Abstract
With increasing fossil fuel price and environmental concern, alternative and renewable resources have become attractive. However, a large amount of the cheapest or negative-cost lignocellulosic biomass produced from agriculture and forest-based industries is the most abundant renewable resource to convert biofuels and value-added chemicals. The aim of this study was to produce carboxymethyl cellulase (CMCase), a key enzyme for utilizing lignocellulosic biomass produced in agriculture and forest-based industries to produce biofuels and value-added bioproduct. Five potential bacterial strains isolated from soil and rotted wood samples from Kingfisher Lake, Thunder Bay, Canada were screened for their cellulolytic activity using carboxymethyl cellulose as the sole carbon source. The most promising strain IM7 was identified as Paenibacillus sp. on the basis of 16S rRNA gene sequence analysis. In this study, the optimal conditions for high production of CMCase were defined in a completely aerobic process. The newly isolated Paenibacillus sp. IM7 strain showed the highest CMCase activity up to 17.7 ± 0.17 IU/mL using 1.5% yeast extract in the culture medium as a nitrogen source. Moreover, when the culture medium supplemented with 2% mannose as an inducer the CMCase activity was markedly boosted up to 24.59 ± 0.09 IU/mL. The optimum pH and temperature of the culture for CMCase production by the strain IM7 were 5.0 and 30 °C respectively, and the significant enzyme production was obtained at a wide temperature range of 25–55 °C. Therefore, this Paenibacillus IM7 strain could be a potential candidate for CMCase production in an industrial bioconversion process.
Graphic Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Statement of Novelty
This Paenibacillus IM7 is a novel and efficient carboxymethyl cellulase (CMCase) producing bacterial strain. This Paenibacillus IM7 has potential for biofuel production using low/negative-cost biomass in industrial bioconversion processes. In this report, a high CMCase activity (24.59 ± 0.09 IU/mL) was obtained by using this newly isolated strain. We have demonstrated that this novel Paenibacillus sp. IM7 is capable of efficiently utilizing carboxymethyl cellulose (CMC) as the sole carbon source to produce sugars, which can be further converted to biofuels and value-added chemicals via microbial fermentation. The research will contribute to the development of biorefining process and production of biofuel industry products. The paper is interesting to popular or scientific audience.
Introduction
With decades of studies on lignocellulose bioconversion, cellulases have been playing an important role in producing fermentable reducing sugars from cellulosic biomass. Typically, cellulases are primarily composed of endoglucanases that hydrolyze the exposed cellulose chains of the cellulose polymer, exoglucanases that act to release cellobiose from the reducing and nonreducing ends, and β-glucosidases that help to cleave the cellobiose and short-chain cello-oligosaccharide into glucose [1]. Bioethanol, a renewable biofuel produced from fermentation of sugar is most commonly used as an additive to gasoline for motor vehicles. It is a form of renewable energy obtained from fermentation of sugars using agricultural feedstocks [2]. Among the many advantages of using bioethanol as a fuel are its low toxicity and susceptibility to biodegradation, making it “eco-friendly” [3]. However, to achieve high production levels, fertile land and ample water supplies are required to grow agricultural feedstocks [4]. To produce bioethanol from the fibrous part of a plant cellulose, a cellulolytic process has been developed that consists of hydrolysis of pretreated lignocellulosic material with the help of cellulase enzymes to break complex cellulose into simple sugars [5]. As an alternative form of energy cellulose has gained importance recently, and it represents millions of dollars to countries with the means to obtain energy from it [6]. Plant cellulose is convenient because it can be easily stored and moved, and is non-toxic. However, cellulose is a linear biopolymer of glucose molecules. The enzymatic hydrolysis of cellulose requires mixtures of hydrolytic enzymes including endo-β-glucanases (EC 3.2.1.4), exo-β-glucanases (EC 3.2.1.91), and β-glucosidase (EC 3.2.1.21), all acting in synergy [7]. Mechanistically, the endo-β-glucanase randomly breaks the internal glycosidic bonds which result in formation of glucan chains of different lengths; the exo-β-gluconase acts on the ends of the cellulose chains resulting the formation of β-cellobiose as end products, and the β-glycosidase finally breaks the glycosidic bond of β-cellobiose or small polysaccharides to produce glucose molecules [8]. These cellulases have been used for both academic research and industrial production. Cellulase enzymes produced by microorganisms could be used for bioconversion of cellulosic biomass to renewable biofuels because they can depolymerize the β-(1–4) linkages in the cellulose molecule into cellobiose and glucose [9].
