Abstract
A series of hydroxylated mesoporous aluminum–boron catalysts with different Al/B molar ratios were synthesized through a sol–gel method, and were characterized by XRD, FT-IR, NH3-TPD, SEM and N2 adsorption–desorption techniques. It was demonstrated that hydroxylated Al2B3 containing more Lewis acidity showed superior activity to the other Al–B catalysts in dehydration of glucose to 5-hydroxymethylfurfural (HMF) with a high yield of up to 39.9 % and glucose conversion of 92.1 % at 140 °C for 2 h. The synergistic effect of Lewis and Bronsted acid sites in Al2B3 catalyst was demonstrated to be crucial for efficient conversion of glucose to HMF.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
5-Hydroxyfurfural (HMF), recognized to be one of the top ten biomass-derived chemicals by the Department of Energy (U.S.), has been widely applied as a valuable platform molecule in production of biofuels and fine chemicals [1–8]. A large quantity of renewable biomass derivatives (e.g., glucose, sucrose, fructose and inulin) can be used to produce HMF. Relatively high HMF yields have been achieved from fructose promoted by a series of acidic catalysts [9]. From the economic point of view, glucose is more readily available from nature and shows great potential in large-scale production of value-added chemicals [10, 11]. Therefore, increasing efforts are being made for the upgradation of glucose.
A variety of homogeneous and heterogeneous catalysts such as Hf(OTf)4 [12], metal phosphates [13], SO3H-functional ionic liquids [14, 15], MCM-41 silica [16], hydroxylated AlF3 [17], sulfated mesoporous carbon [18], Sn-based catalyst [19–21], and graphene oxide-ferric oxide [22] were designed for dehydration of glucose to HMF. Although both heterogeneous and homogeneous acids can efficiently catalyze glucose being converted to HMF, homogeneous catalysts always contain some unavoidable drawbacks such as difficulty in separation, equipment corrosion and environmental pollution [23]. Generally speaking, heterogeneous catalytic materials are able to overcome these shortcomings from homogeneous acids. In general, the procedure for glucose degradation to HMF involves two catalytic steps involving Lewis acid-catalyzed isomerisation of glucose to fructose, and subsequent dehydration over Brønsted acid to produce HMF [24, 25].
The combination of aluminum with boron species was demonstrated to be capable of creating mesoporous architecture and additional acid sites. Importantly, the resulting mixed oxides were effective for various reactions such as methanol dehydration, Beckman rearrangement, oxidation, and esterification [25–33]. In the present study, a series of hydroxylated mesoporous Al–B catalysts with different molar ratios of B/Al were prepared from aluminium isopropoxide and phenylboronic acid by a sol–gel method, which were testified to have large pore volume, and remarkably high surface area and enhanced Lewis acidity. The as-prepared catalysts were further used to produce HMF from carbohydrates especially glucose, wherein several important reaction parameters including molar ratio of Al/B, reaction time and temperature, catalyst dosage, and type of solvents were studied to optimize HMF production.
Experimental Section
Material
Glucose (>99 %) was purchased from Sigma–Aldrich Corporation, F68 [HO(C2H4O) m (C3H6O) n H] and aluminum isopropoxide (>99.5 %) were bought from Zhejiang Maya reagent Company. Other reagents procured from Shanghai Aladdin Industrial Corporation were of analytical grade without any purification, unless otherwise noted.
Catalyst Preparation
A series of hydroxylated aluminum–boron catalysts with different B/Al molar ratios were synthesized via a sol–gel process. In a typical procedure, Pluronic F68 (1.0 g) was added into a mixture containing absolute ethanol (20 g), and kept stirring at 40 °C for 1 h. Then, a certain amount of aluminium isopropoxide (1.02 g) was added. After stirring for 4 h, phenylboronic acid (0.91 g) was slowly dropped into the above mixture. Upon completion, the resulting solution was further stirred for another 1 h. The catalyst (Al2B3) could be obtained through ultrasound treatment for 40 min, drying in vacuum at 40 °C for 3 days, 80 °C for another 3 days, and then calcined at 400 °C for 6 h. Other boron–aluminum catalysts (Al x B y : x & y denote the mole of B and Al, respectively) with molar ratios of 1/1, 2/1, 2/1, and 2/3 were also prepared through the identical method. For comparison, P-Al (Al2P3) and P-Zr (Zr2P3) catalysts were synthesized, according to previously reported procedures [28].
