Abstract
The diphosphate RbGdP2O7 compound has been synthesized by the conventional solid-state reaction method and characterized by X-ray powder diffraction, Raman spectroscopy and impedance spectroscopy. The title compound crystallizes in the monoclinic system with P21/c space group. The AC electrical conductivity was measured in a frequency range from 200 Hz to 1 MHz and temperature range 480–580 K. Impedance plot revealed the presence of two contributions at different temperatures associated with grain and grain boundary. The obtained results were analyzed by fitting the experimental data to an equivalent circuit model based on the ZView software. The temperature dependence of these contributions is found to obey the Arrhenius law with activation energies 0.42 eV and 0.32 eV, respectively. The alternating current (AC) conductivity of grain contribution follows the universal Jonscher’s power law. The temperature dependency of frequency exponent ‘s’ shows that the correlated barrier hopping model (CBH) is the most responsible mechanism for AC conduction in the investigated compound. The theoretical fitting between the proposed model and the experimental data showed good agreement.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Over the past years, phosphate compounds have been the subject of numerous investigations for their potential applications in diverse areas such as solid-state laser materials, sensors, solid-state batteries, catalysts and ionic conductors [1,2,3,4,5,6]. Interestingly, diphosphate compounds with general formula AIMIIIP2O7 (where AI is a monovalent cation and MIII rare earth element) have been a focal point due to their interesting conductivity coming from the mobile AI ions located in the diphosphate group.
The essential structural feature of alkali rare earth diphosphates is a three-dimensional, cation–anion network having an interconnected interstitial space occupied by mobile AI ions [7]. On the other hand, monovalent ions are responsible for the ionic conductivity properties that were observed in many compounds, with the attendant promise of applications such as solid electrolytes in rechargeable batteries [8,9,10,11,12].
Many investigations were carried out for these types of materials through these original properties and their good conductivity. Among the most interesting diphosphates, we have selected the RbGdP2O7 compound with tunnel structure where the Rb ions reside; this is a good character to supply a good conductivity.
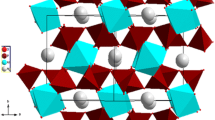
A crystallographic study carried out by Parajón-Costa et al. revealed that RbGdP2O7 material is isostructural with KYP2O7 [13]. It crystallizes in the monoclinic system (P21/c space group) with the following unit cell parameters: a = 7.722 Å, b = 11.095 Å, c = 8.707 Å, and β = 105.281°. The structural arrangement consists of corner-sharing GdO6 octahedral and P2O7 diphosphate groups. The three-dimensional framework delimits tunnels running along [1 0 0] direction where the rubidium ions are located (Fig. 1).
The aim of this work is to report a detailed investigation on the electrical features of this compound as a function of frequency and temperature by using the impedance spectroscopy. Also, this study may give valuable information on the electrical conductivity and to determine the appropriate model responsible for the conduction mechanism.
2 Experimental details
The diphosphate RbGdP2O7 compound was obtained by the conventional solid-state reaction. The raw materials are Rb2CO3 (99.8%, Sigma-Aldrich), NH4H2PO4 (99.999%, Sigma-Aldrich) and Gd2O3 (99.99%, Sigma-Aldrich). The starting reagents with their stoichiometric ratios were ground in an agate mortar and progressively heated from room temperature to 573 K in order to eliminate NH3, H2O and CO2.
The resulting sample was reground, pelletized into cylindrical pellets using a hydraulic press at a pressure of 5 t/cm2 and heated again at 800 K for 12 h in which the relative density obtained was 92%. The following reaction describes the synthesis of compound RbGdP2O7:
X-ray diffraction pattern was recorded at room temperature using a Philips PW1710 diffractometer operating with copper anticathode radiation CuKα (λkα = 1.5418 Å) in a range of Bragg’s angle 10° ≤ 2θ ≤ 90°. Unit cell parameters of the synthesized sample were refined with the Full Prof program by the least square method from the powder data.
