Abstract
Combined pretreatment methods for improving sugar yield as well as the economic aspects of lignoethanol production processes have gained a great deal of interest. However, most investigations involving such methods have been conducted under optimum conditions. Such fully-optimized combinations although improve enzymatic digestion, they could also degrade cellulose structure resulting in reduced sugar yield in some cases. The present study was set to prepare rice straw for the 1-ethyl-3-methylimidazolium acetate pretreatment step (2 h, 120 °C), by mild alkaline extraction (30 min, 80 °C, 0.3 % NaOH) with a focus on only 30 % lignin removal to minimize the hemicelluloses degradation. Moreover, the effect of size reduction (<0.42 mm and <2 cm) was also investigated. The results showed that the novel combination method based on only 30 % lignin removal led to an increased sugar yield by 17 %. Unexpectedly, although IL pretreatment needs material to be ground (<0.42 mm), the difference in sugar yields for the two sized tested was negligible.
Graphical Abstract
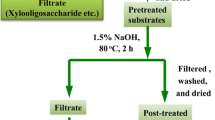
The overall lignocellolusic bioethanol production pathway including the combined AE–IL process proposed in the present study based on 30 % lignin removal through the first AE step.

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The plenty of lignocellulosic materials produced annually are regarded as the most abundant and low cost biomass available to meet the growing global energy demands [1, 2]. For instance, such materials could be converted to bioalcohols such as bioethanol and biobuthanol via appropriate processes [3, 4]. Alternatively, these materials could be combusted in order to generate heat and electricity [5]. Among the agricultural lignocellulosic wastes, rice straw (RS) although produced in large quantities worldwide (1–1.5 kg/kg grain) [6], is mainly useless because its application as animal feedstock is hindered by its high silica content of 7–13 %. Hence, abundantly-produced RS is one of the most favorable feedstock as a substrate for energy and heat generation [7] as it does not spark competitions between the animal feed and energy sectors.
The annual production of RS stands at about 731 million tons with Asia contributing 90 %. This amount of RS could potentially result in the production of 205 billion L of bioethanol per year if the technology was available [8]. Unfortunately, currently an increasing proportion of this RS undergoes field burning. The reasons for considering such a recycling option for RS include its slow degradation in soil, and the fact that it harbors rice stem diseases [9]. This tragic phenomenon i.e. field burning is not only translated into waste of energy (~14 MJ/kg, 10 % moisture), but also poses a serious threat to the environment due to the high levels of greenhouse gases emitted during open burning (CO 70.5, CO2 810.1 and NOx 1.73 g/kg RS, 10 % moisture).
From the composition point of view, RS is a potential feedstock for fuel ethanol production due to its high cellulose (32–47 %), and hemicelluloses (19–27 %) contents that can be readily hydrolyzed into fermentable sugars [10–13]. To achieve that, the main challenges include the complex structure of these materials caused by the presence of lignin, the crystallinity of the cellulose, and the presence of covalent cross-linkages between lignin and hemicelluloses in plant cell walls [14, 15]. Thus, continuous efforts are being made to find cost effective pretreatment methods so that the whole production process becomes commercially competitive with the first generation bioalcohol production [16–18].
Among the various pretreatment techniques, those involving ionic liquids (ILs) have been shown promising. This is ascribed to the fact that ILs are great cellulose solvents owing to their high polarity. This in conjunction with their other unique features i.e. great thermal stability (even above 300 °C), high conductivity and large electrochemical window, negligible volatility and non-flammability have been the driving forces of numerous investigations in which ILs have been used on various lignocellulosic feedstocks [19].
A few attempts have also been made to combine IL pretreatment with conventional procedures e.g. 1-ethyl-3-methylimidazolium acetate (EMIM[Ac])/Ammonia [20], IL/NH4OH–H2O2 [21], and cholinium IL/ultrasound [22]. Such isolated efforts have been made to ensure structural disruption of lingocellulosic materials to consequently make cellulose highly accessible to ILs. For instance, Nguyen et al. [20] applied EMIM[Ac]/Ammonia combined pretreatment to achieve lignin elimination by ammonia pretreatment prior to IL treatment. However, in most of the published reports available, both procedures i.e. IL and the conventional chemical pretreatment such as alkali extraction were conducted under their respective optimal conditions in order to achieve maximal lignin removal and sugar yields. Moreover, these reports do not imply considerable improvements over each pretreatment method alone [21, 22]. This could be ascribed to the fact that the harsh experimental conditions (i.e. the two-stepped pretreatment) must have degraded a portion of the carbohydrates (cellulose and hemicelluloses) and washed them away during the process.