Catalyst Characterization
NH3-TPD measurements of those prepared catalysts were conducted using an AutoChem 2920 chemisorption analyzer. FT-IR spectra were recorded on an IR prestige-21 FT-IR instrument (KBr disks). Scanning electron microscopy (SEM) image was performed using a FESEM XL-30 (Philips) electron microscope. X-ray diffraction (XRD) measurements were carried out using a D/Max-3c X-ray diffractometer with Cu Ka (λ = 0.154 nm), scanning from 5° to 90° and using an operating voltage and current of 40 kV and 30 mA, respectively.
Catalytic Dehydration of Carbohydrates to HMF
All kinds of sugar conversion experiments were conducted in a pressure tube (15 mL) under magnetic stirring condition, unless otherwise mentioned. In a typical procedure, glucose (50 mg), catalyst (20 mg) and DMSO (1.0 g) were added into the pressure tube. The resulting mixture was stirred at 500–700 rpm and heated by a controllable oil bath for a specific time. The zero time of the reaction was recorded when the pressure tube was submerged into the oil bath. After filtration, the solution was decanted into volumetric flask (25 mL) using de-ionized water as diluent. The liquid products in the mixture were quantitatively analyzed by high performance liquid chromatography (HPLC).
Analytical Method
HMF yield and glucose conversion were analyzed by using HPLC (Agilent 1100, USA), which was fitted with a LiChrospher C18 column and an ultraviolet detector at 284 nm, and equipped with an Aminex HPX-87H column (Bio-Rad, Richmond, CA) and a refractive index (RI) detector. The column oven temperature was set at 65 °C, while the mobile phase was CH3CN/H2O (V/V = 80/20) at a flow rate of 1.0 mL/min. The molar concentrations of glucose and HMF were verified according to the external standard method. Glucose conversion rate (X, mol%) and HMF yield (Y, mol%) were calculated as follows:
Result and Discussion
Characterization of Catalyst
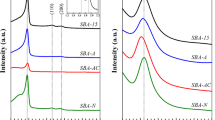
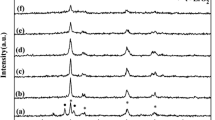
XRD patterns show that all the Al–B catalysts consistently have a low crystallinity, as illustrated in Fig. 1. One broad band located at 16°–27° proved the existence of the boron component [33], while the band at 45° is roughly attributed to aluminium species [33, 34]. Those results indicated the presence of aluminum and boron components in the as-prepared catalysts. The structure of Al2B3 was further examined by FT-IR spectrum (Fig. S1). A big peak in range of 3000–3500 cm−1 possibly belonged to hydroxyl group and water. The peak in the region of 1250–1450 cm−1 was characteristic of Al–O–B bond, while bands in the region of 900–1100 and 580–680 cm−1 were assigned to boron oxide and aluminum oxide, respectively [32]. Those results demonstrated that the Al–B mixed oxides were synthesized successfully.
The texture properties of the aluminum–boron catalysts were examined by N2 adsorption–desorption and BJH pore-size distribution (Fig. 2). It can be seen that the N2 adsorption–desorption isotherms of all aluminum–boron catalysts show a typical type IV patterns and the resulting hysteresis loops are assigned to H3-type, indicating the existence of mesopores structure in the as-prepared catalysts. In addition, the Al2B3 catalyst (Fig. 2) had broader pore size distribution and pore diameter, compared to another four catalysts, which may be responsible for its high catalytic performance. Furthermore, SEM image illustrates that the appearance of Al2B3 catalyst is fluffy (Fig. S2), further confirming the presence of porous structue, which is consistent with the results of N2 adsorption–desorption (Fig. 2).
The acid properties of Al2B3 catalyst were evaluated by NH3-TPD and pyridine-adsorbed FT-IR (Figs. S2 & 3). As shown in NH3-TPD, two bands at 250 and 550 °C strongly illustrate that the acid centres of the Al2B3 catalyst are assigned to medium-strong and strong acid sites, respectively (Fig. S3). Two sharp absorption bands at around 1448 and 1622 cm−1 can be obviously observed from the pyridine-adsorbed FT-IR spectrum of Al2B3 (Fig. 3), which are characteristic of Lewis acid [35]. Brønsted acid sites are also existent, on the basis of the absorption peak at 1590 cm−1 [36]. Moreover, the peak at 1489 cm−1 can be attributed to pyridine-adsorbed Brønsted and Lewis acid sites [37], which clearly proves that the as-prepared Al2B3 catalyst containing both Brønsted acid and Lewis acid sites.
