Abstract
Cerebral damage following cerebral ischemia/reperfusion injury affects the neurological deficits and motor impairment of stroke patients in the long-term period. Angiogenesis, the essential process for restoration of cerebral blood flow (CBF) in the ischemic brain, promotes the recovery of neurological function following ischemia. The aim of this study was to investigate the long-term effects of morin on angiogenesis and functional outcomes in a middle cerebral artery occlusion (MCAO) and reperfusion model. Male Wistar rats were subjected to MCAO, and they were administered 30 mg/kg of morin at reperfusion via i.p. injection daily for 14 days. Fourteen days after I/R injury, the rats were evaluated for the brain damage, and angiogenic factors involved in Ang1/Tie-2 and Wnt/β-catenin signaling. In addition, at 1, 7, and 14 days after reperfusion, rotarod and pole tests were performed to investigate the functional recovery. We found morin significantly reduced the infarct size, blood–brain barrier (BBB) leakage, and apoptotic cells at 14 days after I/R injury. It also promoted angiogenesis via boosting the expression of angiogenic proteins, such as angiopoietin 1 (Ang1), Tie-2, Wnt3α, β-catenin, and cyclin D1. Morin-mediated angiogenesis was confirmed by a significant increase in microvessel’s density in the penumbra area and an increase in von Willebrand factor (vWF) protein expression of the morin-treated rats. Moreover, the rotarod and pole tests also demonstrated morin increased functional recovery in the morin-treated rats compared to the vehicle rats. Therefore, our data exposed that morin promotes angiogenesis and improves functional outcomes in MCAO and reperfusion rats.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Stroke, the leading cause of death in adults worldwide, is the most frequent induction of permanent disability (Donnan et al. 2008; Johnston et al. 2009; Wong et al. 2007; Pundik et al. 2012). The most common type of stroke is cerebral ischemia; cerebral ischemia–reperfusion (I/R) injury triggers the brain damage and long-term disability (Widgerow 2014). The recovery phase of the brain begins 2–14 days after cerebral I/R onset (Pettersson et al. 2000; Zhang and Chop 2002). There are several mechanisms for neurorestorative events and cell survival, including neurogenesis, synaptic plasticity, and angiogenesis. Angiogenesis is a crucial process and a natural defense mechanism helping restore oxygen and nutrient supply to the affected brain tissue (Krupinski et al. 2007; Hayashi et al. 2003). In response to cerebral I/R injury, hypoxic cells trigger the existing vasculature to release angiogenic factors for neurovascularization. Vascular endothelial growth factor (VEGF) is released to bind to vascular endothelial growth factor receptor (VEGFR) on the vascular endothelial cells (Talwar and Srivastava 2014; Marti et al. 2000). Moreover, neuropilin-1 (NRP-1), expressed in neurons, vessels, and astrocytes, also has angiogenic properties, identified as co-receptors for VEGF or to form complexes with VEGFR (Mey et al. 2013). These mechanisms lead to the initiation of an angiogenic response and new vessel formation. Subsequently, angiopoietin 1 (Ang1), an endogenous ligand for the vascular endothelial receptor tyrosine kinase Tie-2, promotes the stabilization and maintenance of vascular endothelial integrity (Chen et al. 2009; Moss 2013). Moreover, the Wnt/β-catenin pathway has an important role in the angiogenic activity by regulating cyclin D1 to promote endothelial cell proliferation for enhancing new vasculature (Martowicz et al. 2019; Xu et al. 2016). This establishes dynamic blood vessel structures and restores blood supply to the affected brain tissue, leading to improved functional outcomes after cerebral ischemia.
Morin (3,5,7,2′,4′-pentahydroxyflavone) is a flavonol isolated from Maclura cochinchinensis (“kae lae” in Thai), belonging to the Moraceae family (Fig. 1). Many studies have reported that morin exhibits multiple pharmacological and physiological effects, including anti-oxidant, anti-inflammatory, anti-apoptotic, and neuroprotective activity (Kim et al. 1999; Lee et al. 2008; Oh et al. 2004; Uyar et al. 2006; Zhang et al. 2008). Our previous study provided evidence that morin attenuated oxidative stress, apoptosis, inflammation, and blood–brain barrier (BBB) disruption during the acute phase of a middle cerebral artery occlusion (MCAO) model (Khamchai et al. 2020). However, its benefits in improving cerebral damage and neurological outcomes via the angiogenesis pathway during the recovery phase of cerebral I/R have not been reported. In the present study, we investigated the neuroprotective effects of morin during the recovery phase of MCAO and reperfusion rats via promotion of angiogenesis and brain recovery.