However, most of the cellulases currently used in industries and laboratories are produced by fungi because of their high yields. Indeed, both bacteria and fungi are able to produce cellulases, but bacterial cellulase production is higher due to their high growth rate compared to fungi [10]. However, unlike fungi, bacteria have a limited capacity to produce a large number of extracellular enzymes. For several years, spectrophotometric methods have been used to detect cellulase activity based on reducing sugars using 3,5-dinitrosalicylic acid (DNS) [11]. In the recent years, enzymatic bioconversion of lignocellulosic wastes to biofuels and value-added chemicals is attracting because of the rapid depletion of fossil fuels and increasing demand for renewable energy. It is a complicated process for the production of cellulases by microorganisms because different strains induced by different substrates might produce different cellulases which are more suitable for acting on the specific substrates [12]. Consequently, even though there are a number of cellulase-producing microbial strains commercially available, it is still necessary to isolate new effectual strains for efficient degradation of specific lignocellulosic biomass. Thus, the aim of our research work is enzymatic cellulose degradation by efficient cellulase producing bacterial strain to produce sugar, which can be further converted to biofuels and other value-added chemicals. In our research work, a novel bacterial strain Paenibacillus sp. IM7 was isolated and characterized which can efficiently produce cellulase (CMCase). Therefore, this research has a significant contribution to the development of biofuel industries.
Materials and Methods
Isolation of Bacterial Strain
Soil samples were collected from Kingfisher Lake, Thunder Bay, Ontario, Canada. One gram of sample was added to an Erlenmeyer flask containing 100 mL of 1% peptone water. The sample was mixed thoroughly using the sterilized magnetic stirrer, then 100 µL of sample was spread on Luria–Bertani (LB) agar (10 g/L peptone, 5 g/L yeast extract, 10 g/L NaCl, and 15 g/L agar) plate using sterilized glass spreader [13]. Plates were incubated at 30 °C for 48 h. From these plates, bacterial colonies were selected based on morphological features such as colony morphology, color and size. Pure bacterial isolates were screened for their CMCase activity.
Screening for CMCase Activity
Bacterial isolates were grown in tubes containing 5.0 mL LB broth (10 g/L peptone, 5 g/L yeast extract, and 5 g/L NaCl) [14] for 24 h at 30 °C, with shaking at 200 rpm. Subsequent incubation, 5.0 µL of culture broth was dropped on the center of CMC agar (5 g/L CMC, 1 g/L NaNO3, 1 g/L K2HPO4, 1 g/L KCl, 0.5 g/L MgSO4, 0.5 g/L yeast extract and 15 g/L agar) plates, incubated for 48 h at 30 °C, stained with Gram’s iodine solution (per 300 mL: 2 g KI and 1 g I) to determine the visible hydrolysed zone of CMC formed by bacterial CMCase [15]. The formation of clear zones after approximately 5 min in the areas around colonies indicate an absence of CMC. These “halos” are signs that the colony exhibits cellulase activity. By using a standard ruler, the halos were measured in centimeters.
Identification of Bacterial Isolates by 16s rRNA Gene Sequencing
16s rRNA gene sequencing is often used for bacterial identification and discovery of novel bacteria strains. In our study, the most promising five bacterial isolates were identified using 16s rRNA sequencing. All five cellulase positive isolates were cultured in LB broth for 24 h where the incubation temperature was maintained at 30 ºC. Following incubation, cells from 1000 µL of broth culture were collected by centrifugation at 12,000×g. The genomic DNA was isolated from these bacterial calls using DNA extraction kit (Ultra-clean Presto™ mini genomic DNA Bacteria kit) following manufacturer protocols. The extracted DNA was amplified by PCR (polymerase chain reaction) using universal 16S primers HDA-1 (5ʹ-GAC TCC TAC GGG AGG CAG CAG T-3ʹ) and E1115R (5ʹ-AGG GTT GCG CTC GTT GCG GG-3ʹ). The PCR reaction mixtures (50 μL) was contained 1.0 μL of each primer, 25.0 μL of 2× PCR master mixture (Taq Mix), 22 μL nuclease-free water and 1.0 μL genomic DNA template. The PCR thermal cycling procedure was as follows: primary denaturation at 94 °C for 3 min; 35 amplification cycles consisting of denaturation at 94 °C for 30 s, annealing at 58 °C for 30 s, and extension at 72 °C for 1 min; and a final extension step at 72 °C for 10 min. The amplified gene product was separated by agarose gel electrophoresis, purified using the Geneaid PCR/Gel Purification Kit following manufacturer protocols. The concentration of purified DNA sample was measured using Nanodrop spectrophotometer. Finally, the purified DNA sample was sent to the Centre for Applied Genomics (Eurofins Genomics, Toronto, Canada) for sequencing, and the sequence was analyzed using nucleotide BLAST program (https://www.ncbi.nlm.nih.gov/).