Effect of Catalyst Calcination Temperature on Production of HMF from Glucose
The effect of calcination temperature (400–700 °C) for Al2B3 with a constant calcination time of 6 h on conversion of glucose to HMF was initially investigated. As shown in Fig. 4, the catalyst calcined at 400 °C displays a relatively higher HMF yield (39.9 %), but further increasing calcination temperature from 400 to 700 °C leads to the gradual decrease of HMF yield (from 39.9 to 24.5 %). It’s implied that the Al–B mixed oxide begins to cluster together at a high temperature of >400 °C, thus resulting in the decrease of its activity [38]. Hence, the Al2B3 catalyst used in this work was prepared by calcining at 400 °C for 6 h.
Effect of Reaction Temperature and Time
Figure 5 indicates that the reaction temperature obviously affects HMF production from glucose over Al2B3. With the increase of reaction time from 1 to 8 h, the yield of HMF raised from 0 to 42 % at 120 °C. On the other hand, as the reaction temperature was further elevated to 140 °C, the HMF yield increased to 39.9 % in only 2 h. At a high temperature of 160 °C, HMF yield could reach 36.9 % within 1 h, but was decreased after reacting for 2 h. It was proposed that relatively high temperature and long reaction time caused the degradation of HMF to form by-products such as levulinic acid [30]. Therefore, the optimal reaction temperature (140 °C) and time (2 h) with the presence of Al2B3 were utilized for subsequent studies.
Effect of the Molar Ratio of B/Al
Figure 6 displays the influence of the molar ratio of B/Al (i.e., 1:1, 1:2, 2:1, 2:3 and 3:2) on synthesis of HMF from glucose. The optimum molar ratio of B/Al for glucose dehydration was found to be 3:2, and a relatively high HMF yield of 39.9 % could be achieved at 140 °C for 2 h. The relatively larger pose size of Al2B3 (Fig. 2) was demonstrated to facilitate the access of substrate to active sites [39], which could be partially resposible for its superior activity to other Al–B catalysts. To examine the effect of acidity on glucose-to-HMF conversion in DMSO, the catalytic performance of Al2P3, Zr2P3 and Al2B3 was also investigated. It was clear to see that the catalytic activity of Al2P3 and Zr2P3 was inferior to that of Al2B3 (Table S1), demonstrating that the higher acid strength and density of Al2B3 led to its enhanced efficiency in catalyzing conversion of glucose to HMF (Fig. S3).
Effect of Catalyst Dosage
Figure 7 shows the influence of catalyst amount (i.e., 0, 10, 20, 30, 40 and 50 mg) on converting glucose to HMF. It’s found that only 8 % HMF yield with 47 % glucose conversion was obtained in DMSO with the absence of any catalyst. When 10 mg Al2B3 was used to catalyze conversion of glucose, 39.5 % yield of HMF was obtained at 140 °C for 2 h. Further increasing the catalyst amount to 20 mg afforded a slightly increased HMF yield (39.9 %). However, the HMF yield did not continue to rise but decline by 4 % when 30 mg Al2B3 was employed. Excess amount of catalyst was likely to cause catalyst aggregation to hinder the mass transfer, thus decreasing the activity of the Al2B3 catalyst. Therefore, 20 mg catalyst was chosen as the best dosage.
Catalytic Degradation of Glucose to HMF in Various Solvents
The type of solvents was reported to have significant effect on producing HMF from sugars. In this regard, dimethyl formamide (DMF), dimethyl sulfone (DMSO), 1-ethyl-3-methyl imidazolium chloride ([EMIM][Cl]), water/[EMIM][Cl], methyl isobutyl ketone (MIBK)/water, and DMSO/acetonitrile (MeCN) were used to investigate the effect of solvent on HMF production from glucose. As shown in Fig. 8, DSMO exhibits the superior activity, and 39.9 % yield of HMF can be obtained. The unique function of DMSO on preventing the formation of byproducts (e.g., levulinic acid and humins) in the reaction systems is helpful to improve the yields of HMF, illustrating that DMSO can be chosen as the optimum solvent.
