Abstract
In this paper, we report some physical properties of AgAlP2O7 compound obtained through the standard solid-state reaction technique. AgAlP2O7 has been studied by X-ray diffraction, Raman spectroscopy and impedance spectroscopy. The title compound crystallized at room temperature (T = 300 K) in the monoclinic system with P21/c space group. The electrical properties were studied over a wide range of temperature (440–640 K) in the frequency range of 40 Hz–10 MHz. Study of frequency dependence of AC conductivity suggests that the material obeys the Jonscher’s universal dynamic law. The conductivity is equal to 9.37 × 10−5 Ω cm−1 at 640 K, and it is thermally activated with activation energy of 0.76 eV. The variation of DC conductivity with temperature follows the Arrhenius behavior. The calculated values of s decreased with temperature. This behavior reveals that the conduction mechanism is correlated with barrier hopping. The binding energy W m and the hopping distance R ω were deduced.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Diphosphates, often also called pyrophosphates, constitute the largest family of condensed phosphates, and a great number of compounds of different stoichiometries, containing a single or different types of cations, are prepared [1]. They are the subject of various research works throughout the world. Some of them are used for their acid–base properties or for their oxidizing character. They can be used as a nonlinear optic materials, high-temperature ionic conductors, solid electrolytes for high-energy density batteries, ion exchange materials and catalysts [2, 9]. Extensive research work has been made in these diphosphate compounds like KAlP2O7, CsAlP2O7, NaInP2O7, and this interesting field has been grown due to the important characterization such as optical characterization [10]. Recent works were published during last two years on diphosphate materials such as KAlP2O7, NaAlP2O7 and AgFeP2O7 [11, 13].
In fact, the study of diphosphates has become more popular particularly after the development of NASICON and LISICON groups of fast ionic conductors [14, 15].
Compositions with general formula AIBIIIP2O7 containing simultaneously an alkaline ion (A+ = Na, K, Li, …) and a trivalent cation (B3+ = Fe, Al, Cr, …) are an important materials, mainly due to their prospective potential technological applications and their highest conductivity [16, 17].
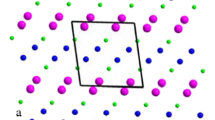
Among these compounds, AgAlP2O7 is isotypic with NaFeP2O7-II [18], and it is one of the monoclinic systems with the space group P21/c. The structure (Fig. 1) containing AlO6 octahedra and the [P2O7]4− anion consists of a pair PO4 tetrahedra. The electric study carried out on phosphate materials is scarce with respect to two different aspects: measurements under continuous mode and under alternative mode. The measurements of AC conductivity have been widely used to understand the conduction process in materials. Different models are based on the relaxation caused by the hopping or tunneling of electrons or atoms between equilibrium sites.
The main objective of this paper is to present and discuss the electrical properties of AgAlP2O7 compound as a function of frequency and temperature.
2 Experimental
The AgAlP2O7 compound has been prepared using the standard solid-state reaction techniques. The starting materials were Ag2CO3, Al2O3 and NH4H2PO4 with purity 99.99 %. These materials were ground, mixed and progressively heated from room temperature to 573 K at first step in order to expel NH3, H2O and CO2. The calcined powder was heated once at 1073 K for 8 h.
X-ray powder diffraction pattern was recorded using a Philips PW 1710 diffractometer operating with copper radiation Kα = 1.5418 Å. Unit cell parameters of the synthesized compound have been refined by the least-square method from the powder data. Raman scattering investigation was performed on powder, using a spectrometer type Horiba Jobin–Yvon T64000.
A pellet with a diameter of 8 mm and thickness of about 1.2 mm using 3 T/cm2 uniaxial pressure was used for electrical conductivity. The measurements were performed using an Agilent 4294A impedance analyzer (two platinum electrodes and we use an evaporation sputtering). The temperature range was between 440 and 640 K.
3 Results and discussion
3.1 X-ray powder diffraction
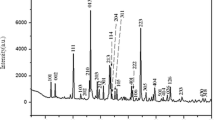
The X-ray powder diffraction was performed at room temperature on powder finely ground in an agate mortar with CuKα radiation and 2θ range from 10° to 60°. All the reflection peaks were indexed in the monoclinic system with P21/c space group. These refined lattice parameters are: a = 7.332(4)Å, b = 7.904(3)Å, c = 9.513(3)Å, and α = 111.830(5)°. These values are in good agreement with the literature [18].
3.2 Raman spectroscopy
The Raman spectrum of the AgAlP2O7 compound at room temperature is shown in Fig. 2. It reveals different peaks which can be attributed according to published results [19–21] in similar compound.