The Raman spectrum was registered at room temperature in the wavenumber range (100–1400) cm−1 with a T-64000 Raman spectrometer (Horiba–Jobin–Yvon), using the 514.5-nm radiation of an Ar/Kr laser as excitation.
A pellet of 8 mm diameter and 1.2 mm thickness was used for electrical measurements. To obtain good contact, the pellet was covered on the opposite surfaces with a thin layer of gold and then mounted between two copper electrodes in a special holder. The performed measurements were taken in the frequency and temperature range of 200 Hz–1 MHz and 480–580 K, respectively, using an Agilent 4294 impedance analyzer.
3 Results and discussion
3.1 X-ray powder analysis
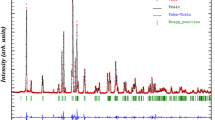
X-ray diffractogram (XRD) pattern of RbGdP2O7 recorded at room temperature is displayed in Fig. 2. Profile refinements using FoolProof suite program indicate that the resulting sample crystallizes in the monoclinic system with P21/c space group. The estimated lattice parameters obtained from Rietveld analysis were found to be: a = 7.6917 Å, b = 11.0553 Å, c = 8.7778 Å and β = 105.13°. The reliability factors are: Rp = 18.1%, Rwp = 14.2% and χ2 = 3.5. The refined lattice parameters of the compound are found to be very much consistent with those reported in the literature [13].
The Rietveld refinement for RbGdP2O7 compound at room temperature. The dotted line indicates experimental data, and calculated data are represented by black continuous lines. The lowest curve in blue shows the difference between experimental and calculated patterns. The vertical bars in blue indicate the Bragg positions (colour figure online)
3.2 Raman spectroscopy
The Raman spectrum of RbGdP2O7 compound, obtained at room temperature, is shown in Fig. 3. A detailed assignment of the most important bands is dared by comparison with similar compounds [14,15,16,17,18]. The frequencies and proposed bands assignment are listed in Table 1. The frequencies of the P2O7 groups are attributed on the basis of the characteristic vibrations of the P–O–P bridge and PO3 groups. Since the P-O bond in the PO3 groups is stronger than that in the P–O–P bridge, we can note that the vibration frequencies of PO3 are expected to be higher than those of P–O–P.
In fact, the bands observed at 1256, 1142 and 1100 cm−1 are attributed to the asymmetric νas (PO3) stretching modes, whereas the symmetric stretching νs (PO3) modes are found at the wavenumber 1072, 1041 and 987 cm−1. The band related to the asymmetric stretching νas (POP) is detected at 719 cm−1. The bands observed between 671 and 629 cm−1 are due to symmetric stretching vibration of νs (POP). As regards the deformation δ (PO3) mode, it is identified in 574– 236 cm−1 region.
3.3 Impedance spectroscopy
Impedance spectroscopy is a useful technique to investigate, analyze and differentiate the role of different microstructures in the conduction process as each region has its unique electrical response within the chosen frequency and temperature domain. This technique allows the estimation grain and grain boundary’s resistivity and capacitance [15, 19, 20].
Figure 4 represents the Nyquist plots (−Z″(ω) vs. Z′(ω)) of RbGdP2O7 compound for several temperatures. All these plots are characterized by the presence of two semicircles revealing the existence of two contributions: the first, detected at high frequency, describes grain contribution, while the second, at low frequency, is due to grain boundary one. The center of those semicircles is localized below the real axis (Z′ axis), indicating a non-Debye-type relaxation [21]. ZView software was used to adjust the impedance data.
The best fits are obtained when we use an equivalent circuit (Fig. 5) that consists of a combination series of grains and grains boundary elements [22, 23]. The first consists of a parallel combination of resistance (R1) and a constant phase element (CPE1), while the second consists of a parallel combination of resistance (R2), capacitance (C) and a constant phase element CPE2. The impedance of CPE is: \(Z_{\text{CPE}} = \frac{1}{{Q(j\omega )^{\alpha } }}\)
The real (Z′) and imaginary (−Z″) components of the impedance have been calculated, respectively, according to Eqs. (1) and (2):
where ω is the angular frequency, Q is the capacitance value of the CPE element, and α is the fractal exponent.