Phylogenetic Relationship of Bacterial Isolates
Phylogenetic relationship of microorganisms helps to provide a better understanding of diversity, systematics, and nomenclature of microbes. The sequencing results were input into the National Center for Biotechnology Information (NCBI) database (https://blast.ncbi.nlm.nih.gov) to identify possible strains. The phylogenetic tree was drawn using the ClustalX Omega software program.
Quantitative CMCase Assay
Caboxylmethylcellulase (CMCase) activity was measured at 50 °C and the released reducing sugar was analyzed using a dinitrosalicylic acid (DNS) method. A modified microplate-based assay method was used for measuring reducing sugars. Briefly, 20 μL of cell-free supernatant (enzyme) was mixed with 80 μL of 0.5% CMC and 50 mM citrate buffer at pH 6.0, and incubated for 30 min at 50 °C. The reaction was terminated by adding 200 μL DNS solution (3.15 g/L DNS, 10.48 g/L NaOH, 91.0 g/L Na–K tartrate, 2.5 g/L phenol, and 2.5 g/L sodium metabisulfite). The mixture was boiled for 5 min. The absorbance was determined at OD540nm using microplate reader spectrophotometer (Epoch™ microplate spectrophotometer, BioTek instrument, USA). All experiments were repeated at least three.
Fermentation Medium and Culture Condition
Bacterial isolates were grown in LB broth medium, and 1.0 mL of an overnight culture was transferred into 200 mL Erlenmeyer flask containing 50 mL of Minimal Salt (MS) medium supplemented with 0.5% CMC as a sole carbon source. When indicated, the MS medium was supplemented with specified concentration of different nitrogen and carbon sources. The MS medium per Litre contained: 1.0 g K2PO4, 0.5 g KCl, 0.5 g MgSO4, 0.5 g NaNO3 and 0.01 g FeSO4. The flasks were incubated in a shaker incubator at 200 rpm. Bacterial growth was observed each 24 h intervals. CMCase activity was determined by measuring the release of reducing sugars from CMC as a substrate. Biomass or cell growth was measured as absorbance at 600 nm (OD600) using microplate spectrophotometer (EPOCH, BioTek).
Optimization of Fermentation Process
Cells from slant culture was inoculated into a LB broth medium to prepare the seed culture. After 24 h incubation at 30 °C under aerobic condition, the seed culture was inoculated into fermentation medium for optimization of fermentation parameters including incubation temperature, incubation time, medium initial pH, and concentrations of carbon and nitrogen sources. In case of CMCase assay, batch fermentations were carried out in 200 mL Erlenmeyer flasks containing 50 mL fermentation medium (MS medium supplemented with 0.5% CMC) with 100 µL of 24 h seed culture, incubated under aerobic condition at 200 rpm using rotary shaker (New Brunswick Scientific, C25 incubator shaker, NJ, USA). When indicated, the medium was supplemented with a specified concentration of different nitrogen and carbon sources. The pH of the medium was adjusted with 1 M NaOH or 1 M HCl depending on the experiment. The cell-free supernatant was collected by centrifugation at 12,000×g, used for the CMCase activity. To optimize the incubation time for maximum enzyme activity, experiments were performed without pH control at 30 ºC for up to 144 h. However, for extracellular enzyme assay, 1.0 mL of sample (culture broth) was taken each 24 h interval.
The effect of initial pH ranging from 4.0 to 8.0 and temperature ranging from 25 to 50 °C was evaluated on CMCase activity. Moreover, different nitrogen sources (malt extract, yeast extract, peptone, sodium nitrate, urea, ammonium sulfate and sodium chloride) and carbon sources (fructose, mannose, sorbitol, glucose, lactose, sucrose, CMC, and galactose) were also used in fermentation medium to optimize the culture for maximum enzyme activity. All experiments were performed in triplicate and the results are expressed as mean values of three replicates.
Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis (SDS-PAGE)
To determine the molecular weight of the enzyme, the zymogram analysis was performed by SDS-PAGE using 10% (w/v) separating gel (0.5 mL of 1% CMC, 3.3 mL water, 3.4 mL of 30% acrylamide with 0.8% bis-acrylamide, 2.6 mL 1.5 M Tris-pH 8.8, 0.1 mL of 10% SDS, 100 μL of 10% ammonium persulfate and 10 μL TEMED) and 5.0% (w/v) of stacking gel (2.975 mL water, 1.25 mL 0.5 M Tris–HCl with pH 6.8, 0.05 mL 10% SDS, 0.67 mL of 30% acrylamide with 0.8% bis-acrylamide, 0.05 mL of 10% ammonium persulfate and 0.005 mL TEMED). TEMED and ammonium persulfate were added immediately before each use [16]. The acrylamide percentage in SDS-PAGE gel depends on the size of the target protein in the sample.
For SDS-PAGE analysis 100 μL of the broth culture was taken from optimized culture condition, centrifuged at 12,000×g for 3 min, and 30 μL of cell-free supernatant was mixed with 10 μL 4× protein loading buffer. From the above loading mixture, 10 μL was loaded into wells along with standard protein marker in the first lane. The gel was run using the Mini-Protean system (Bio-Rad) following manufacturer protocols with some modifications. One part of the gel comprised the samples and molecular marker and was stained with Coomassie brilliant blue R-50 for 1 h, further de-stained with decolorizing buffer for proteins and marker band. The other part was treated with 1% Triton X-100 for 30 min to remove the SDS and allowed refolding of the proteins in the gel. The CMC-Na gel was incubated at 50 ºC for 1 h in 50 mM citrate buffer containing 0.5% CMC at pH 6.0 to detect the cellulase activity. Then, the gel was submerged in 0.1% (w/v) Congo red solution for 30 min, decolorized the protein band using 1 M NaCl until pale-red hydrolysis zones appeared against a red background for zymogram analysis [17].
Statistical Analysis
All the experiments were performed in triplicates, and the results are expressed in terms of mean ± SD (standard deviation). The statistical analysis of data was performed to test the significant difference (P < 0.05) using one-way analysis of variance (ANOVA).
Results and Discussion
Screening for Cellulase Activity
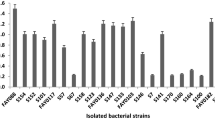
A total of 20 cellulose-producing aerobic bacterial isolates were isolated and screened for their CMCase activities. However, five isolates were exhibited high CMCase activity where they produced hydrolyzing zones on CMC agar plate after treated with Gram’s iodine stain. Results of the CMCase activities of these five bacterial isolates are shown in Fig. 1 along with negative control E. coli JM109 strain. This protocol for enzyme activity is a simple method for preliminary screening of cellulolytic microorganisms [18]. As shown in Fig. 1, the cellulase-producing isolate IM7 exhibited the largest halo (clear) zone of 2.8 cm in diameter after 48 h incubation at 30 ºC, and this isolate IM7 was chosen for further study. The diameters of the halo zones produced by all bacterial isolates on CMC agar plates are shown in Fig. 1. The morphological features of the colony formed by the strain IM7 on CMC agar plate were observed as small, circular, rough, opaque and white.
Identification and Evolutionary Relationship of the Isolates
16S rRNA gene sequencings of the five selected bacterial isolates (IM3, IM6, IM7, IM8B, and IM9) were used for their identification and discovery of novel bacterial strains. 16S rRNA genes were amplified from genomic DNA of all five CMCase producing isolates using PCR. Moreover, the sequences and sequence analysis results of amplified rRNA genes of five different bacterial isolates were successfully obtained, and the sequences of 16S rRNA genes of five isolates IM3, IM6, IM7, IM8B, and IM9 were inputted into the nucleotide BLAST of the NCBI database to get possible identities of the isolates based on homology. Nevertheless, the isolates IM3, IM6 and IM7 reported in this study were identified as Paenibacillus sp. IM3, Paenibacillus sp. IM6 and Paenibacillus IM7 strains respectively which have 99% similarity to the genus Paenibacillus. Similarly, the isolates IM8B, and IM9 have been nominated as Bacillus sp. IM8B and Bacillus sp. IM9 respectively as the new strains of Bacillus sp. The 16S rRNA sequence (717 bp) of the most promising isolate IM7 has been submitted to the GenBank for accession number. The very strain reported in this paper has been designated as Paenibacillus sp. IM7, and its GenBank accession No. is MG844517.
The 16s rRNA gene sequences of the identified bacterial strains were used for the construction of a phylogenetic tree to show the evolutionary relationship. The phylogenetic tree was successfully constructed using gene sequences of different Bacillus sp. and Paenibacillus sp. retrieved from NCBI GenBank by the Neighbor-Joining method (Fig. 2).