Materials and Methods
Reagents
Anti-Ang1, anti-Tie-2, and anti-NRP-1 were purchased from Abcam (Abcam, Cambridge, UK). Anti-cyclin D1 and anti-HIF-1α were purchased from Cell Signaling (Cell Signaling, MA, USA). Anti-VEGF, anti-wnt3α, anti-β-catenin, anti-vWF, and anti-actin were purchased from Millipore (Millipore, MA, USA). All other reagents were obtained from Sigma-Aldrich (St. Louis, MO, USA).
Extraction and Isolation of Morin
Morin was obtained from M. cochinchinensis by the method described previously (Khamchai et al. 2020) with slight modification. Briefly, the dried, pulverized heartwood of M. cochinchinensis (5.0 kg) was extracted successively with n-hexane, ethyl acetate, and methanol at room temperature. To the ethyl acetate extract containing morin was added methanol–water (1:1) with stirring. The solid which was separated out was collected by filtration and recrystallized from methanol–water (1:1) to give pure morin (5.0 g). The spectroscopic (infrared [IR], 1H and 13C nuclear magnetic resonance [NMR] and mass spectra) data were in agreement with those of morin previously isolated by our group (Khamchai et al. 2020).
Animals and Drug Administration
Male Wistar rats (weighing 300–350 g) were obtained from the National Laboratory Animal Center, Mahidol University, Salaya, Nakorn Pathom, Thailand. All were accommodated on a 12/12 h light/dark cycle with a constant ambient temperature (25 ± 1 °C) and fed a standard pellet rat diet and water ad libitum. All experiments in this study were approved by the Institutional Animal Care and Use Committee at the Faculty of Medicine, Chiang Mai University (Permit number: 33/2559), in compliance with National Institutes of Health (NIH) guidelines. Briefly, we randomly divided the 54 rats into three groups (n = 18): (1) Sham group, (2) MCAO group (MCAO rats treated with normal saline at reperfusion), and (3) Morin group (MCAO rats treated with morin at reperfusion). The administration time point and the morin dosage (30 mg/kg BW) were chosen based on our previous study (Khamchai et al. 2020). The vehicle and morin groups were given the treatment via daily intraperitoneal (i.p.) injections for 14 days.
Surgical Preparation of MCAO Model in Rats
All rats were anesthetized with an i.p. injection of zoletil (30 mg/kg) and xylazine (10 mg/kg). An intraluminal monofilament technique was used to conduct MCAO on the rats, as previously described (Longa et al. 1989). Briefly, the right common carotid artery (CCA) and external carotid artery (ECA) were identified, and the 4–0 filament (Doccol Corp., Sharon, MA, USA) was inserted into the internal carotid artery and advanced until we felt a slight resistance. Crucially, we monitored the effective occlusion in cortical perfusion (< 25% of the baseline value) by laser Doppler flowmetry (AD Instruments, Dunedin, New Zealand) (Ansari et al. 2011). After 120 min of MCAO, we carefully withdrew the filament to allow the reperfusion. After surgery, the rats were transferred to a room-temperature environment (25 ± 1 °C) until sacrifice.
Rotarod Test
Six rats from each group were trained for three days before MCAO surgery. They were placed on the apparatus for 30 s without rotation and then after 2 min with a constant low speed (4 rpm). The rats were tested until they successfully stayed on the rotarod spindle for 1 min. This procedure was performed only on the first day of training. After resting for 10 min, the rats underwent a single baseline trial on the accelerating rotarod where the spindle increased in speed from 4 to 40 rpm for 6 min. The animals were evaluated on days 1, 7, and 14 after reperfusion. The maximum duration on the rotarod before falling was recorded for each animal. The rats were tested three rounds per day. The data were presented as the mean of duration times.
Pole Test
The rats were located head-upward on the top of the vertical rough-surfaced pole (diameter 2.5 cm, height 100 cm), and the time until they descended to the floor was recorded with a maximum duration of 120 s. They were trained on a day before MCAO surgery, allowed to descend three times in a single session. The total time until the rat reached the floor with its four paws (time to come down) and the time needed to turn completely head downward (time to turn) were recorded. Could they not turn downward and instead dropped from the pole, the time is recorded as 120 s. The results were shown as the mean of three trials.
