Abstract
In this work, maize residue pellets were torrefied in a macro thermogravimetric analyzer with simulated dry flue gas from mixing of CO2 and N2. The effects of temperature (220–300 °C), residence time (10–40 min), and the presence of CO2 (0–18% v/v) in the reacting gas were investigated on products’ yields, distribution, and torrefied pellet properties such as higher heating value (HHV), elemental composition (C, H, O, N, S, K, and Cl), grindability, and moisture uptake ability. Temperature and residence time were found to affect the distribution of products’ yield and the properties of torrefied pellets considerably. In comparison with the untreated biomass pellets, the torrefied pellets appeared to have improved HHV and grindability but reduced moisture uptake ability. In the presence of CO2, the Boudouard reaction caused slight reductions in the C content and HHV of the torrefied pellets. Changes in torrefaction conditions did not prove to have a statistically significant effect on S, K, and Cl contents of the torrefied biomass materials.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Maize is one of the major economic crops grown worldwide. In Thailand, about five million tons of maize grains is produced annually [1], generating approximately 10 million tons of maize residues such as cobs, stalks, and peels [2]. Maize is harvested either manually or by simple mechanical harvesters where large amounts of maize residues are discarded as agro-wastes. These residues are usually disposed of by open burning, resulting in massive emissions of air pollutants to the atmosphere, which affects public health and the environment negatively. Utilization of maize residues for fuel and/or energy is of great interest to reduce the above-mentioned issues. Modern routes of conversion to transform maize residues to fuel include pyrolysis, gasification, and combustion processes [3,4,5,6,7].
In general, maize residues are usually pressed into cylindrical shapes or pellets prior to being used as solid fuel. Compared with the original form, the pellets are easier to handle in terms of storage and transportation. The pellets have increased density to 800–1200 kg/m3 [8] and reduced moisture content to about 10% w/w. The chemical properties of the biomass compressed into pellets, however, remain the same as the raw biomass; for example, the pellets still have hygroscopic nature and contain high oxygen content, causing a fairly low energy density. They also have poor grindability because the highly fibrous nature of the biomass is maintained through the pelletization process. These properties of the pellets reduce the efficiency of subsequent conversion processes. Interest in torrefaction of biomass pellets has been increasingly growing, since it is a simple process which can overcome these challenges. Torrefaction can also help to create a homogeneous, high-quality energy commodity which could serve as a potential replacement or alternative to coal.
Torrefaction is considered to be a relatively simple technology. It is a mild pyrolysis process, where raw biomass is subjected to thermal treatments at low temperatures between 200 and 300 °C in an oxygen-free environment [9]. During torrefaction, degradation of hemicellulose and glass transition of lignin occur together with de-polymerization of cellulose, and concurrently, the fibrous nature of raw biomass is broken down, leading to much higher grindability [10]. After undergoing torrefaction, pretreated biomass has improved energy densification by at least a factor of 1.3 over the raw biomass. The hygroscopic nature of biomass is changed to hydrophobic for the torrefied biomass, limiting moisture uptake ability to 1–3% w/w of the equilibrium moisture content, and therefore biological decomposition is unlikely to take place. The elemental composition of the torrefied biomass is also observed to be closer to that of coal [10], indicating that the torrefied biomass pellets may be co-fired with the coal in a power plant.
In the previous literature, Na et al. [11], who carried out pelleting of torrefied oil palm mesocarp fiber, found that the pellets could not be prepared from torrefied biomass, since physical flaws were formed in the torrefied biomass at high degrees of torrefaction severity. The well-formed pellets were established at relatively low conditions of the severity. This was in agreement with Rudolfsson et al.’s work [12], where wood chips of Scots pine were torrefied between 291 and 315 °C for 6 to 12 min of reaction time before the torrefied biomass was pelletized. In this work, the torrefied biomass pellets had bulk densities within 558 to 725 kg/m3, durability values in the ranges of 46 to 86%, and fine content in the ranges of 4 to 86%. The quality of torrefied biomass pellets which the biomass was torrefied before making the pellets was also found to be a function of many factors, such as particle size and moisture content of torrefied biomass as well as temperature and pressure of pelletizing [13]. In comparison with untreated biomass, torrefied biomass was difficult to be pressed to pellets; for example, at the same conditions of pelletization, the torrefied biomass pellets exhibited rather low durability and density. This was because the behavior of glass transition was not seen in any dry torrefied biomass lignin [14]. Tu et al. [15], who worked on torrefaction of Camellia shell at 220 °C, showed that the torrefied pellets with density of 1048 kg/m3 could be produced with low energy consumption of 17.6 kJ/kg but low Meyer hardness (6.8 N/mm2). Manouchehrinejad et al. [16] suggested that binders were needed for torrefied energy cane briquettes and torrefied napier grass briquettes in view of producing an excellent solid pellet fuel for a power plant.