In order to confirm the choice of equivalent circuit, we present in Figs. 6 and 7 the variation of the experimental data of (Z′) and (Z″) at several temperatures versus the simulated ones calculated using the parameters of the equivalent circuit. The superposition of these curves reveals a linear behavior with a slope substantially equal to the unity. This behavior indicates that the proposed equivalent circuit well describes the electric properties of the RbGdP2O7 compound.
The temperature dependence of the electrical conductivity of grain (σg) and grain boundary (σgb) is displayed in Fig. 8. It is clear from this figure that both σg and σgb conductivities increase with the rise in temperature, which indicates a typical Arrhenius-type behavior in the sample [24]. The DC conductivity of grain and grain boundaries was calculated from Eqs. (3 and 4):
where e and S are, respectively, the thickness and the area of the pellet. The pure capacitance C can be obtained from the constant phase element CPE, using the following equation:
Following the Arrhenius law, the activation energy for bulk and grain boundary is found to be 0.42 eV and 0.32 eV, respectively. The obtained activation energy Eg is similar to those reported for semiconductor compounds [25, 26].
3.4 AC conductivity analysis
The frequency dependence of AC conductivity in RbGdP2O7 compound at different temperatures is plotted in Fig. 9. In some cases, the phenomenon of the AC conductivity dispersion is analyzed using the Jonscher’s universal power law [27]:
where σdc is the direct current conductivity, A is constant for a particular temperature, and s is an exponent less than or equal to unity which represents the degree of interaction between mobile ions and their surrounding lattices. An overview of the results (Fig. 9) unambiguously illustrates the existence of three distinct regions. In region I, at low frequency, the conductivity is almost constant. This is the evidence for the frequency-independent nature of the conductivity which represents the DC conductivity.
In the middle region of the figure, conductivity begins to increase nonlinearly due to the fact that capacitor admittance becomes numerically larger than resistor admittance with increasing frequency. However, for the third region, high-frequency domain, we notice that conductivity becomes proportional to frequency, involving loss approximately constant [28, 29].
Preliminary attempts to fit the conductivity results to Eq. (6) were unsuccessful. Thus, the frequency-dependent conductivity is analyzed by the following expression [30]:
where σs and σ∞ are the conductivity values at low and high frequencies, respectively, τ is the characteristic relaxation time.
It is clear that experimental and fitted data are very close (Fig. 9). The frequency exponent s obtained by fitting the AC conductivity using Eq. (7) is plotted as a function of temperature in Fig. 10. It can be observed that the exponent s decreases from 0.46 at 490 K to 0.26 at 580 K. In general, the effect of temperature on the exponent (s) plays a key role in the estimation of conduction mechanism in disordered materials. According to the literature, there are different models [31,32,33] explaining the behavior of exponent s and its variation with frequency and temperature.
-
Overlapping large-polaron tunneling (OLPT) model, wherein with the increase in temperature the exponent s first decreases, attains a minimum value and then increases.
-
Non-overlapping small polaron tunneling (NSPT) model, wherein the exponent s increases with the increase in temperature.
-
Quantum mechanical tunneling (QMT) model, where the exponent s is independent of temperature and takes value equal to 0.8.
-
Correlated barrier hopping (CBH) model, where the exponent s decreases with the increase in temperature.