Analysis of Infarct Volume
Six whole brains from each group were collected 14 days after surgery and cut into 2 mm-thick slices. The slices were incubated in 1% 2,3,5-triphenyltetrazolium chloride (TTC) at 37 °C for 20 min and fixed overnight in 4% buffered formaldehyde solution. We determined the infarct size by analyzing the area of the ischemic lesion in each section by using ImageJ software. The infarct volume (%) was calculated as follows: 100% × [(contralateral hemisphere volume – non-infarct ipsilateral hemisphere volume)/contralateral hemisphere volume] (Ashwal et al. 1999).
TUNEL Assay
At 14 days after reperfusion, the brains were fixed in 4% paraformaldehyde (PFA) and cut into 4-μm-thick slices, which, after that, were deparaffinized and rehydrated. Then, TUNEL assay kit (Roche Diagnostics Corp., Indianapolis, IN, USA) was used with nuclei stained with brown particles. Finally, the total cells and TUNEL-positive cells were observed under a light microscope (Olympus AX70, Tokyo, Japan). We randomly chose five fields (× 20 and × 40 magnification) of a coronal section of the cerebral cortex at the same level. Then, we counted the number of apoptotic cells and the total cells of five adjacent sections (Janyou et al. 2017). The average value was used to compute the apoptotic index (Apoptosis index (AI) = number of positive cells/total cells).
Investigation of BBB Disruption
BBB leakage was determined by Evans blue injection, as previously described (Yang and Rosenberg 2011). At 14 days after reperfusion, we anesthetized the rats and administered 2% Evans blue solution (4 ml/kg) via intravenous injection into the jugular vein. Thirty minutes after Evans blue injection, we perfused the rats intracardially with cold phosphate-buffered saline (pH 7.4). The brain tissues were then collected and homogenized in DMSO, and the homogenates were incubated at 50 °C. The samples were centrifuged at 12,000 g at 4 °C for 30 min, and the supernatants were collected. The absorbance was measured at 620 nm using a spectrophotometer (BioTek Instruments Inc, Winooski, VT, USA).
Western Blot Analysis
Fourteen days after reperfusion, the cerebral penumbra of each group was collected and kept at –80 °C until use. To extract total proteins, the brains were homogenized, and the total protein concentrations were determined using the Bradford protein assay (Bio-Rad Laboratories, Hercules, CA, USA) with bovine serum albumin (BSA) as the standard. Twenty-five µg of total protein in each sample was loaded into the lanes of 10–15% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) gels. Then, we transferred isolated proteins to polyvinylidene difluoride (PVDF) membrane (Immobilon-P, Millipore, Bedford, MA, USA), and they were blocked in fresh blocking buffer (containing 5% skim milk in 0.1% Tween-20 in Tris-buffered saline, pH 7.4) for 3 h at room temperature. Subsequently, we incubated the membranes with the primary antibodies (anti-Ang1, anti-Tie-2, anti-wnt3α, anti-β-catenin, anti-HIF-1α, anti-VEGF, anti-NRP-1, anti-cyclin D1, or anti-vWF) overnight at 4 °C. Next, the membranes were washed with Tris-buffer saline and Tween-20 (TBST) and incubated with anti-rabbit IgG or anti-mouse peroxidase-conjugated secondary antibody. The membranes were incubated with Immobilon Western (Millipore, MA, USA), and they were exposed to X-ray film. Densitometric analysis was performed by scanning films with a densitometer. The results were normalized using β actin by Image J analysis.
Determination of the Vascular Element Within Neurovascular
Immunohistochemistry staining was used to visualize von Willebrand factor (vWF)-positive vessels. Fourteen days after reperfusion, the rats from each group were perfused with 4% paraformaldehyde in PBS. The rat brains were harvested and post-fixed overnight. The brain sections were prepared using standard protocols. After incubation with a blocking solution, all sections were incubated with anti-vWF (1:1000) for 48 h at 4 °C. Then, the sections were incubated with secondary antibody for 30 min. Afterward, 3,3-diaminobenzidine tetrahydrochloride (DAB) reagent was added, and the sections were incubated for 10 min at room temperature and then counterstained with hematoxylin for 2 min. The image was observed under light microscopy (AX70 Olympus, Tokyo, Japan). For semi-quantitative measurements of vWF-labeled vessels, only the vWF+ vessels with a clear lumen and those vessels that formed ring-like or tubular structures were counted. Three sections per brain from the same levels were chosen, and the number of vessels in each field was counted.