Effect of Incubation Time on Biomass and CMCase Production
The effect of incubation time on the production of bacterial biomass and CMCase was performed in batch culture at 30 °C using MS medium supplemented with 0.5% CMC. Bacterial growths were observed at different time intervals. The enzyme (CMCase) activity was determined by measuring the release of reducing sugars from CMC as a sole substrate. The time course of the bacterial growth and enzyme activity were performed over a 144 h incubation time. The time courses data of the production of biomass (OD600), as well as CMCase by the strains IM3, IM6, IM7, IM8B and IM9 are presented in Fig. 3a—respectively. The strain IM3 and IM7 showed the highest enzyme activity after 48 h and 72 h incubation respectively compared to that of other strains (Fig. 3). The maximum CMCase production by strain Paenibacillus sp. IM7 was 6.88 ± 0.047 IU/mL after 48 h of incubation (Fig. 3c) which was significantly different (P < 0.05) compared to other strains. Furthermore, the strain IM3 (Paenibacillus sp.) exhibited the highest enzyme activity 6.93 ± 0.0289 IU/mL after 72 h of incubation (Fig. 3a). The maximum enzyme activity of the strain Paenibacillus sp. IM6 was 6.47 ± 0.11 IU/mL after 72 h incubation. Moreover, the strains Bacillus sp. IM8B and Bacillus sp. IM9 produced maximum CMCase 5.36 ± 0.012 IU/mL and 5.05 ± 0.0.04 IU/mL respectively after 120 h incubation. However, the strains IM3, IM6 and IM7 exhibited maximum biomass (OD600) productions 1.14, 1.18 and 1.53 respectively after 72 h incubation. The maximum biomass production (OD600) with the strain IM8B obtained after 48 h incubation was 1.26. Moreover, the biomass (OD600) production of the strain IM9 was increased up to 144 h incubation (Fig. 3e).
Effect of pH and Temperature on Cellulase Activity
CMCase activities have been influenced by the effect of incubation temperature, and the results are illustrated in Fig. 4a. The cellulase producing isolate Paenibacillus sp. IM7 showed cellulase activity at a wide range of culture temperatures (25–50 ºC). The highest enzyme activity 7.015 ± 0.096 IU/mL was obtained at 30 ºC with the strain IM7 which was significantly different (P < 0.05) to other incubation temperatures, and this optimal temperature is similar to the findings of many researchers who reported using other Bacillus sp. and Paenibacillus sp.[19, 20]. The CMCase activity was significantly decreased at 25, 35, 40 and 50 ºC incubation temperatures. Moreover, at 25 ºC culture temperature, the enzyme activity was very low (61.7% of the relative activity) (Fig. 4a) which was significantly lower (P < 0.05) than the control.
However, the initial pH of the culture medium was played a significant role on CMCase activity, and results are shown in Fig. 4b. It was observed that the maximum cellulase activity 7.22 ± 0.13 IU/mL obtained at pH 5.0 which was significantly higher (P < 0.05) that of other pH (Fig. 4b). The cellulase activity was significantly decreased at pH 6.0 which was about 74.53% of relative activity (Fig. 4b). In the literature, the cellulase enzyme activity (U/mL) under the effects of initial pH and temperature during fermentation with 1% CMC as a substrate, the Bacillus pumilus EB3 strain showed the optimum pH 7.0 for maximum CMcase activity (0.058 U/mL) at incubation temperature 37ºC [21]. A similar result was also reported in Bacillus sp. K1 when the optimum pH and the maximum enzyme activity were 6.0 and 5.21 ± 0.2 U/mL respectively [13]. Moreover, for the strain Bacillus sp. K1, the CMCase activity was significantly decreased at pH 5.0, and 60% of the relative activity was attained [13]. On the other hand, the strain B. subtilis subsp. Subtilis A-53 showed a stable cellulase activity at pH 6.5, and CMCase activity of the purified enzyme was 40% of the maximal level under a specific condition between pH 5.0 and 7.5 [22]. Thus, our results indicate that the enzyme activities of the newly isolated strain Paenibacillus sp. IM7 has much wider temperature and pH stability than those reported in the literature. Therefore, our novel strain IM7 could be used as a potential candidate for high production of CMCase in the industry.
