoclaving at 121 °C for 20 min; in order to determine relevant components that would be available for culture growth when grape pomace is employed as the carbon source. Total protein was determined as described in “Determination of Total Proteins” section. Folin–Ciocalteu method was employed for total phenolics estimation [15]. Tannins were determined by Hagerman and Butter method [16].
In addition, after selection of Syrah grape pomace, it was subjected to the following further analysis: crude protein [17], neutral detergent fibre [18], acid detergent fibre [19] and acid detergent lignin [20]. These determinations were done by the service of Experimental Agricultural Station of Rafaela—INTA (Santa Fe, Argentina).
Propagation of Aspergillus niger and Inoculum Preparation
A. niger was grown in potato dextrose broth (PDA), supplemented with 0.01% of tannic acid, being this procedure a requisite to maintain tannic acid tolerance [21]. Grown was performed at 30 °C for 5 days. Spores were resuspended in a Tween-80 solution at 0.02% v/v by magnetic stirring during 30 min. Count of spores was performed in a Neubauer counting chamber.
Production of Tannase by Submerged Fermentation
Tannase production was carried out in 250-mL Erlenmeyer flasks containing 100 mL of modified Czapex minimal medium (pH 5.50) supplemented with tannic acid, grape pomace, or a combination of both. Erlenmeyer flasks with the liquid medium and the grape pomace were sterilized by autoclaving at 121 °C for 20 min. After that, tannic acid was added, being sterilized by filtration with a 0.22 µm regenerated cellulose filter (Sartorius, Minisart RC 4, Goettingen, Germany). The inoculum was added to a final concentration of 106 spores/mL. Cultures were grown on an incubator at 30 °C with an orbital agitation of 120 rpm (Infors HT incubation shaker, Ecotron model, Bottmingen, Switzerland). Aliquots were extracted at regular time intervals to test tannase production.
Determination of Tannase Activity
Tannase enzyme activity was measured with the methanolic rhodanine method [22]. In general, a dilution between 1/5 and 1/10 of the extract was performed before proceeding with the standard protocol, in order to avoid absorbance saturation in the samples corresponding to controls and reactions. One enzyme unit (U) is defined as the amount of enzyme which produces one µmol of gallic acid per min of reaction.
Determination of Total Proteins
Total protein was determined by the bicinchoninic acid method employing Micro BCA Protein Assay Kit (Pierce Chemical Company) [23], after precipitation with trichloroacetic acid (TCA) to avoid polyphenols interference [24].
Determination of Pectinase and Cellulase Activity
Activity was determined by the Miller spectrophotometric method [25], by using the 3,5-dinitrosalicylic acid (DNS) reagent for determination of reducing sugar. In the case of pectinase, polygalacturonase activity was measured by employing polygalacturonic acid as substrate. In the case of cellulase, endoglucanase activity was determined by employing carboxymethylcelullose as substrate. One enzyme unit is defined as the amount of enzyme producing 1 μmol of reducing sugar per min in the described conditions [26, 27].
Determination of α-amylase Activity
Alpha-amylase activity was measured employing the commercial kit Amylase 405 kinetic unites from Wiener Lab, Rosario, Argentina. This kit makes use of a specific substrate of α-amylase: 2-chloro-p-nitrophenyl-α-D-maltotriose (CNP-G3). The enzyme hydrolyses the substrate releasing 2-chloro-p-nitrophenol (CNP) which absorbs at 405 nm (ɛ405 = 12.9 mM−1 cm−1). Thus, the reaction was followed by measuring the absorbance at 405 nm for 5 min and activities were calculated from the initial linear portion of the abs. vs. time curves as described previously [28]. One unit of enzyme activity was defined as the amount of enzyme required to hydrolyse 1 µmol of substrate per minute.