Histology Analysis
Brains from each group were collected and placed in 4% PFA for 48 h. The brain tissues were then embedded in paraffin, processed to 4 μm-thick slices, and stained with hematoxylin & eosin (H&E) and Nissl staining. Morphological changes were detected in the cerebral cortex at the same level in each group. They were observed using a light microscope (Olympus AX70, Tokyo, Japan).
Statistical Analysis
The data are presented as mean ± standard error of the mean (SEM). Statistical differences between the two groups were determined using the Student’s t-test. Other data were analyzed using a one-way analysis of variance (ANOVA), followed by Dunnett’s post-hoc test. A p-value < 0.05 was considered a statistically significant difference between experimental and control groups.
Results
Morin Attenuates Cerebral Damage at 14 days After Cerebral I/R
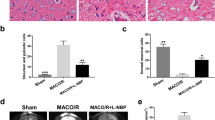
The neuroprotective effect of morin on cerebral I/R at 14 days after onset was first examined by TTC staining. Morin treatment significantly decreased cerebral infarction, as shown by the reduced infarct area and the percentage of infarct volume (p < 0.001 and p < 0.05; Fig. 2A, C). In addition, the Evans blue extravasation assay was performed to evaluate BBB disruption. The dark blue dye color in the brain tissue represented the leakage of the Evans blue extravasation as BBB leakage. The severity of the BBB leakage is shown as OD620nm/g. The result showed the sham group did not have leakage of Evans blue. The extravasation of Evans blue was significantly increased in the vehicle-treated group and significantly decreased with the morin treatment (p < 0.001 and p < 0.01; Fig. 2B, D).
Effects of morin on cerebral damage at 14 days after cerebral I/R. Representative images of TTC staining and percentage of infarct volume, n = 6 (A and C). Representative images of Evans blue leakage in the brain tissues and representation of Evan’s blue leakage absorbance, n = 6 (B and D). Representative images of TUNEL staining, shown as dark brown particles (20 × m agnification). The images were visualized with a light microscope (scale bar = 100 µm) (E). Representation of apoptotic index (AI), n = 6 (F). Representative images of H&E staining (G) and Nissl staining (H) of cerebral cortex (20 × and 40 × magnification). The images were visualized with a light microscope (scale bar = 50 and 100 µm). The data are presented as the mean \(\pm\) standard error of the mean (SEM) from three independent experiments (***p < 0.001 compared with the sham group; #p < 0.05, ##p < 0.01 compared with the vehicle group)
Next, the number of apoptotic cell deaths in the penumbra area in the cerebral cortex was investigated at 14 days after reperfusion via TUNEL staining. The apoptotic cells are presented as dark brown particles. We found that the apoptotic index was significantly reduced in the morin-treated group compared with the vehicle. There were no TUNEL-positive cells in the sham group (p < 0.001 and p < 0.01; Fig. 2E, F).
The histological changes in the cerebral cortex were also assessed by hematoxylin and eosin and Nissl staining. There was a difference in morphological changes between each group. Shrinkage in neuronal cells with pyknotic nucleus and vacuoles were usually seen in the vehicle-treated group. Moreover, there was also loose connective tissue in the vehicle group. There were no histological changes in the sham group, and dense connective tissue was found in this group. The morin-treated groups showed reduced changes in neuronal cells and slightly loose connective tissue (Fig. 2G, H).
Morin Improves the Functional Outcome at 14 days After Cerebral I/R
We evaluated behavioral tests, including the rotarod test and pole test. In the rotarod test, the results showed that the time spent on the rotarod spindle was significantly decreased in I/R rats at 1, 7, and 14 days after I/R compared to the control rats. The time staying on the rotarod in morin-treated rats was significantly increased compared to the vehicle-treated rats (p < 0.001 and p < 0.01; Fig. 3A). In the pole test, the two measurements were taken. Time to turn and the time to down in vehicle-treated rats were significantly increased compared to the control rats and significantly decreased in the morin-treated rats when compared with the vehicle rats (p < 0.001, p < 0.01, and p < 0.05; Fig. 3B, C). These data indicated that morin could ameliorate the behavioral deficits after I/R.