Effect of Different Nitrogen Sources on Cellulase Production
The impact of seven different nitrogen sources on the production of cellulase enzyme was investigated. The results from our experiment showed a clear variation of the relative activity (%) of CMCase using different nitrogen sources viz., peptone, yeast extract, malt extract, sodium nitrate, sodium phosphate, urea, and sodium chloride. The maximum CMCase activity was found by using yeast extract as a source of nitrogen (Fig. 5a). Our results showed that the strain Paenibacillus sp. IM7 can utilize organic nitrogen source yeast extract efficiently, and the maximum CMCase activities were increased (Fig. 5a). When 1.5% of yeast extract was used as a nitrogen source in the culture medium the CMCase activity was boosted up to 17.7 ± 0.17 IU/mL which was significantly different (P < 0.05) to other nitrogen sources. The cellulase production was increased due to decreased of acetic effect in the medium by nitrogen sources [23]. Moreover, the relative activity was decreased about 28.6% and more when inorganic nitrogen sources were used as the nitrogen sources (Fig. 5a). The use of 1.5% yeast extract enhanced 21.1% of the relative activity of CMCase compared with the control. When the concentration of yeast extract was increased from 1.5%, the relative activity of CMCase was significantly decreased (P < 0.05) (Fig. 5b).
Effect of Different Carbon Sources on Cellulase Production
In the present study, 1% of different carbon sources were used in the culture medium to evaluate their effect on cellulase production under optimum condition (culture temperature 30ºC, incubation time 48 h and pH 5.0). The results showed that the maximum CMCase activity obtained after 48 h incubation using IM7 strain was 17.8 ± 0.07 IU/mL when 1.0% of mannose was used in culture medium (Fig. 6a). Furthermore, the concentration of mannose was optimized using different concentrations of mannose in the culture medium. As shown in Fig. 6b, the CMCase activity was expressively increased to 24.59 ± 0.09 IU/mL when 2.0% of mannose was used in the culture medium, and the relative activity was increased 35.9% over control (Fig. 6b) which was significantly different (P < 0.05) to other concentrations of mannose. Therefore, the low-cost mannose was finally selected as an inducing carbon source (co-substrate) for CMCase enzyme production by IM7 strain. This result indicated that the carbon source mannose induced CMCase enzyme activity. Thus, mannose in the culture medium as a co-substrate with CMC has a potential application in large-scale production processes besides a significant advantage over glucose [24].
Therefore, CMSase was substitutive on carbon source cellulose as a substrate. However, the nitrogen source yeast extract and carbon source mannose induced the CMCase production.
SDS-PAGE and Zymogram Analysis
The CMCase enzyme protein from IM7 strain was confirmed by SDS-PAGE and zymogram analysis. The molecular weight of the protein (CMCase) was estimated as ∼50 kDa, and the protein bands including CMCase of IM7 strain are sowed in Fig. 7. The zymogram using CMC as a substrate showed the targeted active bands. However, this molecular weight 50 KDa of the cellulase was similar to the other species reported by Bajaj [25].
However, this study described the cellulase activity of a new cellulase producing bacterium Paenibacillus sp. IM7 isolated from Kingfisher Lake, Thunder Bay, Ontario, Canada. According to the experiments conducted by Sheng [26], Pseudomonas sp. HP207 had maximum endoglucanase production among the optimized fermentation conditions. In his experiment, results showed that the HP207 strain proved to be at the higher enzyme activity as 1.432 U/mL. The endoglucanase enzyme produced in this case proved to be thermostable as well. In fact, Bacillus sp. could utilize both the inorganic and organic nitrogen sources for cellulase production [27]. However, in our report, the production of cellulase in presence of different nutrition factors by Paenibacillus sp. IM7 was 24.59 ± 0.09 IU/mL which is much higher than previously report by many researchers [13, 20]. Also, the enzyme activity of our novel isolate Paenibacillus sp. IM7 was much higher than those studied previously by other workers which have received wide attention for commercial production of cellulase [22, 28, 29].
Nevertheless, cellulases have a potential application for hydrolyzing polysaccharides in biorefining industries which are based on agro-industrial wastes. The bacterial strain IM7 reported in this study was competently able to degrade lignocellulose substrates. This work acts as a step towards a further study on bio-refinery feedstocks that are composed of biomass [30]. Also, the future studies can be done in identification and cloning of the genes β-1,4 endoglucanase in these cellulase producing isolates. The strain improvement for enhancing cellulase production can be achieved by using different techniques such as mutagenesis and metabolic engineering [31, 32].