Shang et al. [17], who carried out torrefaction of Scots pine pellets, pointed out that the HHV of the torrefied pellets increased to 24.37 MJ/kg from 18.37 MJ/kg of the original pellets, while the durability of the torrefied pellets remained higher than 97.5%, as required by the EN Plus standard. These properties indicated that torrefied biomass pellets were much more effective for transportation, handling, and grindability. Similarly, Peng et al. [18], who studied the effects of torrefaction of wood pellets on energy density and hardness, showed that torrefied wood pellets improved HHV and hydrophobicity even though they may significantly reduce pellet density and hardness if the degree of torrefaction severity was increased. Ghiasi et al. [19] claimed that pelletizing biomass followed by torrefaction was of interest, owing to the higher efficiency in the overall energy and material balance. This was in accordance with Chen et al. [20] who carried out torrefaction of oil palm fiber pellets under non-oxidative and oxidative environments. They found that the HHV from the non-oxidative and the oxidative torrefaction of oil palm fiber pellets was intensified by factors of 1.07 to 1.24. Furthermore, the liquid products had improved in the HHV from 92 to 139%, and therefore the retrieval of the liquid product with dehydration enabled the boosting of the energy efficiency of the torrefaction system. It was clear that torrefaction after pelletization was preferred.
Although some information on the torrefaction of biomass pellets in non-oxidative, oxidative, or even pure CO2 environments is available [21,22,23], reported work on application of flue gas from combustion to torrefaction of biomass remains scarce. With this application, raw maize pellets could be torrefied with a low temperature range of flue gas (200–300 °C), exhausting from the combustion process. This could produce torrefied maize pellets, which potentially improve the overall carbon efficiency of the process and also reduce emissions of greenhouse gases. Within this range of temperature, the flue gas may be dry which mainly contains about 8–20% v/v of CO2 balanced by N2 [24]. From energy and supply points of view, the utilization of dry flue gas for torrefaction of maize residue pellets will be of great interest in terms of practicality, affordability, and environmental sustainability rather than deploying inert or pure CO2 gas from compressed gas cylinders.
Therefore, the objective of this work is to experimentally investigate the effect of dry flue gas on torrefaction of maize residue pellets. Torrefaction experiments were conducted in a fixed bed reactor setup with real-time monitoring of weight change. Simulated flue gas containing CO2 in concentrations of 0–18% v/v balanced with N2 was used as the reactive gas. Test conditions were varied for 220, 260, and 300 °C of reaction temperature, and 10, 20, 30, and 40 min of residence time. Performance and properties in terms of distribution and yield of products (solid, liquid, and gas), HHV, elemental composition (C, H, O, N, S, Cl, and K), grindability, and moisture uptake ability were examined.
Materials and Methods
Maize Residue Sample
According to previous works, there are usually two methods used to produce torrefied biomass pellets: either torrefaction prior to pelletization or torrefaction after pelletization. However, recent researches showed that torrefied biomass was more difficult to be compressed into pellets under the same operating conditions than regular pellets [22, 25,26,27,28]. In other words, to make the same pellet quality in terms of density, for example, torrefied sawdust required pre-conditions, such as high die temperature and pressure, while raw sawdust may not. There has also been a limit in an industrial production process for torrefied biomass pellets from torrefied biomass [29]. Yet, the pelletization followed by torrefaction is of greater interest because the process has higher overall energy efficiency and material balance [19]. Additionally, biomass pellets from a traditional pelletization technology may be directly improved by the torrefaction process.
In this study, therefore, maize residual pellets were used for the torrefaction experimentation, in which the raw residues (cobs, stems, leaves, and husks) were gathered from a local farm in highland Chiang Mai, Thailand. Firstly, impurities and contaminants were removed from the maize residues, and then, the pure residues were dried in ambient air in a controlled store room. Subsequently, they were reduced in size, and pelletized into cylindrical shape. Each pellet had the dimension of 8.15 ± 0.1 mm in diameter, 30 ± 1.15 mm in length, and 2 ± 0.05 g in mass. A small batch of representative dried pellets was milled, sieved, and sent for property analyses.
