Abstract
Lignocellulose is found in nature as an alternative source of energy and can be used for the production of bioethanol. Cellulose, one of the most abundant components of lignocellulose can be hydrolyzed using enzymes cellulase to produce glucose, which can be used for the production of ethanol. Cellulase production from cellulolytic bacteria is challenging. Hence, research has been focused on isolation and identification of efficient cellulolytic bacteria for their use in bioethanol production. In the present investigation, the most potent screened bacterial strain was subjected to optimization of its cellulase production by using response surface methodology taking four independent variables such as substrate concentration, pH, temperature, and incubation time. The optimization result showed that the bacteria had a maximum production of cellulase enzyme of 617.71 U/mL in an optimized condition at an incubation time of 42 h, pH 9, carboxy methyl cellulose (CMC) concentration of 15 gm/L, and temperature of 37.5 °C. The strain, SCB9, was identified as Bacillus albus based on the 16S rRNA sequencing and phylogeny analysis. Overall results from this study indicate that the cellulolytic bacteria SCB9 is a potent candidate for cellulase production, which can be exploited for bioethanol production from lignocellulosic biomass through adaptation of further appropriate biotechnological approaches.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
- Lignocellulosic biomass
- Similipal biosphere reserve
- Cellulolytic bacteria
- Cellulase
- Response surface methodology
- Enzyme optimization
1 Introduction
Fossil fuel sources such as coal, oil, natural gas have contributed to the drastic increase in the level of greenhouse gases in the earth’s atmosphere resulting in the need for alternative energy sources that are environmentally friendly, renewable, and sustainable (Ballesteros et al. 2006). Biomass is readily accessible around the world as residual wastes and agriculture biomass can be used as eco-friendly, renewable, and sustainable energy sources. The most important and abundant renewable biomass resources include crop residues such as corn straw, wheat straw, and rice straw. China has abundant biomass resources, as it is one of the largest agriculture-based economics in the world. China produces approximately 216 million metric tons of corn straw per annum and more than half of that remains utilized. Corn straw contains nonedible plant material so-called lignocellulose and is mainly composed of cellulose, hemicelluloses, and lignin.
Bioethanol produced from lignocellulosic biomass promises to achieve a great emphasis; however, the process has several challenges and limitations such as biomass transport, biomass handling, and efficient pretreatment methods for total delignification of lignocellulosic and appropriate fermentative organism. Conventionally, bioethanol is produced from the processing of starch and sucrose-based feedstock, utilizing enzymatic liquefaction and saccharification, leading to the production of relatively clean glucose pool. However, the food and feed crops for energy production crisis have promoted the need for bioethanol production from sources other than the feedstocks with direct food and feed values.
In the current study, soil sample was collected from Similipal Biosphere Reserve (SBR). Similipal Biosphere Reserve located in the northern part of Odisha and spreading over an area of 5578 km2 is one of the prominent biological hotspots enriched in various floral, faunal, and microbial communities. The uneven geophysical condition of this biosphere reserve influences the diversity of floral and faunal distribution. Several studies in relation to flora and fauna diversity have been undertaken in this ecosystem. However, very less attempt has been made to assess the microbial diversity form this unique biosphere reserve. The soils of Similipal forests are associated with lignocellulosic waste materials and are good source of lignocellulolytic microorganisms. Hence, in the present study, an attempt has been made to isolate and identify some of the cellulose-degrading soil bacteria from this biosphere reserve and to assess the cellulose production ability for possible use in bioethanol production from lignocellulosic biomasses. There are attempts being made in the present study to isolate and screening of cellulose-degrading bacteria from the rhizospheric soil sample from SBR. Maximum cellulase enzyme production from the most potent bacterial isolates has also been conducted using various parameters such as temperature, pH, incubation time, and substrate concentration.
2 Materials and Methods
2.1 Collection of Soil Sample
The soil samples were collected from seven different locations of Sitakunda and Lulung regions of Similipal Biosphere Reserve.