In the present case, the temperature dependence of the exponent s for the studied sample is shown in Fig. 10, which clearly shows that s decreases with the increase in temperature. Consequently, the correlated barrier hopping (CBH) [34, 35] is in good agreement with our experimental results, suggesting, thus, that the electrical AC conduction mechanism of RbGdP2O7 compound can be explained by charge-carrier hops between sites over the potential barrier WM separating them. In this model, carrier motion occurs by means of hopping over the Coulomb barrier separating two defect centers, and the AC conductivity is given by [36]:
where n is the number of polarons involved in the hopping process, N is the density of pair sites, and Rω is the hopping distance for conduction (ω τ = 1) and is given by [37]:
where WM is the binding energy of the carrier in its localized sites and τ0 is a characteristic relaxation time which is in the order of an atom vibrational period 10−13 s [38]. The power exponent s obeys the following relation [4]:
Equation (10) can be approximated to take the form:
The value of WM is calculated as 0.21 eV from the slope of the straight line in Fig. 10. According to the CBH model, if the maximum barrier height WM is the quarter of the activation energy (Ea), single polaron is the dominating conduction mechanism, while if WM = Ea/2, the bipolaron hopping is the dominating [39]. From the above consideration, the maximum barrier height WM is approximately the half of the activation energy which indicates that the bipolaron hopping is the dominating conduction mechanism.
The temperature dependence of AC conductivity for the titled compound is given in Fig. 11. Clearly, this figure shows that the theoretical calculations fitted by Eq. (8) are good with the experimental results. It is evident from this figure that σac increases linearly with the temperature, implying that the AC conductivity is a thermally activated process. The calculated fitting parameters are summarized in Table 2. From a qualitative analysis of these values, we can observe that an increase in the frequency causes a decrease in the states density localizes as shown in Fig. 12. Based on the definition given by Mott et al. [40], we can connect the reduction of the localized states density as a function of the frequency by the increase in the disorder, therefore diminishing the stabilities of states from which the non-localization of the latter occurs.
Figure 13 illustrates the variation of the tuning distance Rω with temperature at selected frequencies. It is remarkable that Rω increases with the increase in temperature, suggesting that the increase in temperature provides a contribution of thermal energy to polarons, which, afterward, will move and facilitate the hopping because of the interchain interaction that will occur. This is explained by the fact that the increase in the temperature induces a local disorder within the material. This disorder will, thus, favor the decrease in electrical conductivity.
The only conduction pathway is along tunnels in the a-direction, and the modest conductivity is determined by the dimensions of the tunnels section. So, further investigation on the relation of the electrical conductivity to structure for the RbGdP2O7 compound was done. Consequently, as mentioned earlier, there is one kind of tunnels where Rb+ cations are located. Based on the reported data [13] and using DIAMOND—Visual Crystal Structure software, we are able to present in Fig. 14. Otherwise, the dimensions of the tunnels vary between 4.977 and 6.255 Å, while, according to Shannon radii, the diameter of the rubidium ion is 3.32 Å [41]. We may conclude that this tunnel is of a sufficient dimension for ion transport. Thus, it can be deduced that the conduction process is assured by the movement of the Rb+ cations along a-axis tunnels.
4 Conclusions
In summary, we have synthesized the RbGdP2O7 diphosphate by solid-state reaction. The X-ray diffraction analysis shows that the sample crystallizes in the monoclinic system with space group P21/c. The Raman spectrum was recorded and interpreted on the basis of the structural peculiarities of the P2O74− moieties present in the crystal lattice. In addition, the impedance spectrum was described by two semicircles revealing the presence of two relaxations processes in the studied compound associated with the grain and grain boundary.
Consequently, an equivalent electrical circuit for the electrochemical cell with RbGdP2O7 was proposed. The AC conductivity for grain response is interpreted using the universal Jonscher’s power law. The obtained activation energy for bulk is similar to those reported for semiconductor compounds.
Indeed, the temperature dependence of the exponent s shows that the correlated barrier hopping (CBH) model is the suitable model for explaining the AC conduction mechanism in the investigated compound.