Experimental Setup and Test Procedure for Torrefaction
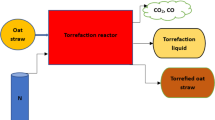
The maize pellets were thermally treated in a fixed bed, flow-through reactor [30], comprising a conditioning gas unit, a main thermal reactor unit, a real-time mass monitoring unit, and a by-product collection unit, as depicted in Fig. 1. The conditioning gas from the pressured gas cylinders was supplied through two flow meters and controllers. It was preheated with a 2.0 kW blanket heater and stirred well in a mixer, prior to being released to the main thermal reactor. The main thermal reactor was cylindrical in shape, made of stainless steel with dimension of 50 mm in diameter and 400 mm high. The main reactor was divided into heating and cooling sections. For the heating section, a 3.5 kW electric heater and a fiber board insulator were installed circumferentially on the outside wall of the main reactor. The power of the two heaters could be adjusted by controllers, coupled with temperature probes connected to a data logger. A peak heating rate of 30 °C/min and a peak temperature of 1100 °C could be achieved in the main thermal reactor. This section was designated for the biomass sample to undergo torrefaction. For the cooling section, a cooling water unit was externally mounted, in which was designated for the biomass sample to rest prior to and after each test. As for the real-time mass monitoring unit, a perforated basket 35 mm in diameter and 80 mm high was suspended inside the reactor with its top linked with a metal wire of 1.5 mm diameter to a digital balance connected to a computer to enable continuous recording of mass change. The digital balance used had a resolution of 0.01 g. For the by-product collection unit, an ice/salt mixing bath was adopted as the condenser to trap any condensable vapors/liquids released from the main reactor.
For each test, a sample mass of 40 ± 0.5 g (about 20 pellets) was used and loaded into the basket. The basket was initially positioned at the cooling section whose temperature remained at no more than 60 °C even with heating on. High purity research grade nitrogen and carbon dioxide gases were supplied at a given CO2 concentration (0, 6, 12, and 18%) as the conditioning gas with a total flow rate of 7 L/min. The conditioning gas was heated to establish a torrefying atmosphere, from room temperature to set point temperatures varying between 220, 260, and 300 °C. The change in the mass and the temperature of the conditioning gas were continually recorded at a frequency of 2 Hz. Once a designated residence time (10, 20, 30, and 40 min) was reached, the basket was moved to the cooling section at the top of the main reactor to stop the reaction. Immediately afterwards, the electric heaters were switched off while a flow of fresh gas was maintained until the temperature of the main reactor dropped to about 60 °C. The torrefied biomass pellets were then taken out and stored prior to subsequent sending for analyses. Each test condition was randomly chosen and reiterated for a minimum of three times. The yields of solid, liquid, and gas are calculated from Eq. (1)
where y is a product yield at any time t % w/w on dry basis, m is a mass of each product represented by subscript i, and m0 means an initial dry mass of raw pellets.
Elemental and Heating Value Analyses
Determination of C, H, and N was carried out using a Thermo Scientific CHNS/O analyzer model Flash 2000, while determination of S, Cl, and K was carried out using a Phillips X-ray fluorescence spectrometer model PW2400. The calorific value of the dried samples was evaluated using a bomb calorimeter, and given as a higher heating value of combustion at constant volume.
Grindability Test
Raw biomass materials are usually tough; hence, they are difficult to be reduced in size, because of their fibrous and tenacious nature. Torrefaction is known to tackle this resistance of biomass. Determination of the size reduction ability or grindability may be performed by determining the energy consumed per unit mass for grinding any material also termed grinding energy, as well as the resultant particle size distribution. The test setup for measurement of the grinding energy consumption for processing raw and torrefied pellets consisted of a Fiorenzota coffee grinder model F5, coupled with a C.A. 8220 power analyzer (000.1 to 999.9 W ± 1%) with an MN93A clamp 5 A (500 mA to 6 A ± 0.7%), and a data acquisition system, similar to Shang et al. [17] and Correia et al. [31]. About 20 ± 0.05 g of pellets was loaded into the grinder. For each batch of pellets, three runs were carried out in the grinder. For all the three intervals, the time taken to grind the pellets again and the power required by the grinder were recorded. The resultant grinding energy was calculated by numerically integrating the area under the curve of power against time. The size distribution of the ground pellets was also determined using the sieve method based on the ASTM E11 standard. Nine different sieves were used corresponding to mesh sizes of 2000, 1180, 850, 600, 425, 300, 212, 150, and 75 μm. The weight of each size fraction was subsequently evaluated.
Moisture Uptake Test
Measurement of the moisture uptake ability for raw and torrefied pellets was carried out using the gravimetric method based on transfer of saturated water vapor at constant pressure. Saturated salt solution in an enclosed chamber was adopted to create a controlled humidity atmosphere, similar to the one discussed in Peng et al.’s work [27]. The resultant humidity was dependent on the temperature. In this work, K2SO4 salt was used. Its saturated solution kept at 35 °C would give relative humidity of 94.8% [32]. Prior to each test, the pellet samples were dried in an oven at 105 °C for 24 h. They were then hung directly above an open container of the chosen salt solution inside the temperature-controlled chamber. To determine the moisture uptake rate, the weight of the pellets was monitored at 1, 2, 3, 6, 12, and 24 h, until no significant change was noticed.