Conclusions
Five cellulase-producing bacterial isolates were obtained from different soil and rotting wood samples, and the novel strain Paenibacillus sp. IM7 exhibited a remarkable cellulase activity. Therefore, we have successfully identified an efficient cellulase producing bacterial strain Paenibacillus sp. IM7 with exceptional potential for industrial use in the bioconversion of lignocellulosic biomass to biofuels and value-added products via reducing sugar. Moreover, many of our isolates characterized here also have potential for industrial use. The most promising strain IM7 reported in this research paper could be a foundation for current exploitation of cellulase enzyme production by further investigation. Also, the strain IM7 may have great potential for developing metabolically engineered strain to increase the biodegradation of lignocellulosic biomass and help overcome expensive hurdles being faced for industrial production of biofuels.
Therefore, Cellulase (CMCase) is a key enzyme for hydrolyzing lignocellulosic biomass to fermentable sugar. This commercially available enzyme produced in industries uses for hydrolyzing cellulosic biomass to produce sugar stream. This fermentable sugar biomass is using to produce bioethanol and other value-added bioproducts (1, 5, 7–9). Thus, our research on high production of cellulase using the newly isolated novel bacterial strain would contribute on the valorization of lignocellulosic biomass waste produced from agriculture and forest-based industries which are contained more than 30% cellulose.
References
Kim, J., Yun, S., Ounaies, Z.: Discovery of cellulose as a smart material. Macromolecules 39, 4202–4206 (2006)
Mussatto, S.I., Dragone, G., Guimarães, P.M., Silva, J.P., Carneiro, L.M., Roberto, I.C., Vicente, A., Domingues, L., Teixeira, J.A.: Technological trends, global market, and challenges of bio-ethanol production. Biotechnol. Adv. 28, 817–830 (2010)
Balat, M., Balat, H.: Recent trends in global production and utilization of bio-ethanol fuel. Appl. Energy 86, 2273–2282 (2009)
Kiran, B., Kumar, R., Deshmukh, D.: Perspectives of microalgal biofuels as a renewable source of energy. Energy Convers. Manag. 88, 1228–1244 (2014)
Haankuku, C., Epplin, F.M., Kakani, V.G.: Industrial sugar beets to biofuel: Field to fuel production system and cost estimates. Biomass Bioenergy 80, 267–277 (2015)
Yang, W., Meng, F., Peng, J., Han, P., Fang, F., Ma, L., Cao, B.: Isolation and identification of a cellulolytic bacterium from the Tibetan pig’s intestine and investigation of its cellulase production. Electronic J. Biotechnol. 17, 262–267 (2014)
Bhat, M.K.: Cellulases and related enzymes in biotechnology. Biotechnol. Adv. 18, 355–383 (2000)
Lamed, R., Setter, E., Bayer, E.A.: Characterization of a cellulose-binding, cellulase-containing complex in Clostridium thermocellum. J. Bacteriol. 156, 828–836 (1983)
Subramaniyan, S., Prema, P.: Cellulase-free xylanases from Bacillus and other microorganisms. FEMS Microbiol. Lett. 183, 1–7 (2000)
Pérez, J., Muñoz-Dorado, J., De La Rubia, T., Martínez, J.: Biodegradation and biological treatments of cellulose, hemicellulose and lignin: an overview. Int. Microbiol. 5, 53–63 (2002)
Maki, M.L., Broere, M., Leung, K.T., Qin, W.: Characterization of some efficient cellulase producing bacteria isolated from paper mill sludges and organic fertilizers. Int. J. Biochem. Mol. Biol. 2, 146–154 (2011)
Fontes, C.M.G.A., Gilbert, H.J.: Cellulosomes: highly efficient nanomachines designed to deconstruct plant cell wall complex carbohydrates. Annu. Rev. Biochem. 79, 655–681 (2010)
Paudel, Y.P., Qin, W.: Characterization of novel cellulase-producing bacteria isolated from rotting wood samples. App. Biochem. Biotechnol. 177, 1186–1198 (2015)
Rahman, M.S., Yuan, Z., Ma, K., Xu, C., Qin, W.: Aerobic conversion of glycerol to 2,3-butanediol by a novel Klebsiella variicola SRP3 strain. J. Microb. Biochem. Technol. 7, 299–304 (2015)
Maki, M., Iskhakova, S., Zhang, T., Qin, W.: Bacterial consortia constructed for the decomposition of Agave biomass. Bioengineerd 5, 165–172 (2014)