Synthesis of HMF from Various Sugars with Al2B3
To expand the substrate scope, other biomass-derived sugars including fructose, inulin, sucrose and cellobiose were also applied as feedstock to produce HMF in the presence of Al2B3 (Fig. 9). Relatively high HMF yields of 45.5, 40.2, 50.1 and 36.4 % could be achieved from fructose, inulin, sucrose and cellobiose, respectively. Notably, a little higher HMF yield was obtained from sucrose than that from fructose. It’s proposed that humins are much easier to be formed from fructose than sucrose during the reactions [40]. These data indicated that the Al–B catalyst containing Lewis and Brønsted acid sites was more helpful for converting sugars that contain glucose units to HMF.
Recycling Experiment
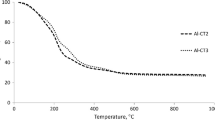
After each cycle of reactions, the used catalyst was separated from the reaction mixture by centrifugation, successively washing with water, acetone and ethanol for three times, and drying at 80 °C overnight. The recovered catalyst was used for the next cycle in producing HMF from glucose. Figure 10 demonstrates that the catalyst can be reused for at least 5 times with only slight decrease in catalytic activity. No obvious changes in the structure and acid density (0.8 vs. 0.6 mmol/g) of the fresh and recovered (after five cycles) Al2B3 catalysts are observed, as illustrated by IR spectra and NH3-TPD patterns (Fig. 11), which clearly indicate the good stability of Al2B3 in the reations. Two possible reasons are speculated to be responsible for the decreased catalytic activity of Al2B3 after five consecutive recycles: (1) the active sites of the catalyst are hindered by humins, and (2) part of active sites in the catalyst may be lost during filtration [17, 22, 32].
Conclusions
In this study, a series of aluminum–boron catalysts with different molar ratios and Bronsted-Lewis acid sites were prepared and employed for converting glucose to HMF in DMSO under mild reaction conditions. A relatively high HMF yield of 39.9 % with glucose conversion of 92.1 % was obtained over Al2B3 at 140 °C for 2 h. Moreover, the catalyst was stable and could be recycled for at least five times without obvious loss in activity.
References
Li, H., Zhang, Q.Y., Liu, X.F., Chang, F., Hu, D.Y., Zhang, Y.P., Xue, W., Yang, S.: InCl3-ionic liquid catalytic system for efficient and selective conversion of cellulose into 5-hydroxymethylfurfural. RSC Adv. 3, 3648 (2013)
Zhou, J., Tang, Z., Jiang, X., Jiang, R., Shao, J., Han, F., Xu, Q.: Catalytic conversion of glucose into 5-hydroxymethyl-furfural over chromium-exchanged bentonite in ionic liquid-dimethyl sulfoxide mixtures. Waste Biomass Valorif. 1–12 (2016). doi:10.1007/s12649-016-9525-1
Tai, Z., Lee, A.F., Wilson, K.: Progress in the development of mesoporous solid acid and base catalysts for converting carbohydrates into platform chemicals. In: Schlaf, M. (ed.) Reaction Pathways and Mechanisms in Thermocatalytic Biomass Conversion I, pp. 123–169. Springer, Singapore (2016)
Alonso, D.M., Bond, J.Q., Dumesic, J.A.: Catalytic conversion of biomass to biofuels. Green Chem. 12, 1493–1513 (2010)
Werpy, T., Petersen, G., Aden, A., Bozell, J., Holladay, J., White, J., Jones, S.: Top value added chemicals from biomass. Volume 1-results of screening for potential candidates from sugars and synthesis gas (No. DOE/GO–102004–1992). Department of energy, Washington, DC (2004)
Nie, G., Tong, X., Zhang, Y., Xue, S.: Efficient production of 5-hydroxymethylfurfural (HMF) from d-fructose and inulin with graphite derivatives as the catalysts. Catal. Lett. 144, 1759–1765 (2014)