Results and Discussion
Products Distribution and Yields
The results of product distribution and yields are shown in Fig. 2, for varying residence time and CO2 concentration at a fixed temperature of 300 °C. Figure 3 illustrates further how the solid yields changed at torrefaction temperatures of 220 and 260 °C with different conditions of varying residence time and CO2 concentration. The average values are shown along with bars representing individual standard errors of mean. At a relatively low temperature of 220 °C, changes in the residence time and the CO2 concentration in the reacting gas did not affect the solid yield much. The loss in the solid yield at this relatively mild condition was expected to be small, less than 5% w/w. At higher temperatures of 260 and 300 °C, the loss in the solid yield increased with temperature and residence time, ranging from 5 up to 55% w/w. This was contributed to the fact that the three main components of the biomass pellets started to thermally decompose in different temperature ranges; for example, hemicellulose decomposed easily within 220–315 °C, but the greatest mass loss occurred at temperatures more than 260 °C [33]. A longer period was needed for the heat to fully distribute inside the pellets [34]. These higher levels of the mass loss were, therefore, anticipated as the degree of torrefaction severity in terms of higher temperature, longer residence time, and higher concentration of CO2 increased [35]. The presence of CO2 in the reacting gas at the same concentration but higher torrefaction temperatures was also expected to reduce more torrefied solid yields because the presence of CO2 at high torrefaction temperature would enable (i) higher overall thermal inertia because the specific heat of CO2 is higher than that of N2, resulting in some heat removal during heating, and (ii) chemical reactions of CO2 with the biomass pellets, including possible catalytic reaction between the ash contained in the biomass pellets [36]. Nonetheless, changes in the solid yields with varying CO2 concentration were observed only within 1–3% w/w at any torrefaction temperature. This finding was in agreement with the findings of previous studies [21, 37]. In contrast, loss in the solid yields was directly revealed as gain in the gas and the liquid by-products, due to the fact that dehydration, initial devolatilization, and/or decarboxylation had occurred. Both increases in temperature and residence time resulted in higher yields of the liquid- and gas-phase products rather than with the presence of CO2 in the reacting gas. For example, at 220 °C, the gas yields were found to be within 3–5% w/w, and the liquid yields were between 0 and 8%. Yet, at 300 °C, the gas yields were about 5 to 20% w/w, and the liquid yields were between 0 and 35% w/w, as shown in Fig. 2 (a) and (b). The presence of CO2 in the reacting gas was found to affect the gas-phase product yields only at any torrefaction temperature. In comparison with the inert condition, at 300 °C for 20 min of residence time with applied CO2 in the reacting gas, the gas yields increased about 5% from 10% w/w of the inert condition. The changes in the liquid and gas yields were also similar to Saadon et al. [37] who explained that the presence of CO2 in the reacting gas limited the formation of liquid products, but boosted up the generation of volatile components, and accordingly caused the gas products were formed in the higher yields.
Elemental and Heating Value Analyses
Figure 4 shows the results from the elemental and the heating value analyses of raw and torrefied biomass pellets, for varying residence time and CO2 concentration at a fixed temperature of 300 °C. The values for raw materials were noted at the residence time of 0 min. The removal of moisture and initial volatile matter in the biomass pellets during torrefaction resulted in the reduction of the O and the H content, leading to increased energy density. An increase in the torrefaction time appeared to increase the amount of elemental C and the heating value in the torrefied pellets. The percentages of gain in the C content and the HHV were in the ranges of 35 to 45% and 29 to 41%, respectively. At the same time, the O content dropped from 50 to about 31–35% w/w, and the H content reduced from 5.6 to about 3.6–4.1% w/w, in similar order of magnitude to those reported in the literature [10, 29, 38, 39].
However, increasing the CO2 content in the carrier gas did not seem to offer any statistically significant change in the elements and the HHV. Uemura et al. [23] claimed that the presence of CO2 could reduce the C content in the solid product and the composition of the gaseous by-products through Boudouard reaction (CO2(g) + C(s) → 2CO(g)). By torrefying activated carbon at 300 °C under 15% v/v CO2 balanced with N2, they found that a CO yield of 0.11% v/v was produced. The present study also investigated the gaseous products from the torrefaction process, using a Shimadzu 8A gas chromatograph equipped with a thermal conductivity detector and a Shin carbon column. The gas samples were collected at every 5 min during the biomass decomposition, and the results are shown in Fig. 5. The amount of CO was found to be about 1.5% of the peak area with no CO2 applied, but it increased to around 4% of the peak area in the case of 18% v/v CO2 in the reacting gas. With these conditions, the C content was found to have decreased to 60 from about 65% w/w with inert atmosphere, as shown in Fig. 4. Reduction in elemental C under CO2 environment has also been reported by Eseltine et al. [21]. Another observation from Fig. 5 is that all the mean values of the generated CO in the gas-phase products were found between 10 and 30 min of residence time, and all the peaks were found at 20 min of residence time (with different values of the peaks). This indicated that the main reactions of maize pellet decomposition occurred within 10–30 min of residence time, and the main reactions had the highest rate at 20 min of residence time. These reasonings were supported by the results of solid yields, as shown in Fig. 2 (c). The solid yields slightly changed at the conditions of residence time between 0–10 min and 30–40 min, but the yields markedly decreased (about 30% w/w, compared with the yields at 10 min of residence time) if residence time was extended to 20 min.