The peaks due to the symmetric and asymmetric stretching frequencies of PO3 in P2O7 4− are generally observed in the regions 994–1209 cm−1. The bands observed in the regions 861–960 and 600–743 cm−1 are attributed to the asymmetric and symmetric P–O–P stretching modes. The bands due to the symmetric and asymmetric binding vibrations of PO3 are generally observed in the regions 200–575 cm−1. Band assignments for the fundamental modes of P2O7 4− anions are listed in Table 1.
3.3 Impedance analysis and equivalent circuit
The complex impedance analysis technique is based on analyzing the AC response of a system to a sinusoidal perturbation and subsequent calculation of impedance as a function of the frequency of the perturbation. The technique can be used for electrical characterization of polycrystalline materials [22]. To understand the contribution of grain and electrode effects observed in structural characterization, we compute from experimental data real Z′ and imaginary Z″ parts of the impedance and we plot Z″ versus Z′.
The complex impedance spectra (Z″ vs. Z′) of the AgAlP2O7 compound at several temperatures are presented in Fig. 3a–c. The center of this semicircle is depressed below the real axis, which indicates a non-Debye type of relaxation [23]. The lower frequency response corresponds to the electrode processes and the higher one to the bulk effects.
To interpret such a diagram, it is necessary to modelize the sample. At higher frequencies, the observed shapes are typical of relaxation mechanisms modelized by a circuit constituted by a resistor R g in parallel with a constant-phase element CPEg. At lower frequencies, electrode polarization effects transduced by constant-phase element (CPEe) are evidenced (inset Fig. 3).
In order to justify the choice of equivalent circuit, we present in Fig. 4 the experimental and calculated Z″ values at different temperature, using the parameters of the equivalent circuit model. The good conformity between the experimental and calculated data indicates that the equivalent circuit describes the compound–electrode interface reasonably well.
Figure 5 shows the variation of imaginary part of impedance (Z″) with angular frequency for some representative temperatures. The spectra are characterized by appearance of peaks, which shift to higher frequencies with increasing temperature. The broadening of peaks in frequency suggests that there is a spread of relaxation times. The merger of Z″ values in the high-frequency region may possibly be an indication of the accumulation of space charge in the material [24].
The obtained fitted values of R g, CPEg and CPEe for different temperatures are summarized in Table 2. We can see that the value of grain resistance (R g) decreases with the increase in temperature. In addition, the value of conductivity increases with the increase in temperature, due to the charge carriers according to the hopping conduction mechanism [25].
The values of capacitance (Q g) are in the range of pico-farad, implying that the single semicircle response is from grain interiors. The values of capacitance electrode Q e are in the range of micro-farad implying the electrode effect in low frequency.
The frequency ωmax corresponding to Z″max gives the most probable relaxation time τm from the condition ωmaxτm = 1. Figure 6 shows a plot of the (ln ωmax) versus 1000/T. It follows Arrhenius relation. The dots are the experimental data, and the solid line is the least-squares straight-line fit. The activation energy calculated from this figure is 0.76 eV.
Figure 7 shows the plot of the impedance Z″ at different temperatures, where frequency axis is normalized by ω max and Z″ axis by Z″ max at various temperatures. The normalized plot shows that all the curves overlap on a single master curve. This indicates that all possible relaxation mechanisms occurring at different frequencies exhibit the same thermal energy and the dynamical processes are temperature independent.
The value of grain conductivity of the material was evaluated using the relation: \(\upsigma_{\text{g}} = \frac{e}{{R_{\text{g}} *S}}\), where R g is the bulk resistance, e is the thickness, and s is the surface of the sample. Figure 8 shows an increase in the DC conductivity with a rise in temperature. This behavior can be explained by thermally activated transport processes governed by an Arrhenius relation \(\upsigma_{\text{g}} T = B\exp \left( {\frac{{ - E_{\text{a}} }}{{K_{\text{b}} T}}} \right)\), where B is the pre-exponential factor and E a = 0.76 eV is the activation energy of mobile charge carrier. The AgAlP2O7 compounds crystallize in the monoclinic system with P21/c space group; in this structure, the Ag+ ions are located in tunnels formed by P2O7 polyhedra and AlO6 octahedra. In short, this suggests that the conduction in this material is due to the motion of the Ag+ ions along [101] tunnels’ direction presented in the structure of the investigated material. The values of the activation energy calculated from conductivity and obtained from the relaxation in Z″ are very close: This improves that the relaxation process and the electrical conductivity are ascribed to the same effect.