Determination of Polyphenols by HPLC-DAD
The analysis was performed employing a Dionex Ultimate 3000 SD HPLC system with a Diode Array Detector (Thermo Fisher Scientific, Waltham, MA, USA). Typically 20 µL of a filtered sample or a convenient dilution was injected on a Hypersil Gold C18 3 µm; 2.1 mm × 100 mm column (Thermo Fisher Scientific, Waltham, MA, USA). Mobile Phase consisted of a mixture of deionized water (solvent A) and acetonitrile (solvent B), both acidified by acetic acid 0.5%; at a flow rate of 0.25 mL/min. The separation method was as follow: 2.5 min at 10% B, gradient from 10% B to 50% B in 6.5 min; gradient to 80% in 2 min; 4 min at 80% B; and then back to 10% B. Chromatograms were recorded at 260, 280, 320 and 520 nm. Quantification was performed by comparison with a calibration curve performed with gallic acid and syringic acid as standards, at 280 nm. Results were expressed as mg/ml.
Results and Discussion
Induction of Tannase Production by Tannic Acid
According to previous studies reported in the literature, tannase production by Aspergillus niger reaches it maximum yield at incubation times varying between 36 and 96 h; and concentrations of tannic acid between 1 nad 10% w/v [29,30,31,32,33,34]. High concentrations of tannic acid have been reported to be inhibitory [34]. We performed a preliminary test at a fixed time of 48 h and tannic acid concentrations between 1 and 15% w/v (Fig. 1). We found that a concentration of 6% w/v was enough to reach a maximum induction, while higher concentrations did not result in higher tannase production. For subsequent experiments, we performed cultures induced with 4 and 6% w/v TA as controls.
Characterization of Red Grape Pomace and their Employment as a Complement in Tannase Induction
The content of tannins, total phenolics, and total proteins were determined in culture mediums obtained with grape pomace as the sole source of carbon (Fig. 2). We found that the liberated tannins content was relatively lower than previously reported natural tannins sources [10, 12]. Tannins content was similar in all the varieties, ranging between 0.70 and 0.85 mg/mL TA eq. The Cabernet Sauvignon variety has the highest total protein content, while Pinot Noir has the highest tannin and total phenolics content.
Characterization of culture mediums prepared with grape pomace of different varieties. The content of: a total protein, b Tannins, and c total Phenolics in culture mediums prepared with grape pomace of five different varieties (Cabernet Sauvignon, Syrah, Malbec, Marselan y Pinot Noir) were determined. These values correspond to the content on the culture medium before inoculation
According to the tannin content determinations, in order to reach at least a concentration of 0.25% w/v of tannins in the culture medium, a concentration of 8.8% w/v of grape pomace would be necessary in the case of Pinot Noir variety. This concentration was tested with all the grape pomace varieties. Culture growth was observed in this condition, but tannase activity was not detected in the extracellular medium. Therefore, we tested whether grape pomace could be employed as a complement in cultures induced with 4% w/v TA. This hypothesis was tested with the five grape pomace varieties and contrasted with the extracellular tannase production profile induced by 4 and 6% w/v TA (Fig. 3). In general, complementation of 4% w/v TA with grape pomace gave rise to significant increments on tannase production at incubation times of 24, 48 and 96 h. In these cases, the values of tannase activity obtained were similar to those corresponding to 6% w/v TA. Only in the case of Cabernet Sauvignon, the increments found were not significant.
Production of tannase induced by tannic acid (TA), grape pomace (GP) and combinations of TA + GP and total protein content. Tannase production (U/mL) was tested in A. niger cultures grown at regular time periods in cultures mediums containing 4 and 6% w/v TA (controls) and combinations of 4% w/v TA and GP of five different grape varieties (Cabernet Sauvignon, Syrah, Malbec, Marselan y Pinot Noir). Those points were a significant difference was observed by supplementation of the 4% w/v TA with GP are marked with *(p ≤ 0.05) ó **(p ≤ 0.001). Total protein content was tested in parallel as a growth indicator
The maximum yields of tannase obtained with the GP complementation ranged between 3.0 and 4.5 U/mL. These values are larger than the one reported previously when a tannin-rich desert plant (Larrea tridentata Cov.) was used in fungal solid-state culture, which was of 1.04 U/mL [12]. In the case of submerged cultures, previous studies reported very low induction by the employment of natural tannin: powder of dried persimmon (Diospyrus kak) fruits, 0.0558 U/mL; powder of dried pomegranate peels (Punica granatum), 0.30 U/mL, 1.60 U/mL powder of dried river-red-gum (Eucalyptus camaldulensis Dehn) leaves; 0.0028 U/mL, powder of dried unripe date; and this fact was attributed to the presence of sugars in these materials, which would be used by the fungus to growth [10]. In that study, maximum yield (6.10 U/mL) could be obtained at 96 hs and 35°C of submerged culture with tannic acid as the carbon source.