Decreases in the H/C and the O/C atomic ratios contributed to the removal of the water content and the light volatiles. The losses in the H/C and the O/C atomic ratios were found to be more than 50%, in which the average atomic ratios of the H/C and the O/C of the torrefied pellets were observed to have improved from the original values of 1.5 and 0.85 to 0.8 and 0.45 at the most severe torrefaction conditions, as shown in Fig. 6. Martín-Lara et al. [40] showed similar changes in the H/C and the O/C ratios of the sample from 0.7 to 0.1 and 1.0 to 0.2, respectively, for biomass torrefied at 300 °C and 60 min. In a non-inert atmosphere, Chen et al. [29] reported decline in the H/C and the O/C ratios from about 1.1–1.5 and 0.6–0.7 for raw materials to approximately 0.2–0.9 and 0.2–0.35, respectively, for torrefied materials. Reductions in H and O contents of the torrefied biomass pellets, relative to the C content, approached the values for charcoal and coals in the van Krevelen diagram linearly. This finding agreed with Li et al. [41]. The release of moisture and light volatiles and the degradation of hemicellulose resulted in the linear profile between the atomic H/C and O/C ratios in the van Krevelen plot, which were a consequence of the devolatilization and pyrolysis of the biomass during the thermal degradation.
Van Krevelen (H/C vs. O/C atomic ratio) diagram of raw and torrefied maize pellets for four different CO2 concentrations. The higher degree of torrefaction refers to that the biomass pellets were torrefied at higher temperatures, longer periods of residence time, and more CO2 contents in the reacting gas
Regarding changes in other elements considered here, the S content was observed to remain at a relatively stable value when undergoing torrefaction, as shown in Fig. 7. At a given temperature but varying residence time, the K content appeared to show a slight shift in value, whereas the Cl content exhibited a slight drift. The opposite changes in the K and Cl contents observed here were similar to that reported in previous work, such as Chen et al. [42], who investigated the release and transformation of K and Cl during mild pyrolysis of rice straw between 200 and 350 °C. In this work, the Cl content was markedly released (between 5–20% w/w of the total Cl in the biomass) from the biomass at 250 and 300 °C, and the K was found to decompose to the gas phase about 6.5–7.3% w/w of the total K compound in the biomass at only temperatures above 300 °C. However, Bläsing et al. [43], who applied torrefaction to upgrade wheat straw, reported that increases in the K and Cl contents occurred together if torrefied. Yet, at higher torrefaction temperatures, the K was found to show higher values, while the Cl exhibited a slight drop. Based on the literature, the K content in biomass fuels during pyrolysis started to be released to the gas-phase products considerably at temperatures over 700 °C [44]. At lower temperatures, it may be thermally decomposed during devolatilization at about 5–10% w/w of the total K in the biomass [45]. On the other hand, the release of Cl was observed at low temperature, mainly resulted from the reaction between metal chlorides and oxygen-containing functional groups, such as –COOH, and/or methyl-esterified carboxyl group, which occurred simultaneously with devolatilization [46]. The Cl compound would be released to the gas-phase products along with volatiles through HCl and/or CH3Cl, after it was initially formed in inner structures of fuel particles and moved to the surfaces [45,46,47].
Grindability
Figure 8 shows the grindability of the torrefied pellets against raw biomass pellets in terms of (a) the relationship between the grinding energy and the mass loss, as well as (b) the mass weighted size distribution of the resultant ground particles. The degree of loss in the solid yield was used to represent the severity of torrefaction experienced under the various conditions applied in this study. Mass loss of up to about 60% was realized, with grinding energy ranging from an average of 690 J/g for raw biomass pellets to about 25 J/g for the maximum mass loss case. It was clearly demonstrated that torrefaction was able to immensely reduce the fibrous and tenacious nature in the treated biomass pellets. As for the raw biomass materials, the fibers could form links between the particles, and consequently, this made the handling of the raw ground samples difficult. In contrast, the torrefied biomass particles tended to be isolated, owing to the fact that devolatilization of its fibrous microstructure caused microporous structures to develop on the surface of the torrefied pellets. Wang et al. [48] reported a 50% reduction in the grinding energy required when the pellets were torrefied at 225 °C. In this work, decreases in the grinding energy by 35% at 10% mass loss, a factor of five at 20% mass loss, and more than 27 times at 60% mass loss were observed, compared with the untreated biomass pellets. This is in agreement with Repellin et al. [49], who presented mass loss as a function of torrefaction severity and noted that torrefied wood (spruce and beech) with 28% mass loss demonstrated lowering in grinding energy by 93%. Together with grinding energy, particle size distribution also determines the grindability of torrefied biomass. Compared with raw materials, the size distribution curves were observed to shift toward smaller particle sizes. Higher severity of the torrefaction reaction appeared to promote finer particle sizes. There was, for example, about 20% of raw pellets with particle sizes between < 600 and 150 μm, while there was over 60% of torrefied pellets in the same particle size range. Increased mass fraction resulting in finer particles for torrefied biomass was confirmed by other studies as well [50,51,52].
