Abstract
The present work is devoted to the experimental study of torrefaction of corn-stover. This biomass was processed in a high capacity thermobalance which can accept a load of approximately 500 g. It allows not only following the weight of the biomass under investigation as a function of time (or temperature) but also to collect and analyze the by-products of the thermal degradation (μ-GC for gas, HPLC for tars). Torrefaction is expected to be of main concern in the preparation of biomass for energy production or for conversion of biomass into liquid fuels. This is one of the reason why this process has been widely studied in the case of wood in the literature. However, so few studies exist on the torrefaction of agricultural residue which could play an important role in the above mentioned fields. The purpose of the present work is to give insights on the transformation of corn stover (which is an important by product of corn) undergoing an operation of torrefaction. To do so, the influence of operational conditions (temperature, residence time, heating rate, weight of sample) on the yield and composition of by-products was studied with a factorial design. It is shown that the main gaseous products are carbon monoxide and dioxide while tars are mainly composed of acetic acid and formaldehyde. It is also shown that the discriminant operating parameter in corn stover conversion is the temperature.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Torrefaction of biomass can be described as a slow pyrolysis at atmospheric pressure in a temperature range between 220 and 280 °C. Its main goal is to produce a dry solid, with a higher heating value than the initial biomass and which can be stored without taking back any moisture content (the produced solid is hydrophobic). Also, an important feature of this product is that it can be easily grinded as required to a powdery fuel. The main expected use of this torrefied biomass is combustion in boilers (either at large scale when it is reduced to powder by a grinding step or directly for lower capacity boilers) or as a feedstock for biomass to liquid process [1–3].
Corn is one of the most commonly grown cereals in the world. Corn stover is a residue of corn once the grain has been collected. It is composed of the cane, leaves and shucks (encasement surrounding the cob) and actually not valorized to its full extent. The main goal of this work is to study the behavior of corn stover during torrefaction in order to make this residue a potential feedstock for energetic applications. The main purpose consists in qualification and quantification of the byproducts obtained after the torrefaction process as a function of the working parameters of the operation [4].
This analysis is performed using in a high capacity thermobalance (conceptually similar to a commercial TGA system) which is fully linked to two analytical systems (gas chromatography and high performance liquid chromatography). It allows following mass losses of the samples under investigation as well as the composition and quantification of the permanent (non condensable) gases and tars resulting from thermal. The influence of the parameters has been investigated using a fractional experimental design based on the analysis of the variance (ANOVA). Such a design allows the determination of the relevant operating parameters of the process.
Materials and Methods
Corn Stover
The properties of the corn stover used in our experiments are reported in Table 1. The initial moisture content of the samples is 12.1 %. Samples have been grinded and sieved in a first step to a resulting average particle size of one millimetre (Fig. 1). Noteworthy that the carbon content is high (which is of importance dealing with energetic application) as well as its ash and hemicellulose contents.
High Capacity Thermobalance
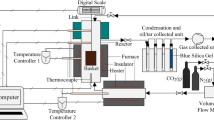
The experimental set up is conceptually equivalent to a commercial thermobalance, but it can accept a load of up to 1 kg of biomass, depending upon its density. Basically, the device is firstly composed of a furnace that can reach up to 850 °C. This commercial furnace has been made airtight in order to avoid leakages of volatile matter produced during pyrolysis, and in order to quantify this production. A basket, placed in the furnace which receives the feedstock to be pyrolysed. The basket is supported by a balance placed outside of the furnace in a tight box, cooled by a nitrogen flow which in turn enters the furnace through the legs of the basket establishing the mechanical connection with the balance. The biomass is inserted in the furnace prior to any heating of the device, which means that it is heated progressively in the furnace. This balance allows the recording of the mass of the sample held within the basket during the experiment. The furnace itself is also fed with a nitrogen flow in order to drag the product of pyrolysis outside of the furnace. Thanks to an electrical heater (EC101, Fig. 2) the temperature of the nitrogen supply is controlled so that it is equal to the actual temperature of the furnace. The gas leaving the furnace (composed of the incoming nitrogen and the products of pyrolysis) leaves the furnace in a pipe (heated to 250 °C using an electrical heating cable) and then it enters a condenser where tars are condensed and collected. After the condensing stage, the resulting gas is routed towards a filter where aerosols are separated from the gas and collected. Uncondensable gases are analyzed using gas chromatography. Figures 2 and 3 show a scheme as well as two photos of the overall equipment.