References
B Louati, F Hlel and K Guidara J. Alloys Compd. 486 299 (2009)
M Megdiche, H Mahamoud, B Louati, F Hlel and K Guidara Ionics 16 655 (2010)
B Louati, M Gargouri, K Guidara and T Mhiri J. Phys. Chem. Solids 66 762 (2004)
Y Ben Taher, A Oueslati and M Gargouri J. Alloys Compd. 668 206 (2016)
R Ben Said, B Louati and K Guidara Ionics 20 703 (2014)
N Dridi, A Boukhari, J M Réau, E Arbib and E M Holt Mater. Lett. 47 212 (2001)
K Horchani-Naifer and M Férid Solid State Ion. 176 1949 (2005)
H Mahamoud, B Louati, F Hlel and K Guidara J. Alloys Compd. 509 6083 (2011)
V Biju and M A Khadar J. Mater. Sci. 36 5779 (2001)
B Louati and K Guidara Ionics 17 633 (2011)
S Nasri, M Megdiche and M Gargouri Physica B 451 120 (2014)
A Daidouh, M L Veiga, C Pico and M Martinez-Ripoll Acta. Cryst. C 53 167 (1997)
J L Yuan, J Wang, Z J Zhang, J T Zhao and G B Zhang Opt. Mater. 30 132 (2008)
B S Parajon-Costa, R C Mercader and E J Baran J. Phys. Chem. Solid 74 354 (2013)
H Mahamoud, B Louati, F Hlel and K Guidara Bull. Mater. Sci. 34 1069 (2011)
R Ben Said, B Louati and K Guidara Ionics 20 1071 (2014)
A B Rhaiem, S Chouaib and K Guidara Ionics 16 455 (2010)
E J Baran, R C Mercader, A Massaferro, E Kremer Spectrochim. Acta. Part A 60 1001 (2004)
M Idrees, M Nadeem and M M Hassan J. Phys. D Appl. Phys. 44 155401 (2011)
H Kchaou, A Ben Rhaiem, K Karoui and F Jomni Appl. Phys. A 122 82 (2016)
Y Ben Taher, A Oueslati, N K Maaloul, K Khirouni and M Gargouri Appl. Phys. A 120 1537 (2015)
M Ram J. Alloys Compd. 509 5688 (2011)
M Sassi, A Oueslati and M Gargouri Appl. Phys. A 119 763 (2015)
C R Mariappan, G Govindaraj, S Vinoth Rathan and G Vijaya Prakash J. Mater. Sci. Eng. B 121 2 (2005)
M I Youssif, F Sh Mohamed and M S Aziz Mater. Chem. Phys. 83 250 (2004)
S A El-Hakim, F A El-Wahab, A S Mohamed, and M F Kotkata. phys. stat. sol (a). 198, 128 (2003)
A K Jonscher, Dielectric Relaxation in Solids (1983)
W Li and R W Schwartz Appl. Phys. Lett. 89 242906 (2006)
S Das, S Banerjee and T P Sinha J. Nanosci. Nanotechnol. Res. 1 1 (2017)
S Nasri, M Megdiche, K Guidara and M Gargouri Ionics 19 1921 (2013)
S Hassairi, B Louati and K Guidara J. Alloys Compd. 715 397 (2017)
S R Elliot Adv. Phys. 36 135 (1987)
A Ghosh Phys. Rev. B 41 1479 (1990)
R Punia, R S Kundu, M Dult, S Murugavel and N Kishore J. Appl. Phys. 112 083701 (2012)
S Nasri, A Oueslati, I Chaabane and M Gargouri Ceram. Int. 42 14041 (2016)
N Mehtaa, D Kumarb, S Kumarc and A Kumard Chalcogenide Lett. 2 103 (2005)
A Kahouli, A Sylvestre, F Jomni, B Yangui and J Legrand J. Phys. Chem. A 116 1051 (2012)
S R Elliott Adv. Phys. 36 135 (1987)
S Hajlaoui, I Chaabane and K Guidara RSC Adv. 6 91649 (2016)
N F Mott, E A Davis, 2nd edn. Clarendon Press, Oxford (1979)
R D Shannon Acta. Cryst. Sect. A 32 751 (1976)
Acknowledgements
This work is financially supported by the Ministry of Higher Education and Scientific Research of Tunisia.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mathlouthi, S., Oueslati, A. & Louati, B. AC conductivity and conduction mechanism study of rubidium gadolinium diphosphate compound. Indian J Phys 93, 603–610 (2019). https://doi.org/10.1007/s12648-018-1324-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12648-018-1324-z