Moisture Uptake Ability
Figure 9 shows the effects of varying torrefaction temperature, residence time, and CO2 concentration on the uptake of humidity from the surrounding air. Even with drying, biomass fuels tend to recapture moisture from the air when stored. This particular property of biomass makes long-term storage very costly, since it requires controlled storage conditions and moisture removal during subsequent combustion or gasification. Water uptake ability is inversely proportional to the hydrophobicity of biomass. The torrefaction process was found to positively affect the water uptake of the biomass pellets. At a given CO2 concentration and residence time, a higher temperature was observed to have an adverse influence on the water uptake ability of torrefied pellets. The moisture uptake of around 15% in raw pellets was observed to decrease to about 6% in torrefied pellets at 300 °C. Similarly, at a given CO2 concentration and temperature, a higher residence time reduced the saturated water uptake of the torrefied pellets, compared with the untreated pellets. At 300 °C, raising the residence time to 40 min caused the water uptake ability to drop by over 60%, compared with raw materials. For a fixed temperature of 260 °C, and residence time of 40 min, changes in the CO2 concentration did not affect a change in the water uptake ability markedly. Nonetheless, the torrefied biomass pellets were found to be more hydrophobic than the untreated raw biomass pellets. A reduction in the moisture uptake capacity from about 15% for raw biomass pellets to 5% for the torrefied pellets was evident. The observation was similar to those reported in the literature [25, 27, 41, 53, 54]. Peng et al. [27] noted a reduction of about 36% in moisture uptake when torrefaction temperature increased from 240 to 300 °C. Li et al. [25] reported a decrease in water uptake ability from about 21 to 14% in torrefied sawdust pellets. Bergman [53] claimed that torrefaction of biomass went through total dehydration, after which uptake of moisture was very limited, 1 to 6%, depending on the torrefaction treatment conditions. Li et al. [41] confirmed that the temperature at which the torrefaction process was carried out showed more significant influence than the other factors. The moisture uptake of raw materials, which is over 20%, was found to decline to about 5% for torrefied materials at 340 °C. It was postulated that torrefaction destroyed the OH groups within the hemicellulose and the cellulose of the biomass material, consequently weakening the ability of the torrefied materials to establish hydrogen bonds with water. Furthermore, non-polar and unsaturated structures were reported [54].
Conclusions
In this work, torrefaction of maize pellets was experimentally carried out using simulated dry flue gas as the reacting gas with the presence of CO2 (0–18 v/v) at 220–300 °C for 10–40 min. The torrefaction of biomass pellets with the dry flue gas was demonstrated to be practical, in which the torrefaction offered remarkable improvement of biomass pellets by removing moisture content and initial volatile matter, leading to increases in C and energetic contents as well as modifying mechanical and water uptake characteristics. Among all the conditions considered here in this study, the overall best conditions of torrefaction were either at 300 °C for 20 min or at 260 °C for 30 min, with any CO2 concentration in the reacting gas. These torrefaction parameters resulted in the torrefied pellets with 70–85% w/w yields, 18–21 MJ/kg HHVs, 0.75–0.90 H/C, and 0.45–0.60 O/C atomic ratios, and over 50% reduction in grinding energy consumption. The findings provide a favorable outlook for energy utilization of agro-residues via torrefaction with dry flue gas as a pretreatment method.
References
Office of Agricultural Economics. Thailand agricultural production information. Available: www.oae.go.th/. Accessed: 16-Feb-2018.