Here we found that a combination of a natural tannin source with tannic acid resulted in productive induction of tannase. We hypothesize that an initial tannase induction by tannic acid is necessary to initiate culture growth. In this way, tannase and other enzymes can degrade the grape pomace to liberate more tannins and other trapped molecules that can be employed as inducers and as carbon source.
Effect of Grape Pomace on Fungal Growth and Other Proteins Production
The employment of grape pomace as a complement on the induced culture may improve the fungus growth, since it provides additional nitrogen and carbon sources. When insoluble substrates are employed in fungal cultures, determination of growth with direct methods such as dry weight method is precluded. Indirect methods, such as total protein content have been employed to circumvent this problem [35]. Therefore, total protein concentration on the extracellular medium was determined for the cultures described in the previous section (Fig. 3). Higher total protein contents were found in general in the case of complementation with grape pomace than those corresponding to 4% w/v TA, and at shorter incubation times. Therefore we can conclude that the favourable effect of grape pomace on the fungus growth contributes to the higher tannase production.
In some cases, the protein content was also higher than that corresponding to 6% w/v TA. This effect was observed in all the tested incubation times in the case of Syrah variety. The characterization of this grape pomace variety was therefore expanded.
Syrah grape pomace has a considerable proportion of cellulose, hemicellulose, and lignin (Table 1). These vegetal components can induce hydrolytic enzymes in fungus. We tested cellulose, amylase, and pectinase activities in cultures were tannic acid, Syrah grape pomace or tannic acid complemented with Syrah grape pomace, were employed as carbon source (Fig. 4).
Evaluation of relevant enzymatic activities in the extracellular medium of A. niger cultures aimed to produce tannase enzyme. Pectinase, alpha-amylase, and cellulase activities were tested in the extracellular medium of A. niger cultures grown for 48 h, containing 4 and 6% w/v of TA (controls) and combinations of TA 4% w/v, Syrah GP + 4% w/v TA, and Syrah GP
Production of pectinase was found with 4 and 6% w/v TA, though not being a specific inductor. Production levels were greater with Syrah grape pomace alone. Though the maximum activity achieved was lower than the one obtained with other natural carbon sources in optimized conditions, such as mango and banana peels [36, 37], our results indicate that grape pomace is a plausible natural carbon source for pectinase production.
Alpha-amylase was induced in all the tested cultures, being greater in the case of 6% w/v TA.
In the case of cellulase, no activity was detected with tannic acid as the carbon source, but there was a specific induction in the case of Syrah grape pomace. Though activity values are low in comparison to the ones obtained with other biomass inductors in optimized conditions [38]; these results show that grape pomace can induce cellulase production in A. niger cultures.