3.4 AC conductivity
Figure 9 shows the plot of σac versus angular frequency at various temperatures. The conductivity σac depends on frequency according to the universal dynamic response given by the phenomenological law:
where σdc is the DC conductivity, A is a temperature-dependent parameter, and s is an exponent function of temperature and frequency and is related to the degree of correlation among moving ions. It is clear that two different regions are observed from Fig. 9. At the lower frequency (ω < 105 rad s−1), the DC conductivity shows the existence of a plateau (σ dc) independent on the frequency. At the higher frequency, the AC conductivity increases in parallel with the increase in frequency and obeying to power law Aω s. The point at which the change in slope occurs is known as hopping frequency [26], which shifts toward higher-frequency side with the increase in temperature. In the higher-frequency region, the increase in conductivity is due to the hopping of charge carrier in finite clusters.
The temperature dependence of conductivity at various frequencies is displayed in Fig. 10. As seen from the figure, σ ac increases linearly with the reciprocal of absolute temperature, indicating that AC conductivity is a thermally activated. Besides, an Arrhenius-type behavior is shown. The value of the activation energy derived from the slope is determined and shown in the inset in Fig. 8.
Such a decrease in activation energy can be attributed to the contribution of the applied frequency to the conduction mechanism. The increment of the applied field frequency enhances the electronic jumps between localized states; hence, the activation energy decreases rapidly with an increase in the applied frequency (higher frequency). The smaller activation energy values and increase in AC conductivity with increasing frequency suggest the hopping to be conduction mechanism [27].
3.5 Theory investigation of the correlated barrier hopping (CBH) conduction mechanism
The behavior of the exponent factor s as a function of temperature can be used to determine the origin of the conduction mechanism. Values of the frequency exponent s at different temperature were calculated from the slopes of linear part of the relation of log (σ –ac σac) = f(ω) at the higher-frequency range (Fig. 11). The variation of s with temperature for the investigated compound is shown in Fig. 13. It is observed from this figure that the frequency exponent s decreases with increasing temperature.
To precise the predominant conduction mechanism under AC field for the studied device, different theoretical models have been proposed. In the overlapping-large polaron tunneling (OLPT) conduction mechanism [28], the exponent s decreases with increasing temperature to a minimum value at a certain temperature and then begins to increase with increasing temperature. Therefore, the OLPT conduction mechanism is also not applicable to the obtained results. In the non-overlapping small polaron tunneling (NSPT) conduction mechanism, the exponent s increases with increasing temperature [29]. In the quantum mechanical tunneling (QMT) conduction mechanism [30], the exponent s is almost equal to 0.8 and increases slightly with increasing temperature or is independent of temperature. Therefore, the NSPT and QMT conduction mechanism are also not applicable to the obtained results.
It is clear in Fig. 12 that the value of s decreases with the increase in temperature. Such behavior suggests that the correlated barrier hopping (CBH) conduction mechanism is the predominant conduction mechanism. In CBH model, the conduction occurs via single polaron or bipolaron hopping process over the Coulomb barrier separating two defect centers. The frequency exponent s can be calculated in this model by [31].
where k B is the Boltzmann constant, T is the absolute temperature, W M is the binding energy, ω is the angular frequency, and τ0 is the characteristic relaxation time, which is in the order of atom vibrational period τ0 = 10−13 s. For large values of W M/kB T, the exponent s becomes expressed by [32]:
W M the binding energy is defined as the energy required to move an electron completely from one site to another site. Values of W M are calculated from Eq. (2) and shown in Fig. 12. W M decreases with increasing temperature which corresponds to the decrease in the exponent s. So, the number of free carriers which can jump over the barrier will be increased. Consequently, this behavior confirms that the σ ac increases with the increasing temperature.
The value of the hopping distance (R ω) is given by [33]:
where ε′ is the dielectric constant and ε 0 is the permittivity of free space.
The variation of R ω as a function of the frequency at various temperatures is shown in Fig. 13. It can be seen that the hopping distance (R ω) decreases as the frequency increases. This behavior is in harmony with the increment of the conductivity as the frequency increases.
It is obvious that the hopping distance R ω increases with increasing temperature. As a matter of fact, the increasing temperature gives a contribution of thermal energy to polarons, which then will move and facilitate the jump because of the interchain interaction that will occur.
An additional consideration in the crystal structure view point is that this compound induces a significant a polaronic conductor character of the silver sites. As well, the 3D framework is formed with tunnels (Fig. 1), where silver cation resides which is in favor of the conductivity along these tunnels.