Effects of morin on functional outcome after cerebral I/R. Representative rotarod time (min) at 1 day, 7 days, and 14 days after I/R (A). Representative pole test including time to turn (sec) (B) and time to down (sec) (C). The data are presented as the mean \(\pm\) standard error of the mean (SEM) from three independent experiments (**p < 0.01, ***p < 0.001 compared with the sham group; #p < 0.05, ##p < 0.01 and ###p < 0.001 compared with the vehicle group; n = 6)
Morin Promotes Angiogenic Factors After Cerebral I/R
We next determined the capacity of morin to induce angiogenesis in I/R rats; we first studied the expression levels of angiogenic factors, including HIF-1α, VEGF, Ang1, Tie-2, and NRP-1, by Western blotting analysis (Fig. 4A). HIF-1α, VEGF, and NRP-1 were significantly decreased in the vehicle group compared to the sham group. Treatment with morin significantly increased the expression of NRP-1 compared with the vehicle-treated rats (p < 0.001; Fig. 4D). However, HIF-1α and VEGF expressions in rats treated with morin tended to increase but with no significant differences when compared to the vehicle-treated group (Fig. 4B, C). In vehicle-treated rats, the expressions of Ang1 and Tie-2 were also significantly reduced compared to the sham group, and the morin-treated group showed significantly increased Ang1 and Tie-2 expressions compared to the I/R group (p < 0.001; Fig. 4E, F).
Effects of morin on the expression of angiogenic factors at 14 days after cerebral I/R. Western blot analysis of HIF-1α, VEGF, Ang1, Tie-2, and NRP-1 (A). Quantitative analysis of the expression of the proteins normalized using β-actin (B–E). The data are presented as the mean \(\pm\) standard error of the mean (SEM) from three independent experiments (*p < 0.05 and ***p < 0.001 compared with the sham group; #p < 0.05 and ###p < 0.001 compared with the vehicle group; n = 6)
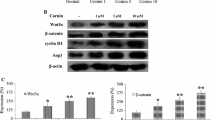
Morin Promotes Vascular Endothelial Cell Proliferation Via Wnt Signaling
To investigate the ability of morin to promote vascular endothelial cell proliferation in I/R rats, we examined the expression levels of Wnt/β-catenin pathway proteins by Western blotting analysis (Fig. 5A). Vehicle-treated rats had significantly reduced Wnt3α and β-catenin levels compared with the control rats. Expression of both proteins was significantly increased in morin-treated rats compared to the vehicle group (p < 0.001 and p < 0.01; Fig. 5B, C). In addition, β-catenin up-regulates pro-proliferative proteins such as cyclin D1. The results showed cyclin D1 was significantly reduced in the vehicle group compared to the control group, and the morin-treated group showed significantly increased cyclin D1 expression compared to the vehicle group (p < 0.001 and p < 0.05; Fig. 5D).
Effects of morin on the expression of levels of Wnt/β-catenin pathway proteins at 14 days after cerebral I/R. Western blot analysis of Wnt3α, β-catenin, and cyclin D1 (A). Quantitative analysis of protein expression normalized using β-actin (B–D). The data are presented as the mean \(\pm\) standard error of the mean (SEM) from three independent experiments (***p < 0.001 compared with the sham group; #p < 0.05, ##p < 0.01, and ###p < 0.001 compared with the vehicle group; n = 6)
Morin Enhances Microvasculature Density After Cerebral I/R
VWF is commonly expressed in endothelial cells and identified as a biomarker of angiogenesis. We investigated the expression of vWF protein by Western blotting analysis. The result showed that vWF expression was significantly decreased in the vehicle group compared with the control group. The morin-treated group revealed significantly increased vWF expression when compared to the vehicle rats. These results indicated that morin treatment could increase angiogenesis in the penumbra of MCAO rats (p < 0.001 and p < 0.01; Fig. 6A, B).
Effects of morin on the microvasculature density at 14 days after cerebral I/R. Western blot analysis of vWF, n = 6 (A). Quantitative analysis of vWF normalized using β-actin (B). Representative images of vWF positive cells in the penumbra, shown as ring-like or tubular structures (20 × and 40 × magnification).The images were visualized with a light microscope (scale bar = 50 and 100 µm) (C). Representative vWF positive cells as a percent of control, n = 6 (D). The data are presented as the mean \(\pm\) standard error of the mean (SEM) from three independent experiments (**p < 0.01, ***p < 0.001 compared with the sham group; ##p < 0.01 compared with the vehicle group)
Moreover, vWF-positive cells represented the microvasculature density in the cerebral cortex. In the vehicle group, vWF-positive cells were significantly decreased in the brain tissue on day 14 compared with the control group. Treatment with morin significantly increased the vWF-positive cells compared with vehicle group (Fig. 6C, D).