Grape Pomace as a Source of Phenolic Acids
Grape pomace is a known source of valuable polyphenols. Therefore, we tested the effect of the A. niger on the liberation of phenolics during the culture grown for tannase production. Samples of cultures containing 4% w/v TA, Syrah GP, and 4% w/v TA + Syrah GP, without inoculum and with inoculum after 48 h incubation, were analyzed by HPLC. Gallic acid and syringic acid were detected in the samples at quantitative amounts in all the samples (Table 2). The gallic acid concentration was increased in all the cases by the fungus growth after 48 h. This increment can be related to the production of tannase enzyme, since it was greater in the cases where tannic acid was present. In the case of the culture induced with 4% w/v TA, the gallic acid comes from the tannic acid. In the case of the culture induced with 4% w/v TA + Syrah GP, the gallic acid comes from the tannic acid and the grape pomace. In this last case, it was found that the final concentration and the relative increment of gallic acid were greater than in the case of tannic acid induction. Therefore, we can conclude that the effect of tannase enzyme in situ liberates gallic acid from the grape pomace. The absolute quantities of recovered gallic acid from grape pomace (Table 2) are similar to those reported when a tannin-rich desert plant (Larrea tridentata Cov.) was used in fungal solid-state culture (0.33 mg/mL) [12]. When tannic acid is present in the culture this value is substantially larger (Table 2).
Therefore, we demonstrate that a process intended to produce tannase enzyme can at the same time be employed to produce gallic acid from a tannin-rich agricultural waste.
Conclusion
The employment of grape pomace as a natural source of tannin was tested in the production of tannase by A. niger. We found that grape pomace was not able to induce quantitative amounts of tannase. However, supplementation of tannic acid with grape pomace allows reducing the concentration of tannic acid employed for the induction. This effect was significant in 4 out of the 5 tested grape varieties. Our results indicate that the positive effect on tannase induction by grape pomace complementation can be related to the provided tannins, which serve as inductor; and also to other nutrients that promote the fungus growth.
In addition, we found that Syrah grape pomace can also induce the production of alpha-amylase, pectinase, and cellulase activity. Moreover, we found that during the culture growth gallic acid is liberated from grape pomace.
Globally, our results indicate a great potential of the grape pomace to be employed as a substrate in the production of industrial enzymes and in the production of gallic acid, being these procedures routes of valorisation of this agroindustrial waste.
References
Zacharof, M.-P.: Grape winery waste as feedstock for bioconversions: applying the biorefinery concept. Waste Biomass Valor. 8, 1011–1025 (2017). https://doi.org/10.1007/s12649-016-9674-2
Fontana, A.R., Antoniolli, A., Bottini, R.: Grape pomace as a sustainable source of bioactive compounds: extraction, characterization, and biotechnological applications of phenolics. J Agric. Food Chem. 61, 8987–9003 (2013). https://doi.org/10.1021/jf402586f
Belur, P.D., Mugeraya, G.: Microbial production of tannase: state of the art. Res. J. Microbiol. 6, 25–40 (2011). https://doi.org/10.3923/jm.2011.25.40
Boadi, D.K., Neufeld, R.J.: Encapsulation of tannase for the hydrolysis of tea tannins. Enzyme Microb. Technol. 28, 590–595 (2001)
Lekha, P.K., Ramakrishna, M., Lonsane, B.K.: Strategies for the isolation of potent fungal cultures capable of producing tannin acyl hydrolase in higher titres. Chem. Mikrobiol. Technol. Lebensm. 15, 5–10 (1993)