Therefore, the aim of the present study was to perform a basic pretreatment method at under-optimal conditions in advance not only to maximize the accessibility of RS cellulose to EMIM[Ac] and consequently to increase sugar yields by the combined pretreatment method, but also to minimize cellulose and hemicelluloses degradation and wash-out. More specifically, lignin removal through the first basic pretreatment step was not performed under optimal conditions (highest sugar yield) and only 30 % lignin removal was targeted based on a report published by Hamaguchi et al. [23]. Their study concerned pulp mill industries and not lignoethanol production and indicated that in general only 30 % lignin removal guaranteed highest cellulose accessibility. This strategy i.e. limiting lignin removal to only 30 % through a moderate alkali step of a combined pretreatment method involving IL in order to maximize cellulose accessibility and consequently sugar yield has never been put to test in the lignoethanol production domain before. Moreover, limiting alkali usage through this strategy would enhance the sustainability of lignoethanol production from both economic and environmental point of views; through less water usage in the water-washing step.
Materials and Methods
Materials
RS was obtained from a rice paddy field located in the north of Iran. The straws were dried under sun before shredded into pieces. Then, the shredded straws were sieved to obtain fractions with a particle size of 0.420 mm [20]. EMIM[Ac], sodium hydroxide 97 %, Cellulase (40 FPU/mL) from Trichoderma reesei ATCC 26921 and Cellobiase from Aspergillus niger were purchased from Sigma-Aldrich (Germany). Other chemicals used in this study included sulfuric acid 95–97 % (Fluka) used for compositional analysis and sodium azide (Sigma-Aldrich) used to prevent microbial growth during enzymatic hydrolysis.
The Composition of Rice Straw (RS)
The composition of the untreated-RS, AE–RS, IL–RS, and AE–IL–RS, i.e. cellulose, hemicellulose and lignin contents, was determined based on the protocol of the National Renewable Energy Laboratory (NREL/TP-510-42618) [24]. Briefly, cellulose and hemicellulose were measured after two steps of sulphuric acid hydrolyzation (72 % solution at 30 °C for 1 h and 4 % solution at 121 °C for 1 h). After nutralizing the solution with calcium carbonate and filtering the liquid, the filtrate was injected into a high performance liquid chromatography (HPLC) equipped with an RI detector (Knauer, Germany), and a Eurokat H carbohydrate analysis column (Knauer, Germany) to obtain the concentration of glucose and xylose. The mobile phase was acidified water (0.01 N sulfuric acid, pH 2) at a flow rate of 1 mL/min with a column temperature of 65 °C. Then the amount of glucose and xylose were used to calculate the amounts of cellulose and hemicellulose, respectively. All experiments were conducted in triplicates.
Dissolution and Regeneration of RS by Ionic Liquid (IL)
Commercial EMIM[Ac] was heated at 130 °C for 30 min to remove any moisture in the IL. Then, 0.25 g of the air-dried untreated RS and AE–RS were separately mixed with 5 mL IL (1:20, RS:IL) and incubated at 100–130 °C for 30 min to 3 h while stirring with a magnetic stirrer (150 rpm). The dissolved RS mixtures were turned into gels and the gels were poured into hot deionized (DI) water (20 mL, 85 °C) under rapid stirring (150 rpm) to regenerate (precipitate) the materials. The regenerated RS flocs were collected by vacuum filtration through Whatman filter paper No. 2 and were thoroughly washed with hot DI water. The regenerated materials were referred to as IL–RS (without the alkali-extraction step) and AE–IL–RS (with the alkali-extraction step). IL–RS and AE–IL–RS samples were vacuum dried at 40 °C and were stored in sealed containers at 4 °C for enzymatic hydrolysis, scanning electron microscopy (SEM), and Fourier transform infrared spectroscopy (FTIR) experiments.
Alkali Extraction of RS
The air-dried RS was soaked in NaOH (0.3–1 wt%) at 80 °C for 30 min to 3 h at a constant solid/liquid (S/L) ratio of 1:15 to find the conditions under which 30 % lignin elimination could be achieved. The alkali-pretreated RS was then filtered through Whatman filter paper No. 2 and was thoroughly washed with hot DI water until filtrate at neutral pH was obtained. The resulting material left on the filter was referred to as alkali-extracted rice straw (AE–RS). A portion of the AE–RS was stored in a sealed container at 4 °C for structural analysis, while the remainder was dried at 40 °C, and stored in a sealed container at ambient temperature till used for the IL pretreatment.