Laemmli, U.K.: Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227, 680–685 (1970)
O’Farrell, P.H.: High resolution two-dimensional electrophoresis of proteins. J. Biol. Chem. 250, 4007–4021 (1975)
Maki, M.L., Idrees, A., Leung, K.T., Qin, W.: Newly isolated and characterized bacteria with great application potential for decomposition of lignocellulosic biomass. J. Mol. Microbiol. Biotechnol. 22, 156–166 (2012)
Chellapandi, P., Jani, H.M.: Production of endoglucanase by the native strains of Streptomyces isolates in submerged fermentation. Braz. J. Microbiol. 39, 122–127 (2008)
Asha, B.M., Revathi, M., Yadav, A., Sakthivel, N.: Purification and characterization of a thermophilic cellulase from a novel cellulolytic strain Paenibacillus barcinonensis. J. Microbiol. Biotechnol. 22, 1501–1509 (2012)
Ariffin, H., Hassan, M.A., Shah, U.K., Abdullah, N., Ghazali, F.M., Shirai, Y.: Production of bacterial endoglucanase from pretreated oil palm empty fruit bunch by Bacillus pumilus EB3. J. Biosci. Bioeng. 106, 231–236 (2008)
Kim, B.K., Lee, B.H., Lee, Y.J., Jim, I.H., Chung, C.H., Lee, J.W.: Purification and characterization of carboxymethylcellulase isolated from a marine bacterium, Bacillus subtilis subsp. subtilis A-53. Enzyme Microbial. Technol. 44, 411–416 (2009)
Lee, Y.J., Kim, B.K., Lee, B.H., Jo, K.I., Lee, N.K., Chung, C.H., Lee, Y.C., Le, J.W.: Purification and characterization of cellulase produced by Bacillus amyoliquefaciens DL-3 utilizing rice hull. Bioresour. Technol. 99, 378–386 (2008)
Berrios, J., Altamirano, C., Osses, N., Gonzalez, R.: Continuous CHO cell cultures with improved recombinant protein productivity by using mannose as carbon source: metabolic analysis and scale-up simulation. Chem. Eng. Sci. 66, 2431–2439 (2011)
Bajaj, B.K., Pangotra, H., Wani, M.A., Sharma, P., Sharma, A.: Partial purification and characterization of a highly thermostable and pH stable endoglucanase from a newly isolated Bacillus strain M-9. Indian J. Chem. Technol. 16, 382–387 (2009)
Sheng, P., Huang, S., Wang, Q., Wang, A., Zhang, H.: Isolation, screening, and optimization of the fermentation conditions of highly cellulolytic bacteria from the hindgut of Holotrichia parallela larvae (Coleoptera: Scarabaeidae). App. Biochem. Biotechnol. 167, 270–284 (2012)
Acharya, S., Chaudhary, A.: Effect of nutritional and environmental factors on cellulases activity by thermophilic bacteria isolated from hot spring. J. Sci. Ind. Res. 70, 142–148 (2011)
Kang, S.W., Park, Y.S., Lee, J.S., Hong, S.I., Kim, S.W.: Production of cellulases and hemicellulases by Aspergillus niger KK2 from lignocellulosic biomass. Bioresour. Technol. 91, 153–156 (2004)
Gao, J., Weng, H., Zhu, D., Yuan, M., Guan, F., Xi, Y.: Production and characterization of cellulolytic enzymes from the thermoacidophilic fungal Aspergillus terreus M11 under solid-state cultivation of corn stover. Bioresou. Technol. 99, 7623–7629 (2008)
Fitzpatrick, S.W.: The Biofine Technology: A “Bio-Refinery” Concept Based on Thermochemical Conversion of Cellulosic Biomass. Feedstocks for the Future, pp. 271–287 (2006)
Lin, C., Shen, Z., Qin, W.: Characterization of xylanase and cellulase produced by a newly isolated Aspergillus fumigatus N2 and its efficient saccharification of barley straw. Appl. Biochem. Biotechnol. 182, 559–569 (2017)
Lin, H., Wang, Q., Shen, Q., Ma, J., Fu, J., Zhao, Y.: Engineering Aspergillus oryzae A-4 through the chromosomal insertion of foreign cellulase expression cassette to improve conversion of cellulosic biomass into lipids. PLoS ONE 9(9), e108442 (2014)
Acknowledgements
The research was supported by the Canada’s NSERC-RDF to WQ and Saudi Ministry of Higher Education Scholarship to IA.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests
The authors declare that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Almuharef, I., Rahman, M.S. & Qin, W. High Production of Cellulase by a Newly Isolated Strain Paenibacillus sp. IM7. Waste Biomass Valor 11, 6085–6094 (2020). https://doi.org/10.1007/s12649-019-00832-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-019-00832-5