2.2 Isolation of Cellulolytic Bacteria
Bacterial isolates were isolated by serial dilution method (Aneja 2007). 1 gm of each of the respective soil samples was homogenized in 9 mL of sterilized sterile distilled H2O taken in test tube and the suspension was then serially diluted up to 10–5 and 10–6, respectively.
2.3 Screening of Cellulolytic Bacteria
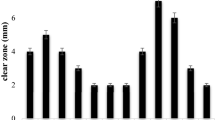
Each bacterial isolates were screened for cellulolytic activity using CMCase plate assay (Muhammad et al. 2012). 10 μL of each bacterial broth was inoculated inside 4 mm pore made on the CMC agar plate. The CMC agar plates containing bacteria inoculums were then incubated at 37 °C for 72 h. After incubation, CMC agar plates were stained by 0.1% Congo Red solution for 1 h followed by distain using 1 N NaCl solution for the determination of cellulose hydrolyzing zone surrounding the bacterial growth on CMC agar plate.
2.4 Quantitative Assay of Bacterial Cellulase Activity
Quantitative assay of cellulase activity was estimated using DNS method (Miller 1959) by measuring the amount of reducing sugar hydrolyzed by individual bacterial isolates. Each selected bacterial strain was cultured in nutrient broth medium for overnight incubation at 37 °C. Incubated bacterial broths were individually transferred to sterilized 1-mL Eppendorf tube. Each Eppendorf tube was centrifuged at 10,000 rpm for 10 min at 4 °C. Substrate solution was prepared by adding 1% of CMC with phosphate buffer (0.1 M) at pH 6.8. 0.5 mL of substrate solution and 0.5 mL of each bacterial supernatant (crude enzyme) were taken in assay tube to form 1 mL reaction mixture. Assay tubes containing the reaction mixture were then incubated at 55 °C for 15 min in water bath. After the incubation of reaction mixture, 1.5 mL of DNS solution was added in each assay tube and boiled at 100 °C for 10 min in water bath to stop the reaction. Amount of reducing sugar released in the hydrolysis by each bacterial supernatant was measured using spectrophotometer at an absorbance value of 540 nm. During the spectrophotometric analysis, 1.5 mL DNS and 1 mL ddH2O were taken as reference. 1 unit of cellulase activity was expressed as 1 μ/mol of glucose liberated per mL of enzyme per minute.
2.5 Optimization of Cellulase Enzyme
2.5.1 Experimental Design of Process Parameters for Cellulase Optimization Using RSM
For determining the optimum condition of cellulase activity, Box–Behnken designs (BBD) was used. Box–Behnken designs are used to generate higher order response surfaces using fewer required runs than a normal factorial technique. This technique essentially suppresses selected runs in an attempt to maintain the higher order surface definition. Using four independent variables and BBD approach, the combinatorial effects of the incubation time, pH, substrate concentration, and temperature were studied on cellulase enzyme production by the cellulolytic strain SCB9. Using Box–Behnken method, the first and higher order may be the effects of each factor and the interaction among them on cellulase activity was investigated. A total of 27 experimental conditions were designed using Design Expert 12 software optimization toolbox to perform response surface methodology (RSM). The minimum and maximum ranges of all the independent variables are given in Table 1.
Mathematical relationship of response (enzyme activity) and variables, i.e., A, B, C, and D was approximated by a quadratic polynomial model equation.