Structural Analyses of RS
FT-IR Spectroscopy
FT-IR spectra of the dry and ground untreated and treated rice straw samples were acquired with an Equinox 55 FT-IR (Bruker, Germany). Spectra were obtained using 16 scans for KBr pellets containing 1 wt% sample with a resolution of 4 cm−1.
Scanning Electron Microscope (SEM)
A ZEISS ΣIGMA VP scanning electron microscope (SEM) (Germany) was used to examine the untreated and treated rice straw samples. Untreated-RS was soaked in DI water at room temperature overnight before the SEM observation. The samples (untreated-RS, AE–RS, IL–RS, and AE–IL–RS) were mounted on the SEM stub with a carbon tape, vacuum dried, and then coated with a thin layer (5 nm) of gold. All images were obtained at an acceleration voltage of 5 kV and magnifications of 500 and 5000.
Enzymatic Hydrolysis of Pretreated RS
Pretreated RS (0.25 g of AE–RS, IL–RS, or AE–IL–RS) and sodium azide 2 % (20 mg/mL in distilled water) were mixed with citrate buffer (pH 5.0, 50 mM) in a 50 mL flask in order to prevent microbial growth during the digestion phase. Meanwhile, the final total solid (TS) loading was controlled at 2.5 %. Cellulase (10 FPU/g) and Cellobiase (200 CBU/g) were added to the mixture and the enzymatic hydrolysis was carried out at 50 °C and 200 rpm in an illuminated shaking incubator (n-biotek,inc). The hydrolysate was used to determine the sugar content using HPLC as mentioned earlier [see Sect. “The composition of rice straw (RS)”]. These values were then used to calculate glucose release as well as cellulose digestibility.
Statistical Analysis
The mean comparisons were conducted by SAS 9.1 (SAS, Cary, NC). The graphs were plotted by Excel 2010.
Results and Discussion
Chemical Composition of Untreated and Pretreated RS
The composition of the RS used in this study was determined as follows: 42 % Cellulose, 9 % hemicellulose, 15 % lignin, 23 % extractives, and 11 % ash. The solid recovery rate after the AE step as in AE–RS was at 85.5 % wt (i.e. 14.5 % mass loss) (Table 1). In fact, the alkali treatment partially degraded lignin by breaking the linkages between hemicellulose and lignin and solubilizing a portion of the hemicellulose [25, 26]. This would explain the increase achieved in the cellulose/hemicellulose ratio in the AE–RS sample. As a result of the mass loss of 14.5 % wt which was in fact caused by the removal of lignin, hemicellulose and water-soluble components, the total carbohydrate content (%) was increased from 51 to 58 %.
The untreated-RS and AE–RS were dissolved separately in EMIM[Ac] and were regenerated with hot DI water. The solids obtained through this dissolution/regeneration step were 68 % for the untreated-RS (Table 2) and 82 % for the AE–RS (Table 3). Therefore, given the solid recovery rate achieved through the AE step (i.e. 85.5 % wt) and the solids obtained after the IL treatment (82 %), the overall solids recovery through the combined AE–IL treatment stood at 70 % wt.
Optimization of IL Pretreatment of RS
Since the presence of water in IL could diminish IL solubility and reduce pretreatment efficiency, all the experiments were carried out at temperatures above 100 °C [19]. Moreover, IL pretreatment at temperatures above 130 °C could destruct carbohydrates [19], and thus, 110, 120 and 130 °C were chosen to find out the optimum pretreatment conditions. Table 2 tabulates the experiments conducted and their biomass recovery rates.
After pretreatment, the samples were hydrolyzed for 48 h to convert cellulose to glucose. Figure 1 presents cellulose digestion rates and consequent glucose yields achieved through the experiments.
As clearly seen, the IL pretreatment at 120 °C for 2 h resulted in the highest sugar yield (Fig. 1). In comparison with the untreated-RS, IL pretreatment under optimal conditions improved sugar yield by four times and within 24 h, a sugars yield as high as 85 % was obtained.
Alkali Extraction of RS
In order to determine the conditions under which lignin removal of 30 % could be achieved, AE was carried out under moderate conditions (80 °C for 0.5–1.5 h) at low alkali (NaOH) concentrations ranging from 0.3 to 1 wt%, (Table 1). The conditions were set as stated so that no pressure reactor was required and hemicellulose loss was minimized. As seen, pretreatment with 0.3 % NaOH at 80 °C within 30 min resulted in a favorable outcome.