Kerdsuwan S, Laohalidanond K (2015) Approach of using corn residue as alternative energy source for power production: a case study of the northern plain area of Thailand. Energy Procedia 79:125–130
Wongsiriamnuay T, Tippayawong N (2015) Effect of densification parameters on the properties of maize residue pellets. Biosyst Eng 139:111–120
Tippayawong N, Rerkkriangkrai P, Aggarangsi P, Pattiya A (2018) Characterization of biochar from pyrolysis of corn residues in a semi-continuous carbonizer. Chem Eng Trans 70:1387–1392
Jaroenkhasemmeesuk C, Tippayawong N (2015) Technical and economic analysis of a biomass pyrolysis plant. Energy Procedia 79:950–955
Punnarapong P, Promwungkwa A, Tippayawong N (2017) Development and performance evaluation of a biomass gasification system for ceramic firing process. Energy Procedia 110:53–58
Sittisun P, Tippayawong N, Wattanasiriwech D (2015) Thermal degradation characteristics and kinetics of oxy combustion of corn residues. Adv Mater Sci Eng 304395:1–8
Piboon P, Tippayawong N, Wongsiriamnuay T (2018) Densification of corncobs using algae as a binder. Chiang Mai Univ J Nat Sci 16:175–182
Srinivasan V, Adhikari S, Chattanathan SA, Tu M, Park S (2014) Catalytic pyrolysis of raw and thermally treated cellulose using different acidic zeolites. BioEnergy Res 7(3):867–875
Tumuluru JS, Sokhansanj S, Hess JR, Wright CT, Boardman RD (2011) A review on biomass torrefaction process and product properties for energy applications. Ind Biotechnol 7(5):384–401
Na B-I, Kim Y-H, Lim W-S, Lee S-M, Lee H-W, Lee J-W (2013) Torrefaction of oil palm mesocarp fiber and their effect on pelletizing. Biomass Bioenergy 52:159–165
Rudolfsson M, Borén E, Pommer L, Nordin A, Lestander TA (2017) Combined effects of torrefaction and pelletization parameters on the quality of pellets produced from torrefied biomass. Appl Energy 191:414–424
Rudolfsson M, Stelte W, Lestander TA (2015) Process optimization of combined biomass torrefaction and pelletization for fuel pellet production – a parametric study. Appl Energy 140:378–384
Reza MT, Uddin MH, Lynam JG, Coronella CJ (2014) Engineered pellets from dry torrefied and HTC biochar blends. Biomass Bioenergy 63:229–238
Tu R, Jiang E, Yan S, Xu X, Rao S (2018) The pelletization and combustion properties of terrified Camellia shell via dry and hydrothermal torrefaction: a comparative evaluation. Bioresour Technol 264:78–89
Manouchehrinejad M, Yue Y, de Morais RAL, Souza LMO, Singh H, Mani S (2018) Densification of thermally treated energy cane and napier grass. BioEnergy Research 11(3):538–550
Shang L et al (2012) Quality effects caused by torrefaction of pellets made from Scots pine. Fuel Process Technol 101:23–28
Peng J et al (2015) Effects of thermal treatment on energy density and hardness of torrefied wood pellets. Fuel Process Technol 129:168–173
Ghiasi B et al (2014) Densified biocoal from woodchips: is it better to do torrefaction before or after densification? Appl Energy 134:133–142
Chen W-H, Zhuang Y-Q, Liu S-H, Juang T-T, Tsai C-M (2016) Product characteristics from the torrefaction of oil palm fiber pellets in inert and oxidative atmospheres. Bioresour Technol 199:367–374
Eseltine D, Thanapal SS, Annamalai K, Ranjan D (2013) Torrefaction of woody biomass (juniper and mesquite) using inert and non-inert gases. Fuel 113:379–388
Wang C, Peng J, Li H, Bi XT, Legros R, Lim CJ, Sokhansanj S (2013) Oxidative torrefaction of biomass residues and densification of torrefied sawdust to pellets. Bioresour Technol 127:318–325
Uemura Y, Saadon S, Osman N, Mansor N, Tanoue K (2015) Torrefaction of oil palm kernel shell in the presence of oxygen and carbon dioxide. Fuel 144:171–179
Lasek JA, Kopczyński M, Janusz M, Iluk A, Zuwała J (2017) Combustion properties of torrefied biomass obtained from flue gas-enhanced reactor. Energy 119:362–368
Li H, Liu X, Legros R, Bi XT, Jim Lim C, Sokhansanj S (2012) Pelletization of torrefied sawdust and properties of torrefied pellets. Appl Energy 93:680–685
Peng JH, Bi HT, Sokhansanj S, Lim JC (2012) A study of particle size effect on biomass torrefaction and densification. Energy Fuel 26(6):3826–3839
Peng JH, Bi HT, Lim CJ, Sokhansanj S (2013) Study on density, hardness, and moisture uptake of torrefied wood pellets. Energy Fuel 27(2):967–974
Peng JH, Bi XT, Sokhansanj S, Lim CJ (2013) Torrefaction and densification of different species of softwood residues. Fuel 111:411–421
Chen W-H, Peng J, Bi XT (2015) A state-of-the-art review of biomass torrefaction, densification and applications. Renew Sust Energ Rev 44:847–866
Onsree T, Tippayawong N, Zheng A, Li H (2018) Pyrolysis behavior and kinetics of corn residue pellets and eucalyptus wood chips in a macro thermogravimetric analyzer. Case Stud Thermal Eng 12:546–556