2.5.2 Preparation of Bacterial Growth Medium and Extraction of Crude Enzyme
Growth medium for bacterial isolates was prepared in 250-mL Erlenmeyer flask containing culture broth medium containing (gm/L) K2HPO4, 1.0, KCl, 0.2; MgSO4.7H2O, 1.0; yeast extract, 1.0; and pH adjusted to three different values (4.5, 6.75, and 15) with a range of substrate concentration of 2, 8.5, and 15 gm/L. All the flasks containing CMC culture broth were autoclaved at 121 °C for 15 min. After sterilization, the flasks were set to cool in room temperature and placed under laminar flow keeping the UV light on. After few minutes, each flask was inoculated with 1% standard inoculum (V/V) of the SCB9 cellulolytic bacterial strain. The inoculated flasks were then incubated on a rotary shaker (120 rpm) at specific temperatures (30, 37.5, and 45 °C) and for specific time (12, 42, and 72 h) as given in the experimental design from the RSM calculation.
2.5.3 Quantitative Estimation of Cellulase Production
After incubation, according to the design experimental conditions, 1 mL of bacterial from each flask was taken in the Eppendorf tube. In this study, cellulase activity was measured in triplicate by preparing three Eppendorf tubes containing bacterial cultures for each experimental condition. Each Eppendorf tube containing 1 mL bacterial culture broth was centrifuged at 10,000 rpm for 10 min at 4 °C. After centrifugation, the bacterial supplements were collected to perform the DNS assay for the estimation of cellulase activity (Miller 1959). The predicted value of cellulase activity obtained from each experimental condition was then used to generate three-dimensional contour plots, regression analysis, and analysis of variance (ANOVA).
2.5.4 Statistical Analysis and Analysis of Variance (ANOVA)
Design Expert 12 (Stat Ease, Inc., Minneapolis, USA) was used for regression analysis of the experimental data to obtain working parameters and to generate polynomials and the surface contour plots. A second-order polynomial equation was established based on the analysis of variance, and the optimum ratio of experimental variable was found using the same software. Standard deviation, p value, F value, and R2 value were also analyzed.
2.5.5 Molecular Identification and Phylogeny Analysis
The most potent cellulolytic bacterial strain was identified using 16S rRNA sequencing. The partial contig sequence obtained was further analyzed through phylogeny tree in MEGA 7 (Kumar et al. 2016).
2.5.6 Model Validation
The mathematical generated during RSM implementation was validated by conducting checkpoint studies. Experimentally obtained data were compared with the predicted one and the prediction error was calculated.
2.5.7 Molecular Identification of Cellulolytic Bacteria by 16S rRNA Gene Sequencing
Molecular identification of the bacterial strain SCB9 was performed using 16S rRNA gene sequencing of the DNA isolated from the bacterium. For the DNA extraction purpose, 1.5 mL of bacterial culture was centrifuged at 10,000 rpm to obtain bacterial cell pellets. The 16S rRNA gene was amplified using Applied Biosystem Verti Thermal Cycler. The primers for amplification were provided by Sigma-Aldrich. Universal primers, 27 forward (5AGAGTTTCCTGGCTCAG3) and 1492 reverse (5ACGGCTACCTTGTTACGATT3), were used for gene amplification. The presence of amplified gene products was determined by 2.5% agarose gel electrophoresis, and to analyze the size of amplified gene product, DNA markers of 100 bp were used that were provided by the Puregene. Amplified 16S rRNA gene was purified using a Gel DNA extraction kit (Qiagen, Seoul, South Korea) and sent for sequencing to Applied Bioscience Eurofins, Bangalore.
3 Results and Discussion
3.1 Quantitative Assay of Bacterial Cellulase Activity
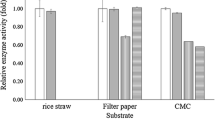
In accordance with the cellulose hydrolyzing plate assay, bacterial isolates SCB7, SCB9, and SCB24 showed the highest cellulose-hydrolyzing activity as measured in DNS method. Obtained spectrometric absorbance values of the three bacterial isolates implied maximum amount of reducing sugar after the catalytic activity of cellulase on the substrate carboxy methyl cellulose (Fig. 1). Lynch et al. (1981) determined the cellulase activities of some aerobic microorganisms isolated from soil. Cellulose extracted from wheat straw, avicel, and CF11 cellulose powder contained 90% glucose, whereas filter paper and carboxy methyl cellulose contained 18% and 28% hemicelluloses, respectively. Among the microorganisms isolated, only fungi had cellulase activities and this activity was the greatest cultures of Cladosporium cladosporoides. Therefore, the current study is in well accordance with the above study conducted by Lynch et al. (1981) and it can be implied that bacterial strains with higher cellulolytic efficiency are abundant in the soil sample.