Combined Pretreatment of RS
The AE–RS (i.e. after 30 % lignin removal) underwent IL pretreatment under the previously-determined optimal conditions (i.e. 120 °C, 2 h). In addition, the AE–RS (40 % lignin removal) was also included in the experiment to ensure that the elimination of 30 % of lignin was sufficient in achieving the highest cellulose accessibility during the IL pretreatment. Moreover, it has been frequently indicated in the literature that size reduction down to 0.42 mm is necessary to maximize IL pretreatment efficiency [20, 27]. Since size reduction could be costly and if less ground materials could be used, the economic viability of the process would be improved, therefore, the combined pretreatment was repeated using both 0.42 mm- and 2 cm-sized RS samples. As presented in Table 3, both higher lignin elimination more than 30 % and the conventionally-recommended size reduction (i.e. screened with #40 mesh) negatively affected solid recovery rates after the combined AE–IL pretreatment. The combined AE–IL process used in the present study based on 30 % lignin removal through the first AE step is schematically demonstrated in Fig. 2.
After the combined pretreatment, the samples obtained were hydrolyzed by cellulase for 48 h and their respective glucose yields were measured (Fig. 1). The combined AE–IL pretreatment resulted in higher glucose yield by 17 % compared to the IL pretreatment alone. Moreover, the impacts of lignin elimination beyond 30 % and size reduction on final glucose yield achieved were negligible and therefore, both could be disregarded in order to improve the economic aspects of the process.
As displayed in Fig. 1, the enzymatic hydrolysis rate and glucose yield for IL–RS were lower than those of the AE–IL–RS. As for the IL–RS sample, despite of the IL-caused disruption of the crystalline structure of cellulose, the redistribution of lignin during cellulose regeneration could have acted as a sheath on the fiber surface preventing the enzymes from accessing much of the cellulose surface [28]. However, by removing the lignin through the mild AE, higher surface area of the fibers was exposed to the enzymes and that is why the AE–IL–RS had a higher hydrolysis rate and glucose yield than the IL–RS. In fact, the higher rate of enzymatic hydrolysis for the AE–IL–RS (i.e. only 12 h) was likely resulted from the increase in surface area caused by IL dissolution and the mild lignin removal by the AE which in turn prevented cellulose/hemicellulose degradation.
Overall, one of the biggest challenges in the process of converting biomass to bioethanol is increasing the accessibility of cellulose to the enzymes for subsequent enzymatic hydrolysis. Beside lignin removal to achieve this, reducing particle size is also considered by many [29]. Interestingly, the sugar yields obtained for the combined method applied on both fine- and coarse-particle RS (i.e. <0.42 mm and <2.0 cm, respectively) were different by only 5.7 % in favor of the fine-particle RS (Fig. 3). Hence, it could be recommended to use coarse-particles instead of fine-particles RS which could reduce the processing cost and consequently enhance the economic viability of the lignoethanol production.
In conclusion, the results showed that cellulose digestion increased from 29 % for the untreated-RS to 99 % for IL–RS. Also, sugar yield increased from 22.7 % for the untreated-RS to 79 and 92.6 % for the IL–RS and AE–IL–RS, respectively. These results obtained herein showed an improvement in sugar yield, even with less size reduction and a significant decrease in process time compared to the previous studies conducted on RS [30, 31]. For instance, Poornejad et al. [30] pretreated RS with N-methyl morpholine N-oxide (NMMO) and 1-buthyl-3-methylimidazolium acetate ([BMIM][OAc]) at 120 °C for 5 h with 5 % RS loading to enhance its sugar yield. Their hydrolysis results indicated a hydrolysis yield of 96 % for the NMMO-treated samples, while the conversion was only 27.7 % for the untreated straw. In a different study, Poornejad et al. [31] pretreated RS by [EMIM][Ac], prior to enzymatic hydrolysis and ethanol production. The pretreatment was carried out at 120 °C for 5 h under atmospheric pressure. They reported that glucose yield increased from 25.7 % for the untreated straw to over 75 % for the treated straw. Nguyen et al. [20] conducted an efficient combined pretreatment method using ammonia and ionic liquid ([Emim][Ac]). The combined method exhibited a synergy effect on RS with 82 % cellulose recovery and 97 % enzymatic glucose conversion. They also pointed out that the cooperative effect led to the conversion of over 90 % of the glucose even with a reduced enzyme usage and incubation time [31]. In a single step pretreatment, Jeya et al. [32] pretreated RS by 2 % aqueous solution of NaOH at 85 °C for 1 h and achieved only 86 % saccharification.