Tong, X., Wang, Y., Nie, G., Yan, Y.: Selective dehydration of fructose and sucrose to 5-hydroxymethyl-2-furfural with heterogeneous Ge (IV) catalysts. Environ. Prog. Sustain. Energ. 34, 207–210 (2015)
Tong, X., Yu, L., Nie, G., Li, Z., Liu, J., Xue, S.: Antimony-meditated efficient conversion of carbohydrates to 5-hydroxymethylfurfural in a simple THF–H2O binary solvent. Environ. Prog. Sustain. Energ. 34, 1136–1141 (2015)
Li, H., Fang, Z., Smith, R.L., Yang, S.: Efficient valorization of biomass to biofuels with bifunctional solid catalytic materials. Prog. Energ. Combust. 55, 98–194 (2016)
Torres, A.I., Tsapatsis, M., Daoutidis, P.: Biomass to chemicals: design of an extractive-reaction process for the production of 5-hydroxymethylfurfural. Comput. Chem. Eng. 42, 130–137 (2012)
Tong, X., Ma, Y., Li, Y.: Biomass into chemicals: Conversion of sugars to furan derivatives by catalytic processes. Appl. Catal. A 38, 1–13 (2010)
Li, J.J., Ma, Y.B., Wang, L., Song, Z., Li, H.P., Wang, T.F., Li, H.Y., Eli, W.J.: Catalytic conversion of glucose into 5-hydroxymethylfurfural by Hf(OTf)4 Lewis acid in water. Catalysts 6, 1 (2016)
Dibenedetto, A., Aresta, M., Bitonto, L.B., Pastore, C.: Organic carbonates: efficient extraction solvents for the synthesis of HMF in aqueous media with cerium phosphates as catalysts. ChemSusChem 9, 118–125 (2016)
Li, J.J., Li, J.H., Zhang, D.J., Liu, C.B.: Theoretical elucidation of glucose dehydration to 5-hydroxymethylfurfural catalyzed by a SO3H-functionalized ionic liquid. J. Phys. Chem. B 119, 13398–13406 (2015)
Li, H., Zhang, Q.Y., Liu, X.F., Chang, F., Zhang, Y.P., Xue, W., Yang, S.: Immobilizing Cr3+ with SO3H-functionalized solid polymeric ionic liquids as efficient and reusable catalysts for selective transformation of carbohydrates into 5-hydroxymethylfurfural. Bioresour. Technol. 144, 21–27 (2013)
Jiménez-Morales, J., Moreno-Recio, M., Santamaría-González, J., Maireles-Torres, P., Jiménez-López, A.: Production of 5-hydroxymethylfurfural from glucose using aluminium doped MCM-41 silica as acid catalyst. Appl. Catal. B 164, 70–76 (2015)
Lu, Y.M., Li, H., He, J., Liu, Y.X., Wu, Z.B., Hu, D.Y., Yang, S.: Efficient conversion of glucose to 5-hydroxymethylfurfural by using bifunctional partially hydroxylated AlF3. RSC Adv. 6, 12782–12787 (2016)
Thombal, R.S., Jadhav, V.H.: Biomass derived β-cyclodextrin-SO3H carbonaceous solid acid catalyst for catalytic conversion of carbohydrates to 5-hydroxymethylfurfural. Appl. Catal. A 499, 213–216 (2015)
Li, L., Ding, J.H., Jiang, J.G., Zhu, Z.G., Wu, P.: One-pot synthesis of 5-hydroxymethylfurfural from glucose using bifunctional [Sn, Al]-beta catalysts. Chin. J. Catal. 36, 820–828 (2015)
Tian, G., Tong, X., Cheng, Y., Xue, S.: Tin-catalyzed efficient conversion of carbohydrates for the production of 5-hydroxymethylfurfural in the presence of quaternary ammonium salts. Carbohydr. Res. 370, 33–37 (2013)
Wang, Y., Tong, X., Yan, Y., Xue, S., Zhang, Y.: Efficient and selective conversion of hexose to 5-hydroxymethylfurfural with tin–zirconium-containing heterogeneous catalysts. Catal. Commun. 50, 38–43 (2014)
Zhang, M.L., Su, K.M., Song, H.M., Li, Z.H., Cheng, B.W.: The excellent performance of amorphous Cr2O3, SnO2, SrO and grapheme oxide–ferric oxide in glucose conversion into 5-HMF. Catal. Commun. 69, 76–80 (2015)
Wang, Y.H., Tong, X.L., Yan, Y.T., Xue, S., Zhang, Y.Y.: Efficient and selective conversion of hexose to 5-hydroxymethylfurfural with tin–zirconium-containing heterogeneous catalysts. Catal. Commun. 50, 38–43 (2014)
Li, H., Zhang, Q.Y., Bhadury, P.S., Yang, S.: Furan-type compounds from carbohydrates via heterogeneous. Curr. Org. Chem. 18, 547–597 (2014)