3.2 Optimization of Cellulase Enzyme from SCB9 Strain
3.2.1 Optimization of Physical Factors by Response Surface Methodology
From the RSM study, a highest cellulase activity for the cellulolytic bacterial strain SCB9 in an optimized condition when the independent variables were maintained at incubation time 42 h, pH 9, substrate concentration 15 gm/L and temperature 37.5 °C (Fig. 2).
3.2.2 Regression Analysis
Regression analysis was carried out to fit the mathematical model to the experimental data in order to determine the optimum conditions that result in the maximum enzyme activity. By applying multiple regression analyses, on the experimental data, a second-order polynomial equation was found to represent the relationship between enzyme activity, incubation time, pH, substrate concentration, and temperature adequately. This second-order polynomial equation obtained from multiple regression analysis is shown to explain cellulase activity of the cellulolytic bacterial strain SCB9 regardless of the significance of the coefficients. If Y is the response value of cellulase activity (Fig. 2), then the fitted response surface model is:
3.2.3 Statistical Analysis
It is evident from the obtained statistical analysis that the cellulase activity model of SCB9 strain was highly significant, as suggested by the model F-value and low probability value. The model F-value of 230.17 as calculated from this system implies that the model is significant. There is only a 0.01% chance that an F-value larger than this could occur due to noise. The lack of fit F-value of 0.3782 implies that the lack of fit is not significant relative to the pure error. There is an 88.03% chance that a lack of fit F-value larger than this could occur due to noise. This nonsignificant lack of fit as represented from the ANOVA table evidences the optimization of model system. The coefficient of determination (R2) was calculated as 0.9963 for enhanced cellulase activity, indicating that the statistical model can explain adequate variability in the response. Similarly, Doddapaneni et al. (2007) suggested that closer the value of R2 to 1.0, stronger the model and better it predicts the response of tested enzyme activity according to the designed experimental conditions. The predicted R2 value of 0.9831 was in reasonable agreement with the adjusted R2 value of 0.9920. This indicated a good agreement between the experimental and predicted values for cellulase activity. If there are many terms in the model and the sample size is not very large, the adjusted R2 value may be noticeably smaller than the R2. In the present study, in concur with the above statement, adjusted R2 0.9920 is also less than the R2 value 0.9963.
Saravanan et al. (2012) studied the optimization of cellulase production using Tricoderma reesei by RSM and comparison with genetic algorithm. The potential of Trichoderma reesei for cellulase production using pineapple waste as substrate has been investigated. A maximum cellulase activity of 569.23 U/mL is obtained under the optimum experimental conditions; pH 5.5, temperature 37 °C, initial substrate concentration 3%, inoculum concentration, and culture time. Box–Behnken design (BBD) statistical tool and genetic algorithm are used to optimize the process parameters. The BBD study of linear and quadratic interactive effects of experimental variables on the desired response of cellulase activity showed that the second-order polynomial is significant. Therefore, it can be depicted from the optimization of cellulase activity that both the current study and the study undertaken by Saravanan et al. (2012) that BBD is a suitable technique and it properly determines as well as validate the optimum condition for maximum enzyme activity.
3.3 Identification of Cellulolytic Bacteria
The most potent cellulolytic strain SCB9 was identified as Bacillus albus by its 16S rRNA sequencing. Shajahan et al. (2016), in their study, identified high cellulose-producing thermophilic bacteria as Bacillus licheniformes. Therefore, the current study and the study of Shajahan et al. (2016) implied that the bacterial strains from the genera Bacillus are common with high cellulolytic activity and this bacterial class is diversified according to their habitat.