The findings of the present study also confirmed that the combined EMIM[Ac] and mild NaOH was advantageous over the single stage pretreatment of RS with [EMIM][Ac]. More specifically, the combined method developed resulted in a sugar yield increase by 17 % compared to the [EMIM][Ac] alone. Moreover, although single stage pretreatment of RS with NaOH was reported as an efficient useful method with a relatively high sugar yield [32], it requires a huge amount of water for the nutralization step. On the contrary, water consumption in the combined method presented herein was significantly less than that of NaOH method. This is ascribed to the fact that only a 0.3 % aqueous solution of NaOH was used in contrast to the 1 or 2 % NaOH solutions conventionally used elsewhere [32]. In addition to that, the process time in the present combined method (2.5 h) was half of that reported by Poornejad et al. [31] (5 h) who used ([EMIM][Ac] for RS pretreatment. Moreover, it is worth quoting that the combined process resulted in approximately similar sugar yields for both the course RS (<2 cm) and the fine RS (<0.42). This would be of economic importance as further size reduction would impose additional operational cost on the process.
FTIR Analysis
FT-IR spectroscopy was used to examine the chemical modifications of the RS surface during the AE and IL dissolution steps (Fig. 4). The peak observed at 897 cm−1 was more intense in the case of the AE–IL–RS and IL–RS compared to those of the AE–RS and untreated RS. In fact, the peak at 897 cm−1 characterizing the C–O–C stretching at b-1,4-glycosidic linkage is indicative of the presence of amorphous cellulose [33]. This revealed the more efficient generation of amorphous cellulose by the AE–IL pretreatment. Moreover, the peak obtained at 1430 cm−1 could be assigned to the bending vibration of CH2. This bond is strong in crystalline cellulose, and weak in amorphous cellulose. On such basis, it could be concluded that the amount of crystalline cellulose was higher in the AE–RS as well as in the untreated-RS than in the IL–RS and AE–IL–RS samples. This further confirmed the above-mentioned explanation concerning the peak obtained at 897 cm−1. Overall, the highest quantities of amorphous cellulose were achieved when AE–IL and IL treatments were applied, respectively, and the lowest amount was found in the untreated-RS.
The AE of the untreated-RS (i.e. in AE–RS) separated lignin from the other fibers, resulting in a decrease in the absorbance of the 1595 and 1510 cm−1 bands associated with the aromatic ring vibrations of lignin [26, 34]. On the other hand, after the dissolution and regeneration of the untreated-RS through the IL–RS treatment, the absorbance values of the lignin bands increased. This unexpected increase could be explained by the fact that as the cellulose and hemicellulose were dissolved into the EMIM[Ac], the lignin and other wax-like components in the RS must have been formed into spheres and aggregates [35, 36]. These aggregated lignin did not remain in the aqueous solution with the EMIM[Ac] during the regeneration step when the polysaccharides precipitated from the solution and were redistributed onto the cellulose fiber surface [36]. This redistributed lignin may explain why the IL–RS sample had a higher absorbance at 1595 cm−1 than the untreated-RS. Nonetheless, the redistributed lignin was not as recalcitrant as the lignin originally found in the cell wall matrix.
The combined pretreatment i.e. AE–IL led to considerable changes in the vibrations of the aromatic ring bands indicating the successful removal of the remaining lignin in the AE–RS by the IL pretreatment applied. In fact, the AE was conducted under rather mild conditions in order to only achieve 30 % lignin removal reflecting highest cellulose accessibility [23], while least cellulose/hemicellulose degradation was caused. This could also be observed through the cellulose/hemicellulose ratio obtained for the AE–RS (5.03) which was only slightly higher than that of the untreated-RS (4.66). This revealed only slight degradation of hemicelluloses by the mild AE applied. This was in agreement with the insignificant decrease noted in the absorbance of the 1735 cm−1 band assigned to the carbonyl functional groups of hemicellulose [26]. More specifically, compared to the untreated biomass, the 1735 cm−1 hemicellulose band diminished insignificantly for the AE–RS samples, but increased in the cases of the IL–RS and AE–IL–RS samples (Fig. 4).