Analysers
Tars collected after condensation were analysed using a high performance liquid chromatography (HPLC), following the protocol developed by Prins et al. [5]. Once they have been filtered, tars are introduced within the HPLC composed of a column Hi-Plex-H (Varian) and a refractometre. The overall device had been calibrated for quantification of formaldehyde, formic acid, acetic acid, lactic acid, hydroxyacetone, acetaldehyde, methanol, phenol and furaldehyde [4].
Uncondesable gases were quantified using a micro chromatography device (Varian CP-4900) and which allowed quantification of hydrogen, oxygen, nitrogen, carbon monoxide and dioxide, methane, ethane, and ethylene within the exhaust gases. The analyses were performed every 5 min once the experiment has started [4].
Experimental Design
The influence of operating parameters [temperature (T), residence time (t), initial mass of the sample to be torrefied (m) and heating rate (β)] has been checked using a fractional experimental design “24−1” of 8 experiments. It is generated considering m, T and β as main parameters while t is “aliased” with the weakest interaction. The resulting set of required experiments are summed up in Table 2.
Results and Discussion
The overall mass balance of the experiments has been quantified within five to ten percent error margin. In order to close the mass balance, the amount of tars collected has been adjusted accordingly to the other fractions. Such assumptions were based on the possibility that losses of tars could result from condensation in cold spots in the output pipes of the systems.
Distribution of Products and By products
Figure 4 gives a representation of the distribution of the products and by products obtained in the above mentioned experiments. It is expressed as the yield of product (or by-product) over the initial dry sample mass. Results show that the yield in torrefied biomass decreases as the temperature increases, while the corresponding by products increases. It can also be stated that the production of tars is always greater than the permanent gases one.
Composition of Permanent Gases
Figures 5 and 6 propose an illustration of the influence of the operating conditions on the global composition of the permanent gas generated during experiments. The latter is expressed as a yield of gas (carbon monoxide or dioxide or other permanent gases) over the initial dry sample mass. Two conclusions can be drawn from the analysis of these figures: first is that the yields of permanent gases other than carbon monoxide and dioxide is quite insignificant and second is that it seems that the repartition between carbon monoxide and dioxide is mainly affected by temperature.
Composition of Tars
Figures 7 and 8 give a representation of the evolution of the tars collected during the experiments as a function of the operating conditions. This figure illustrates that the main condensable gases obtained during the operation are water and acetic acid. It should be noticed here that water includes the initial moisture content of the sample. hydroxyacetone, methanol and formaldehyde are also detected, and are characteristics of hemicelluloses thermal degradation [5–7]. Once again, the great influence of temperature can be stated.
Results of the Analysis of Variance (ANOVA)
Considering the section “Experimental design”, it has been possible to express the yields of products and by products as a mathematical expression of the operating parameters. These expressions have been obtained using the analysis of variance of the results and neglecting effects that have a coefficient proximate from the experimental error (see for example [4, 8, 9] for further information about this procedure). It has also to be noticed that, in the range studied, it could not be observed any influence of the heating rate.
Table 3 illustrates such results. More precisely, this table sums up the different coefficients that were obtained en that represent the yields of torrefied biomass, permanent gases and tars as a function of the operating conditions. These coefficients have to be used as follows in order to compute the yield of the corresponding products on a dry mass basis:
where \( \eta_{x} \) stands for the yield of \( x \) (kg of \( x \) per kg of dry initial solid), \( T \) stands for the operating temperature (°C), \( t \) stands residence time at the operating temperature (min), \( m \) stands for the initial mass sample.
Because of the value of the different coefficients that were obtained, it can be concluded first that the discriminant parameter is the operating temperature (the value of \( \beta_{x} \) is always higher than the other coefficients). The influences of the residence time and of the initial mass of the sample are of the same order of magnitude.
The influence of the initial mass of the sample leads to the conclusion that heat and mass transfer within the sample are relevant and that they should be considered if the high capacity thermobalance was used to extract kinetic information. A model including these phenomena should be written to do so, and this will be the scope of a future work.
Evolution of the Mass of the Sample Versus Time
Important information which is brought by the experimental device is the mass loss versus time as a function of the temperature of the sample. This temperature is measured 10 mm above the centre of the sample for every experiment what mean that even though we do not measure the temperature inside the sample, the thermal conditions can be expected to be equivalent from one experiment to another. Figure 9 shows the evolution of this temperature which represents the following thermal history:
-
Nitrogen supply to the device until O2 percentage in the outlet gas is less than 3 %.