Belmares, R., Contreras-Esquivel, J.C., Rodrı́guez-Herrera, R., Coronel, A.R., Aguilar, C.N.: Microbial production of tannase: an enzyme with potential use in food industry. LWT Food Sci. Technol. 37, 857–864 (2004). https://doi.org/10.1016/j.lwt.2004.04.002
Deschamps, A.M., Lebeault, J.-M.: Production of gallic acid from tara tannin by bacterial strains. Biotechnol. Lett. 6, 237–242 (1984). https://doi.org/10.1007/BF00140043
Murugan, K., Al-Sohaibani, S.A.: Biocompatible removal of tannin and associated color from tannery effluent using the biomass and tannin acyl hydrolase (E.C.3.1.1.20) enzymes of mango industry solid waste isolate Aspergillus candidus MTTC 9628. Res. J. Microbiol. 5, 262–271 (2010). https://doi.org/10.3923/jm.2010.262.271
Abdulla, J., Rose, S.P., Mackenzie, A.M., Mirza, W., Pirgozliev, V.: Exogenous tannase improves feeding value of a diet containing field beans (Vicia faba) when fed to broilers. Br. Poult. Sci. 57, 246–250 (2016). https://doi.org/10.1080/00071668.2016.1143551
Aboubakr, H.A., El-Sahn, M.A., El-Banna, A.A.: Some factors affecting tannase production by Aspergillus niger Van Tieghem. Braz. J. Microbiol. 44, 559–567 (2013). https://doi.org/10.1590/S1517-83822013000200036
Sabu, A., Pandey, A., Daud, M.J., Szakacs, G.: Tamarind seed powder and palm kernel cake: two novel agro residues for the production of tannase under solid state fermentation by Aspergillus niger ATCC 16620. Bioresour. Technol. 96, 1223–1228 (2005). https://doi.org/10.1016/j.biortech.2004.11.002
Treviño-Cueto, B., Luis, M., Contreras-Esquivel, J.C., Rodríguez, R., Aguilera, A., Aguilar, C.N.: Gallic acid and tannase accumulation during fungal solid state culture of a tannin-rich desert plant (Larrea tridentata Cov.). Bioresour. Technol. 98, 721–724 (2007). https://doi.org/10.1016/j.biortech.2006.02.015
Kumar, R., Sharma, J., Singh, R.: Production of tannase from Aspergillus ruber under solid-state fermentation using jamun (Syzygium cumini) leaves. Microbiol. Res. 162, 384–390 (2007). https://doi.org/10.1016/j.micres.2006.06.012
Sharma, N.K., Beniwal, V., Kumar, N., Kumar, S., Pathera, A.K., Ray, A.: Production of tannase under solid-state fermentation and its application in detannification of guava juice. Prep. Biochem. Biotechnol. 44, 281–290 (2014). https://doi.org/10.1080/10826068.2013.812566
Singleton, V.L., Rossi, J.A.: Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 16, 144–158 (1965)
Hagerman, A.E., Butler, L.G.: Protein precipitation method for the quantitative determination of tannins. J. Agric. Food Chem. 26, 809–812 (1978). https://doi.org/10.1021/jf60218a027
AOAC International: AOAC: Official methods of analysis (vol 1). https://archive.org/details/gov.law.aoac.methods.1.1990. (1990)
ANKOM Technology: Neutral detergent fiber in feeds—method 6 (2011)
ANKOM Technology: Acid detergent fiber in feeds—method 5 (2011)
ANKOM Technology: Acid detergent lignin—PROMEFA V2 protocol (2005)
Mangrola, A.V., Patel, H.V., Chudasama, C.J., Vavadia, C.N., Shah, H.: Optimization of cultural conditions for tannase production in submerged fermentation by Aspergillus niger avm-1. Pharm. Res. 8
Sharma, S., Bhat, T.K., Dawra, R.K.: A spectrophotometric method for assay of tannase using rhodanine. Anal. Biochem. 279, 85–89 (2000). https://doi.org/10.1006/abio.1999.4405
Smith, P.K., Krohn, R.I., Hermanson, G.T., Mallia, A.K., Gartner, F.H., Provenzano, M.D., Fujimoto, E.K., Goeke, N.M., Olson, B.J., Klenk, D.C.: Measurement of protein using bicinchoninic acid. Anal. Biochem. 150, 76–85 (1985). https://doi.org/10.1016/0003-2697(85)90442-7
Brown, R.E., Jarvis, K.L., Hyland, K.J.: Protein measurement using bicinchoninic acid: elimination of interfering substances. Anal. Biochem. 180, 136–139 (1989)
Miller, G.L.: Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 31, 426–428 (1959). https://doi.org/10.1021/ac60147a030