In a study, Geng and Henderson [36] suggested a combined pretreatment method involving 1 % NaOH (90 °C, 1 h) and EMIM[Ac] for corn stover. The same procedure was also repeated for RS in the AE step of the combined AE–IL pretreatment in the present study resulting in 60 % lignin removal. However, this led to a considerably lower sugar yield (data not shown). As a result, more moderate conditions (0.3 % NaOH, 80 °C, 30 min) or in another word, lower lignin removal rate was targeted herein i.e. 30 %. Moreover, 40 % lignin removal was also taken into consideration by increasing the pretreatment time to 90 min rather than increasing the NaOH concentration. This could be justified by the fact that increasing NaOH concentration would lead to an increased water consumption in the neutralization step and would consequently jeopardize the environmental viability of the suggested process. Therefore, instead of increasing the alkaline concentration, experimental duration was increased from 30 to 90 min to increase the lignin removal from 30 to 40 %.
SEM
Figure 5a, b demonstrate the untreated-RS and the physical changes caused after 30 min pretreatment by 0.3 % NaOH at 80 °C, respectively. The results obtained indicated that the untreated-RS had a highly fibrillar and intact morphology, and that the mild alkali pretreatment applied herein only had superficial effects on the biomass structure making it flaky. This could explain the observation made after the AE pretreatment i.e. the inflation of the biomass (Fig. 6). Figure 5c, d also show the untreated and the pretreated RS under mild alkaline condition at a lower magnification, respectively, confirming that the mild alkali pretreatment led to insignificant structural changes on the RS.
SEM images of rice straw. a Raw rice straw (×5000), b AE pretreated rice straw at 80 °C in 30 min (×5000), c raw rice straw (×500), d AE pretreated rice straw at 80 °C in 30 min (×500), e IL pretreated rice straw (×500), f combined AE–IL pretreated coarse (<2 cm) rice straw (×500) (NaOH 0.3 % in 80 °C and 30 min, IL in 120 °C and 2 h), g Combined AE–IL pretreated fine (<0.42 mm) rice straw (×500)(NaOH 0.3 % in 80 °C and 30 min, IL in 120 °C and 2 h), h combined AE–IL pretreated (NaOH 0.3 % in 80 °C and 30 min, IL in 120 °C and 1 h) fine rice straw (×500)
On the other hand, Fig. 5e shows the effect of the IL pretreatment on the RS at 120 °C after 2 h indicating complete structural changes of the RS. More specifically, after pretreatment by [EMIM][Ac], no fibrous structure was visible and the surface became swollen and loose with many pores observed throughout the surface. Figure 5f–h represent harsh changes on the RS caused by the combined AE–IL pretreatment. During the first AE step, not only the lignin and other non-cellulosic components were removed from the fibers, but also the cellulose fibers swelled, and the sample achieved after the AE became more accessible to the ions in the dissolution step. Thus, the sample was dissolved in the IL more completely than the untreated-RS. Overall, the combined pretreatment caused both structural and superficial effects on the RS as shown in Fig. 5f–h. These structural differences from the original RS fibers caused the AE–IL–RS sample to be more accessible to the enzymes leading to much improved enzymatic hydrolysis efficiency i.e. sugar yield.
Conclusions
A novel pretreatment process based on only 30 % lignin removal through the first AE process followed by IL-dissolution/regeneration steps was developed. As a result, an increased enzymatic hydrolysis of RS polysaccharides (17 % increase in sugar yield compared to the EMIM[Ac] alone) was achieved. Moreover, applying the AE procedure under mild conditions (aiming at only 30 % lignin removal), instead of the optimized conditions recommended in other studies, led to less water consumption during the neutralization step. In addition to these, the findings achieved by comparing the course RS (<2 cm) and the fine RS (<0.42) with regards to their corresponding sugar yields (11.05 and 11.68 mg/mL, respectively) showed an insignificant difference. Therefore, it could be concluded that the fine RS recommended in most previous studies could be replaced by the course RS which could lead to a more cost-effective procedure as further shredding of RS could be avoided.