4 Conclusion
In this work, we have synthesized the AgAlP2O7 diphosphate compound by the classic ceramic method. From the impedance data, it is concluded that this compound can be modeled by the simple equivalent circuit by R-CPE in series with CPE circuits. An investigation of conductivity properties due to a possible Ag+ ions mobility among structure has been carried out. The AC conductivity for grain response is interpreted using the universal Jonscher’s power law. The correlated barrier hopping (CBH) conduction mechanism successfully explained the behavior of temperature conductivity mechanism.
References
E.J. Baran, R.C. Mercader, A. Massaferro, E. Kremer, Spectrochim. Acta A 60, 1001 (2004)
M.E. Hagerman, K.R. Poppelmeyer, Chem. Mater. 7, 602 (1995)
H. Aono, E. Sugimoto, Y. Sadaoka, N. Amanaka, G. Adaki, Solid State Ionics 62, 309 (1993)
J.P. Boilot, G. Collin, P. Colomban, J. Solid State Chem. 73, 160 (1988)
S. Villain, E. Nigrelli, G. Nihoul, Solid State Ionics 116, 73 (1999)
F. Sanz, C. Parada, J.M. Rojo, C. Ruiz-Valero, R. Saez-Puche, J. Solid State Chem. 145, 604 (1999)
C. Delmas, A. Nadini, J.L. Subeyrou, Solid State Ionics 28, 419 (1988)
A. Clearfeld (ed.), Inorganic ionic exchange materials (CRC Press, Boca Raton, 1982)
S. Arsalane, M. Ziyad, G. Coudurier, J.C. Vedrine, J. Catal. 159, 162 (1996)
Y. Hizhnyi, O. Gomenyuk, S. Nedilko, A. Oliynyk, B. Okhrimenko, V. Bojko, Radiat. Meas. 42, 719 (2007)
Y. Ben Taher, A. Oueslati, M. Gargouri (2014) Ionics. doi: 10.1007/s11581-014-1288-8
Y. Ben Taher, R. Hajji A. Oueslati, M. Gargouri (2014) J. Clust. Sci. doi: 10.1007/s10876-014-0812-3
S. Nasri, M. Megdiche, M. Gargouri, K. Guidara, Ionics (2013). doi:10.1007/s11581-013-0969-z
H.Y.P. Hong, Mater. Res. Bull. 11, 173 (1976)
Goodenough JB (1980) Fast ionic conductors, in UNESCO Course in Materials Science, Erice, Italy
A. Daidouh, M.L. Veiga, C.P. Marin, M. Martinez-Ripoll, Acta Crystallogr. C 53, 167 (1997)
S.R.S. Prabaharan, M.S. Michael, S. Radhakrishna, C. Julien, J. Mater. Chem. 7, 1791 (1997)
J.P. Gamaondes, F. d’Yvoire, A. Boulle, C R Acad. Sci. (Paris) 272, 49 (1971)
J. Belkouch, L. Monceaux, E. Bordes, P. Courtine, Mater. Res. Bull. 30, 149 (1995)
B.S. ParajÓn-Costa, R.C. Mercaderb, E.J. Baran, J. Phys. Chem. Solids 74, 354 (2013)
G.T. Stranford, R.A. Condrate Sr, B.C. Cornilsen, J. Mol. Struct. 73, 231 (1981)
A.R. West, D.C. Sinclair, N. Hirose, J. Electroceram. 1, 65 (1997)
C.G. Koops, Phys. Rev. 83, 121 (1951)
H. Rahmouni, M. Nouiri, R. Jemai, N. Kallel, N. Rzigua, A. Selmi, K. Khirouni, S. Alaya, J. Magn. Magn. Mater. 316, 23 (2007)
Moti Ram, Solid State Sci. 12, 350 (2010)
K. Srinivas, P. Sarah, S.V. Suryanarayana, Bull. Mater. Sci. 26, 247 (2003)
Okutan M, Basaran E, Bakan HI, Yakuphanoglu F (2005) J Phys B 364:300
A.R. Long, Adv. Phys. 31, 553 (1982)
A. Ghosh, Phys. Rev. B 42, 5665 (1990)
M. Pollak, Phil. Mag. 23, 519 (1971)
V. Chithambaram, S. Jerome Das, S. Krishnan, J. Alloys Compd. 509, 4543 (2011)
K.H. Mahmoud, F.M. Abdel-Rahim, K. Atef, Y.B. Saddeek, Curr. Appl. Phys. 11, 55 (2011)
I.A. Niel, Proc SPIE 237, 422 (1980)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ben Taher, Y., Oueslati, A., Maaloul, N.K. et al. Conductivity study and correlated barrier hopping (CBH) conduction mechanism in diphosphate compound. Appl. Phys. A 120, 1537–1543 (2015). https://doi.org/10.1007/s00339-015-9353-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00339-015-9353-3