4 Conclusion
Cellulases are the most important enzymes that are responsible for the bioethanol production. It is one of the most widely used enzymes having great industrial application. Microorganisms are main source of cellulase for which research has been focused on isolation and identification of potent cellulose-producing bacterial to meet the growing demand for cellulases. In Similipal Biosphere Reserve, soil-decomposed plant biomasses are rich in lignocellulolytic bacteria. In this study, 24 cellulolytic bacteria were isolated from the Similipal soil samples that were screened for their cellulose-hydrolyzing capacity and cellulase activity. The screening of cellulase activity revealed that SCB9 strain has the highest turnover of glucose production by degrading CMC with a value of 73.94 U/mL in unoptimized condition. When subjected to optimization of parameters through RSM, the enzyme production showed (617.71 U/mL) cellulase activity over the unoptimized conditions with 8.35-fold increase. The present study reveals the potential for high cellulase production by the identified strain Bacillus albus. Further studies with regard to enzyme characterization and the potential of the enzyme in bioethanol production need to be evaluated.
References
Aneja KR (2007) Experiments in microbiology, plant pathology and biotechnology new age international 1-602
Ballesteros I, Negro MJ, Oliva JM, Cabañas A, Manzanares P, Ballesteros M (2006) Ethanol production from steam-explosion pretreated wheat straw. Appl Biochem Biotechnol Spring 129–132:496–508
Doddapaneni KK, Tatineni R, Potumarthi R, Mangamoori LN (2007) Optimization of media constituents through response surface methodology for improved production of alkaline proteases by Serratia rubidea. J Chem Technol Biotechnol 82(8):721–729
Kumar S, Stecher G, Tamura K (2016) MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol 33(7):1870–1874
Lynch JM, Slater JH, Jacqueline A, Bennett SH (1981) Cellulase activities of some aerobic microorganisms isolated from soil. J Gen Microbiol 127:231–236
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31(3):538–542
Muhammad I, Asma S, Quratulain S, Muhammad N (2012) Isolation and screening of cellulolytic bacteria from soil and optimization of cellulase production and activity. Tur J Biochem 37(3):287–293
Saravanan P, Muthuvelayudham R, Kannan RR, Viruthagiri T (2012) Optimization of cellulase production using Trichoderma reesei by RSM and comparison with genetic algorithm. Front Chem Sci Eng 6:443–452
Shajahan S, Muthu GMI, Natesan S, Gopal S (2016) Statistical modeling and optimization of cellulase production by Bacillus licheniformis NCIM 5556 isolated from the hot spring, Maharashtra, India. J King Saud Univ Sci 29
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
This chapter does not contain any studies with human participants or animals performed by any of the authors. Collection of soil sample from the buffer areas of Similipal Biosphere Reserve, Odisha with a condition that no plants and animals would be disturbed and following all the provision of Biodiversity Act has been permitted by Odisha Forest and Environment Department, Government of Odisha.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Singapore Pte Ltd.
About this paper
Cite this paper
Paul, M., Meher, S.R., Giri, S., Thatoi, H. (2021). Isolation, Screening, and Evaluation of Cellulase-Producing Bacteria from the Soil of Similipal Biosphere Reserve for Biofuel Production from Lignocellulosic Biomass. In: Ramkrishna, D., Sengupta, S., Dey Bandyopadhyay, S., Ghosh, A. (eds) Advances in Bioprocess Engineering and Technology . Lecture Notes in Bioengineering. Springer, Singapore. https://doi.org/10.1007/978-981-15-7409-2_46
Download citation
DOI: https://doi.org/10.1007/978-981-15-7409-2_46
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-15-7408-5
Online ISBN: 978-981-15-7409-2
eBook Packages: EngineeringEngineering (R0)