Abbreviations
- RS:
-
Rice straw
- AE–RS:
-
Alkali extracted rice straw
- IL–RS:
-
Ionic liquid (pretreated) rice straw
- AE–IL–RS:
-
Alkali extracted and ionic liquid (pretreated) rice straw
References
Sassner, P., Martensson, C., Galbe, M., Zacchi, G.: Steam pretreatment of H2SO4-impregnated Salix for the production of bioethanol. Bioresour. Technol. 99, 137–145 (2008)
Pothiraj, C., Arun, A., Eyini, M.: Simultaneous saccharification and fermentation of cassava waste for ethanol production. Biofuel Res. J. 2(1), 196–202 (2015)
Edama, N.A., Sulaiman, A., Rahim, S.N.A.: Enzymatic saccharification of Tapioca processing wastes into biosugars through immobilization technology. Biofuel Res. J. 1(1), 2–6 (2014)
Salehi Jouzani, G., Taherzadeh, M.J.: Advances in consolidated bioprocessing systems for bioethanol and butanol production from biomass: a comprehensive review. Biofuel Res. J. 2(1), 152–195 (2015)
Delivand, M.K., Barz, M., Garivait, S.: Overall analyses of using rice straw residues for power generation in Thailand-project feasibility and environmental GHG impacts assessment. J. Sust. Energy Environ. Special Issue, 39–46 (2011)
Binod, P., Sindhu, R., Singhania, R.R., Vikram, S., Devi, L., Nagalakshmi, S., Kurien, N., Sukumaran, R.K., Pandey, A.: Bioethanol production from rice straw: an overview. Bioresour. Technol. 101, 4767–4774 (2010)
Rehman, M.S.U., Umer, M.A., Rashid, N., Kim, I., Han, J.I.: Sono-assisted sulfuric acid process for economical recovery of fermentable sugars and mesoporous pure silica from rice straw. Ind. Crops Prod. 49, 705–711 (2013)
Balat, M., Balat, H., Öz, C.: Progress in bioethanol processing. Prog. Energy Combust. 34, 551–573 (2008)
Mussatto, S.I., Roberto, I.C.: Optimal experimental condition for hemicellulosic hydrolyzate treatment with activated charcoal for xylitol production. Biotechnol. Prog. 20, 134–139 (2004)
Maiorella, B.L.: Ethanol industrial chemicals. Biochem. Fuels 861–914 (1983)
Zamora, R., Crispin, J.A.S.: Produccion de un extracto acido de paja de arroz. Acta Cient. Venez. 46, 135–139 (1995)
Garrote, G., Domínguez, H., Parajó, J.C.: Interpretation of deacetylation and hemicellulose hydrolysis during hydrothermal treatments on the basis of the severity factor. Process Biochem. 37, 1067–1073 (2002)
Saha, B.C.: Hemicellulose bioconversion. J. Ind. Microbiol. Biotechnol. 30, 279–291 (2003)
Blanch, H.W., Wilke, C.R.: Sugars and chemicals from cellulose. Rev. Chem. Eng. 1, 71–119 (1982)
Werle, L.B., Garcia, J.C., Kuhn, R.C., Schwaab, M., Foletto, E.L., Cancelier, A., Sérgio, L.J., Mazutti, M.A.: Ultrasound-assisted acid hydrolysis of palm leaves (Roystonea oleracea) for production of fermentable sugars. Ind. Crops Prod. 45, 128–132 (2013)
Jafri, U.A., Javed, M.T., Chugtai, I.R.: Process investigation for conversion ofmunicipal solid waste into liquid fuel. JPIChE 39, 23–27 (2011)
Yaqoob, M., Mehmood, S., Rehman, M.S.U., Rashid, N., Han, J.-I.: Optimization of dilute sulfuric acid pretreatment and enzymatic hydrolysis of industrial hemp (Cannabis sativa). Environ. Process. Eng. 1–2, 9–15 (2012)
Barchyn, D., Cenkowski, S.: Process analysis of superheated steam pre-treatment of wheat straw and its relative effect on ethanol selling price. Biofuel Res. J. 1(4), 123–128 (2014)
Mood, S.H., Golfeshan, A.H., Tabatabaei, M., Jouzani, G.S., Najafi, G.H., Gholami, M., Ardjmand, M.: Lignocellulosic biomass to bioethanol, a comprehensive review with a focus on pretreatment. Renew. Sust. Energy Rev. 27, 77–93 (2013)
Nguyen, T.A.D., Kim, K.R., Han, S.J., Cho, H.Y., Kim, J.W., Park, S.M., Park, J.C., Sim, S.J.: Pretreatment of rice straw with ammonia and ionic liquid for lignocellulose conversion to fermentable sugars. Bioresour. Technol. 101, 7432–7438 (2010)