Correia R, Gonçalves M, Nobre C, Mendes B (2017) Impact of torrefaction and low-temperature carbonization on the properties of biomass wastes from Arundo donax L. and Phoenix canariensis. Bioresour Technol 223:210–218
Choudhury D, Sahu JK, Sharma GD (2011) Moisture sorption isotherms, heat of sorption and properties of sorbed water of raw bamboo (Dendrocalamus longispathus) shoots. Ind Crop Prod 33(1):211–216
Yang H, Yan R, Chen H, Lee DH, Zheng C (2007) Characteristics of hemicellulose, cellulose and lignin pyrolysis. Fuel 86(12-13):1781–1788
Onsree T, Sittisun P, Sasaki R, Tippayawong N (2018) Pyrolysis of corn residues: kinetic analysis using discrete distributed activation energy model. IOP Conf Ser: Earth Environ Sci 159:1–7
Onsree T et al (2019) Torrefaction of pelletized corn residues with wet flue gas. Bioresour Technol 285:121330. https://doi.org/10.1016/j.biortech.2019.121330
Thanapal SS, Chen W, Annamalai K, Carlin N, Ansley RJ, Ranjan D (2014) Carbon dioxide torrefaction of woody biomass. Energy Fuel 28(2):1147–1157
Saadon S, Uemura Y, Mansor N (2014) Torrefaction in the presence of oxygen and carbon dioxide: the effect on yield of oil palm kernel shell. Procedia Chemistry 9:194–201
Acharya B, Sule I, Dutta A (2012) A review on advances of torrefaction technologies for biomass processing. Biomass Conversion and Biorefinery 2(4):349–369
Nunes LJR, Matias JCO, Catalão JPS (2014) A review on torrefied biomass pellets as a sustainable alternative to coal in power generation. Renew Sust Energ Rev 40:153–160
Martín-Lara MA, Ronda A, Zamora MC, Calero M (2017) Torrefaction of olive tree pruning: effect of operating conditions on solid product properties. Fuel 202:109–117
Li M-F, Li X, Bian J, Xu J-K, Yang S, Sun R-C (2015) Influence of temperature on bamboo torrefaction under carbon dioxide atmosphere. Ind Crop Prod 76:149–157
Chen H, Chen X, Qiao Z, Liu H (2016) Release and transformation characteristics of K and Cl during straw torrefaction and mild pyrolysis. Fuel 167:31–39
Bläsing M, Tanner J, Winters T, Müller M (2017) Brief evaluation of selected fuel characteristics of thermochemically upgraded wheat straw: torrefaction and hydrothermal carbonization. Energy Fuel 31(12):14426–14429
Knudsen JN, Jensen PA, Dam-Johansen K (2004) Transformation and release to the gas phase of cl, k, and s during combustion of annual biomass. Energy Fuel 18(5):1385–1399
Johansen JM, Jakobsen JG, Frandsen FJ, Glarborg P (2011) Release of K, Cl, and S during pyrolysis and combustion of high-chlorine biomass. Energy Fuel 25(11):4961–4971
van Lith SC, Jensen PA, Frandsen FJ, Glarborg P (2008) Release to the gas phase of inorganic elements during wood combustion. Part 2: Influence of fuel composition. Energy Fuel 22(3):1598–1609
Jensen PA, Frandsen FJ, Dam-Johansen K, Sander B (2000) Experimental investigation of the transformation and release to gas phase of potassium and chlorine during straw pyrolysis’. Energy Fuel 14(6):1280–1285
Wang L et al (2017) Impact of torrefaction on woody biomass properties. Energy Procedia 105:1149–1154
Repellin V, Govin A, Rolland M, Guyonnet R (2010) Energy requirement for fine grinding of torrefied wood. Biomass Bioenergy 34(7):923–930
Gil MV, García R, Pevida C, Rubiera F (2015) Grindability and combustion behavior of coal and torrefied biomass blends. Bioresour Technol 191:205–212
Phanphanich M, Mani S (2011) Impact of torrefaction on the grindability and fuel characteristics of forest biomass. Bioresour Technol 102(2):1246–1253
Arias B, Pevida C, Fermoso J, Plaza MG, Rubiera F, Pis JJ (2008) Influence of torrefaction on the grindability and reactivity of woody biomass. Fuel Process Technol 89(2):169–175
P. C. A. Bergman (2005) Combined torrefaction and pelletisation: the TOP process. Netherlands Energy Research Foundation: Petten, Report ECN-C-05-073.
Iroba KL, Baik O-D, Tabil LG (2017) Torrefaction of biomass from municipal solid waste fractions II: Grindability characteristics, higher heating value, pelletability and moisture adsorption. Biomass Bioenergy 106:8–20
Funding
This work received support from the Thailand Research Fund via the Research and Researcher for Industry program (grant no. PHD57I0059), the National Research Council of Thailand (grant no. 2559-149), and Chiang Mai University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that there is no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Onsree, T., Tippayawong, N. Torrefaction of Maize Residue Pellets with Dry Flue Gas. Bioenerg. Res. 13, 358–368 (2020). https://doi.org/10.1007/s12155-019-10058-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12155-019-10058-x