Gupta, M.N., Dong, G., Mattiasson, B.: Purification of endo-polygalacturonase by affinity precipitation using alginate. Biotechnol. Appl. Biochem. 18(Pt 3), 321–327 (1993)
Ncube, T., Howard, R.L., Abotsi, E.K., van Rensburg, E.L.J., Ncube, I.: Jatropha curcas seed cake as substrate for production of xylanase and cellulase by Aspergillus niger FGSCA733 in solid-state fermentation. Ind. Crops Prod. 37, 118–123 (2012). https://doi.org/10.1016/j.indcrop.2011.11.024
Porfiri, M.C., Farruggia, B.M., Romanini, D.: Bioseparation of alpha-amylase by forming insoluble complexes with polyacrylate from a culture of Aspergillus oryzae grown in agricultural wastes. Sep. Purif. Technol. 92, 11–16 (2012). https://doi.org/10.1016/j.seppur.2012.03.004
Lekha, P.K., Lonsane, B.K.: Comparative titres, location and properties of tannin acyl hydrolase produced by Aspergillus niger PKL 104 in solid-state, liquid surface an submerged fermentations. Process Biochem. 29, 497–503 (1994). https://doi.org/10.1016/0032-9592(94)85019-4
Bradoo, S., Gupta, R., Saxena, R.K.: Parametric optimization and biochemical regulation of extracellular tannase from Aspergillus japonicus. Process Biochem. 32, 135–139 (1997)
Sharma, S., Agarwal, L., Saxena, R.K.: Statistical optimization for tannase production from Aspergillus niger under submerged fermentation. Indian J. Microbiol. 47, 132–138 (2007). https://doi.org/10.1007/s12088-007-0026-6
Darah, I., Sumathi, G., Jain, K., Hong, L.S.: Involvement of physical parameters in medium improvement for tannase production by Aspergillus niger FETL FT3 in submerged fermentation. Biotechnol. Res. Int. 2011, 897931 (2011)
Aissam, H., Errachidi, F., Penninckx, M.J., Merzouki, M., Benlemlih, M.: Production of tannase by Aspergillus niger HA37 growing on tannic acid and olive mill waste waters. World J. Microbiol. Biotechnol. 21, 609–614 (2005)
Aguilar, C.N., Augur, C., Favela-Torres, E., Viniegra-González, G.: Production of tannase by Aspergillus niger Aa-20 in submerged and solid-state fermentation: influence of glucose and tannic acid. J. Ind. Microbiol. Biotechnol. 26, 296–302 (2001)
Favela-Torres, E., Cordova-López, J., García-Rivero, M., Gutiérrez-Rojas, M.: Kinetics of growth of Aspergillus niger during submerged, agar surface and solid state fermentations. Process Biochem. 33, 103–107 (1998). https://doi.org/10.1016/S0032-9592(97)00032-0
Yadav, K.K., Garg, N., Kumar, D., Kumar, S., Singh, A., Muthukumar, M.: Application of response surface methodology for optimization of polygalacturonase production by Aspergillus niger. J. Environ. Biol. 36, 255–259 (2015)
Barman, S., Sit, N., Badwaik, L.S., Deka, S.C.: Pectinase production by Aspergillus niger using banana (Musa balbisiana) peel as substrate and its effect on clarification of banana juice. J. Food Sci. Technol. 52, 3579–3589 (2015). https://doi.org/10.1007/s13197-014-1413-8
Dias, L.M., Dos Santos, B.V., Albuquerque, C.J.B., Baeta, B.E.L., Pasquini, D., Baffi, M.A.: Biomass sorghum as a novel substrate in solid-state fermentation for the production of hemicellulases and cellulases by Aspergillus niger and A. fumigatus. J. Appl. Microbiol. 124, 708–718 (2018). https://doi.org/10.1111/jam.13672
Acknowledgements
This work was supported by grants from Agencia Nacional de Promoción Científica y Tecnológica, Argentina (PICT-2016-4463 and PICT-2016-1170). The authors would like to thank BordeRío Bodega & Viñedos, AER INTA-Victoria, and Bodegas Salentein, Argentina for supplying the grape pomace.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Meini, MR., Ricardi, L.L. & Romanini, D. Novel Routes for Valorisation of Grape Pomace Through the Production of Bioactives by Aspergillus niger. Waste Biomass Valor 11, 6047–6055 (2020). https://doi.org/10.1007/s12649-019-00844-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-019-00844-1