Zhu, Z., Zhu, M., Wu, Z.: Pretreatment of sugarcane bagasse with NH4OH–H2O2 and ionic liquid for efficient hydrolysis and bioethanol production. Bioresour. Technol. 119, 199–207 (2012)
Ninomiya, K., Kohori, A., Tatsumi, M., Osawa, K., Endo, T., Kakuchi, R., Ogino, C., Shimizu, N., Takahashi, K.: Ionic liquid/ultrasound pretreatment and in situ enzymatic saccharification of bagasse using biocompatible cholinium ionic liquid. Bioresour. Technol. 176, 169–174 (2015)
Hamaguchi, M., Vakkilainen, E.K., Ryder, P.: The impact of lignin removal on the dimensioning of eucalyptus pulp mills. Appita J. 64, 433 (2011)
Sluiter, A., Hames, B.R., Ruiz, R., Scarlata, C.J., Sluiter, J., Templeton, D.W., Crocker, D.: Determination of structural carbohydrates and lignin in biomass. Laboratory Analytical Procedure, NREL/TP-510-42628. http://www.nrel.gov/biomass/pdfs/42618.pdf (2011)
Koullas, D.P., Christakopoulos, P.F., Kekos, D., Koukios, E.G., Macris, B.J.: Effect of alkali delignification on wheat straw saccharification by Fusarium oxysporum cellulases. Biomass Bioenergy 4, 9–13 (1993)
Kristensen, J.B., Thygesen, L.G., Felby, C., Jorgensen, H., Elder, T.: Cell-wall structural changes in wheat straw pretreated for bioethanol production. Biotechnol. Biofuels 1, 5–13 (2008)
Mood, S.H., Golfeshan, A.H., Tabatabaei, M., Abbasalizadeh, S., Ardjmand, M.: Comparison of different ionic liquids pretreatment for barley straw enzymatic saccharification. 3 Biotech 3, 399–406 (2013)
Jorgensen, H., Kristensen, J.B., Felby, C.: Enzymatic conversion of lignocellulose into fermentable sugars: challenges and opportunities. Biofuels Bioprod. Biorefin. 1, 119–134 (2007)
Zhu, J.Y., Wang, G.S., Pan, X.J., Gleisner, R.: Specific surface to evaluate the efficiencies of milling and pretreatment of wood for enzymatic saccharification. Chem. Eng. Sci. 64, 474–485 (2009)
Poornejad, N., Karimi, K., Behzad, T.: Improvement of saccharification and ethanol production from rice straw by NMMO and [BMIM][OAc] pretreatments. Ind. Crops Prod. 41, 408–413 (2013)
Poornejad, N., Karimi, K., Behzad, T.: Ionic liquid pretreatment of rice straw to enhance saccharification and bioethanol production. J. Biomass Biofuels 1, 5–18 (2014)
Jeya, M., Zhang, Y.W., Kim, I.W.: Lee, J.K: Enhanced saccharification of alkali-treated rice straw by cellulase from Trametes hirsuta and statistical optimization of hydrolysis conditions by RSM. Bioresour. Technol. 100(21), 5155–5161 (2009)
Mood, S.H., Golfeshan, A.H., Tabatabaei, M., Abbasalizadeh, S., Ardjmand, M., Jouzani, G.S.: Comparison of different ionic liquids pretreatment for corn stover enzymatic saccharification. Prep. Biochem. Biotechnol. 44, 451–463 (2014)
Stewart, D., Yahiaoui, N., McDougall, G.J., Myton, K., Marque, C., Boudet, A.M., Haigh, J.: Fourier-transform infrared and Raman spectroscopic evidence for the incorporation of cinnamaldehydes into the lignin of transgenic tobacco (Nicotiana tabacum L.) plants with reduced expression of cinnamyl alcohol dehydrogenase. Planta 201, 311–318 (1997)
Donohoe, B.S., Decker, S.R., Tucker, M.P., Himmel, M.E., Vinzant, T.B.: Visualizing lignin coalescence and migration through maize cell walls following thermochemical pretreatment. Biotechnol. Bioeng. 101, 913–925 (2008)
Geng, X., Henderson, W.A.: Pretreatment of corn stover by combining ionic liquid dissolution with alkali extraction. Biotechnol. Bioeng. 109, 84–91 (2012)
Acknowledgments
The authors would like to thank Agricultural Biotechnology Research Institute of Iran (ABRII) and Biofuel Research Team (BRTeam) for financing this project.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Raeisi, S.M., Tabatabaei, M., Ayati, B. et al. A Novel Combined Pretreatment Method for Rice Straw Using Optimized EMIM[Ac] and Mild NaOH. Waste Biomass Valor 7, 97–107 (2016). https://doi.org/10.1007/s12649-015-9437-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-015-9437-5