Sato, S., Hasebe, S., Sakurai, H., Urabe, K., Izumi, Y.: Vapor-phase Beckmann rearrangement over alumina-supported boria catalyst prepared by vapor decomposition method. Appl. Catal. 29, 107–115 (1987)
Sato, S., Kuroki, M., Sodesawa, T., Nozaki, F., Maciel, G.E.: Surface structure and acidity of alumina-boria catalysts. J. Mol. Catal. A 104, 171–177 (1995)
Cucinieri Colorio, G., Auroux, A., Bonnetot, B.: Acidty and surface behavior of alumina-boria catalysts studied by adsorption microcalorimetry of probe molecules. J. Therm. Anal. Calorim. 40, 1267–1276 (1993)
Xiu, T.P., Wang, J.C., Liu, Q.: Ordered bimodal mesoporous boria-alumina composite: one-step synthesis, structural characterization, active catalysis for methanol dehydration. Microporous Mesoporous Mater. 143, 362–367 (2011)
Myers, T.W., Berben, L.A.: Aluminium–ligand cooperation promotes selective dehydrogenation of formic acid to H2 and CO2. Chem. Sci. 7, 2771–2777 (2014)
Márquez-Alvarez, C., Žilková, N., Pérez-Pariente, J., Čejka, J.: Synthesis, characterization and catalytic applications of organized mesoporous aluminas. Catal. Rev. 50, 222–286 (2008)
Kirszensztejn, P., Jurek, K., Tolińska, A., Kawałko, A.: Formation and characterization of a SnO2–Al2O3 system derived from a sol–gel process based on different tin precursors. J. Non-Cryst. Solids 357, 1671–1676 (2011)
Delmastro, A., Gozzelino, G., Mazza, D., Vallin, V.: Characterization of microporous amorphous alumina-boria. Chem. Soc. Faraday Trans. 88, 2065–2070 (1992)
Colorioa, G., Védrinea, J.C., Auroux, A., Bonnetot, B.: Partial oxidation of ethane over alumina-boria catalysts. Appl. Catal. A 137, 55–68 (1996)
Dumeignil, F., Rigole, M., Guelton, M., Grimblot, J.: characterization of boria-alumina mixed oxides prepared by a sol-gel method. 2. characterization of the calcined xerogels. Chem. Mater. 17, 2369–2377 (2005)
Carniti, P., Gervasini, A., Bossola, F., Dal Santo, V.: Cooperative action of Brønsted and Lewis acid sites of niobium phosphate catalysts for cellobiose conversion in water. Appl. Catal. B 193, 93–102 (2016)
Stošić, D., Bennici, S., Rakić, V., Auroux, A.: CeO2–Nb2O5 mixed oxide catalysts: Preparation, characterization and catalytic activity in fructose dehydration reaction. Catal. Today 192, 160–168 (2012)
Parry, E.P.: An infrared study of pyridine adsorbed on acidic solids. Characterization of surface acidity. J. Catal. 2, 371–379 (1963)
Ramasamy, K.K., Gray, M., Job, H., Santosa, D., Li, X.S., Devaraj, A., Wang, Y.: Role of calcination temperature on the hydrotalcite derived MgO–Al2O3 in converting ethanol to butanol. Top. Catal. 59, 46–54 (2016)
Liu, J., Li, H., Liu, Y.C., Lu, Y.M., He, J., Liu, X.F., Wu, Z.B., Yang, S.: Catalytic conversion of glucose to 5-hydroxymethylfurfural over nano-sized mesoporous Al2O3-B2O3 solid acids. Catal. Commun. 62, 19–23 (2015)
Ilgen, F., Ott, D., Kralisch, D., Reil, C., Palmberger, A., König, B.: Conversion of carbohydrates into 5-hydroxymethylfurfural in highly concentrated low melting mixtures. Green Chem. 11, 1948–1954 (2009)
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (21576059 & 21666008), Key Technologies R&D Program (2014BAD23B01), Research Project of Chinese Ministry of Education (213033A), International Science & Technology Cooperation Program of China (2010DFB60840), and Key S&T Projects of Guizhou Province ([2012]6012, [2011]3016 & [2008]70011).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhao, W., Yang, T., Li, H. et al. Efficient Production of 5-Hydroxymethylfurfural from Carbohydrates Catalyzed by Mesoporous Al–B Hybrids. Waste Biomass Valor 8, 1371–1378 (2017). https://doi.org/10.1007/s12649-016-9689-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-016-9689-8