-
Heating at 10 °C/min until the temperature reaches 110 °C.
-
Stage at 110 °C during 90 min.
-
Heating at 12.5 °C/min until the temperature reaches 250 °C.
-
Stage at 250 °C during 40 min.
-
End of heating.
Figure 10 shows the corresponding mass loss of the sample. The initial size of the sample was 301.8 g with a moisture content of 6.92 % on a raw basis (a part of the sample was weighted before and after a stay of 24 h in a ventilated oven at 105 °C). As it can be seen, the sample begins to dry during the first 60 min under the effect of being in an environment with dry nitrogen. This drying step becomes much more intense as the heating of the furnace is switched on and during the stage at 110 °C. It has to be noticed here that the sample does not seem to be dry at the end of this “drying stage”. Then, as the temperature is further increased to 250 °C, the mass of the sample still decreased under the effect of the end of drying (for less than 1 % of the initial raw mass of the sample) and under the effect of thermal degradation. The final mass loss on a raw basis if 17.36 % what leads to a production of 88.78 % of the initial dry sample of Torrefied corn stover. Figure 11 shows the comparison between fresh corn stover and Torrefied one. It shows that the obtained Torrefied biomass is quite homogeneous.
Conclusions
Torrefaction of corn stover has been studied in this work. This study relies on the use of a high capacity thermobalance, which is a device that has been developed in the Laboratory for Thermal Sciences and Process (LaTEP). Couples with analysis apparatus, this device allows the determination of yields of products and by products as a function of operating parameters with an overall mass balance in the range of 5–10 % error.
It can be concluded that the main parameter of the operation is the temperature at which the sample is raised and that the main permanent gases produced are carbon monoxide and dioxide while the main collected tars are water and acetic acid, and to a lower extent, hydroxyacetone, methanol, formaldehyde and formic acid.
Because of heat and mass transfer within the sample, the high capacity thermobalance could not be used at present time to extract kinetic information on the operation of torrefaction. This is the reason why a mathematical model based on the theory of volume averaging is being construct, and should allow the determination of such information in future work.
References
Bergman P.C.A., Kiel J.H.A.: Torrefaction for biomass upgrading. 14th European Biomass Conference & Exhibition, Paris (2005)
Kiel J.H.A., Verhoeff F., Gerhauser H., Daalen W.V., Meuleman B.: BO2-technology upgrading into solid fuel—an enabling technology for IGCC and gazification based BtL. 4th International Conference on Clean Coal Technologies, Dresden (2009)
Repellin, V., Govin, A., Rolland, M., Guyonnet, R.: Energy requirement for fine grinding of torrefied wood. Biomass Bioenergy 34, 923–930 (2010)
Casajus, C.: Torréfaction de biomasses lignocellulosiques. PhD thesis, Université de Pau et des pays de l’Adour (2010)
Prins, M.J., Ptasinski, K.J., Janssen, F.J.: Torrefaction of wood: part 2. Analysis of products. J. Anal. Appl. Pyrol. 77, 35–40 (2006)
Shen, D.K., Gu, S., Bridgwater, A.V.: Study on the pyrolytic behaviour of xylan-based hemicellulose using tg-ftir and py-gc-ftir. J. Anal. Appl. Pyrol. 2, 199–206 (1987)
Simkovic, I., Varhegyi, G., Antal, M.J. Jr., Ebringerova, A., Szekeley, T., Szabo, P.: Thermogravimetric/mass spectrometric characterization of the thermal decomposition of (4-O-methyl-D-glucurono)-D-xylan. J. Appl. Polym. Sci. 36(3), 721–728 (1988)
Cascarosa, E., Becker, J., Ferrante, L., Briens, C., Berruti, F., Arauzo, J.: Pyrolysis of meat-meal and bone-meal blends in a mechanically fluidized reactor. J. Anal. Appl. Pyrol. 91, 359–367 (2011)
Cascarosa, E., Gasco, L., Garcia, G., Gea, G., Arauzo, J.: Meat and bone meal and co-gasification: environmental advantages. Resour. Conserv. Recycl. 59, 32–37 (2012)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Marias, F., Casajus, C. Torrefaction of Corn Stover in a Macro-thermobalance: Influence of Operating Conditions. Waste Biomass Valor 5, 157–164 (2014). https://doi.org/10.1007/s12649-013-9235-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-013-9235-x