Discussion
In our previous study, Wistar rats were subjected to 2 h of MCAO and then reperfusion for 24 h and 72 h. Our findings revealed morin significantly diminished neurological deficit scores and cerebral infarct volume through attenuating oxidative stress, inflammation, and apoptosis at 24 h after MCAO. Moreover, at 72 h after MCAO, we found morin improved BBB disruption by increasing tight junction proteins and reducing BBB leakage (Khamchai et al. 2020). The present study demonstrates that MCAO rats with morin treatment improve functional outcomes and enhance angiogenesis via promoting the Ang1/Tie-2 axis and Wnt/β-catenin pathway after administration for 14 days.
Angiogenesis is the crucial mechanism for the restoration of cerebral blood flow (CBF) in the penumbra area, which promotes the recovery of neurological function following ischemia (Zhang and Chop 2002; Krupinski et al. 2007; Hayashi et al. 2003). After I/R injury, hypoxic cells trigger the existing vasculature to release angiogenic factors to promote new microvascular formation (Marti et al. 2000). VEGF, a key signaling molecule involved in angiogenesis, is initially released to bind to vascular endothelial growth factor receptor (VEGFR) and NRP-1 on the vascular endothelial cells. NRP-1 also has angiogenic properties, such as being a co-receptor for VEGF or forming complexes with VEGFR. The expression of NRP-1 also corresponds with the expression of VEGF (Mey et al. 2013). The previous studies demonstrated that VEGF peaks at 24–72 h after cerebral ischemia (Croll and Wiegand 2001). However, the expression of VEGF is reduced at 14 days after onset (Janyou et al. 2020). Our data showed that I/R could suppress the expression of HIF-1α, VEGF, and NRP-1 in the vehicle group at 14 days after I/R. However, morin administration increased the expression of HIF-1α, VEGF, and NRP-1 to promote the formation of new microvessels in MCAO rats at 14 days after I/R (Fig. 4B, C).
In the last step of angiogenesis, Ang1 is up-regulated, which binds to the receptor Tie-2 for stabilization and remodeling of newborn blood vessels. Previous studies indicated that Ang1/Tie-2 is expressed 2–21 days after I/R and is also involved in vessel stability (Chen et al. 2009; Beck et al. 2000). Our results revealed that the expressions of Ang1 and Tie-2 were suppressed in the vehicle group. In the morin-treated group, the level of both proteins was significantly increased at 14 days after MCAO compared to the vehicle group (Fig. 4D, E). Moreover, the Wnt/β-catenin pathway also plays a key role in angiogenesis. β-catenin activates the pro-proliferative protein cyclin D1, helping to enhance vasculature maturation (Martowicz et al. 2019; Xu et al. 2016). Recent evidence revealed that Wnt signaling is crucial in vascular endothelial cells and functions involved in angiogenesis. Our data demonstrated that morin treatment significantly increased the expression of Wnt/β-catenin and cyclin D1 in the morin-treated rat at 14 days after I/R (Fig. 5). The new microvessels formation is started from 14 to 21 days after cerebral ischemia (Chen et al. 2017; Gandin et al. 2016). However, at 14 days after I/R, our study showed that MCAO rats had a decrease in the microvascular density, but we found an increase of microvessels in the morin-treated rats, corresponding with an increase in the expression of vWF protein (Fig. 6). These results suggest that morin induced angiogenesis and promoted vasculature stability.
In rodents, as in humans, MCAO rats have the predominant characteristic and functional deficit, such as hemiparesis. Accordingly, the rotarod and pole tests, widely used to assess the long-term functional outcomes in MCAO rats, were performed in the present study (Janyou et al. 2020). Our data demonstrated MCAO rats had a decrease in the duration on the rotarod and an increase in the time to turn and time to down in the pole test. Nevertheless, daily administration of 30 mg/kg morin for 1, 7, and 14 days significantly improved the functional deficit in MCAO rats (Fig. 3). Morin administration also reduced cerebral damage at 14 days after I/R by decreasing cerebral infarction and BBB leakage. Additionally, we observed fewer TUNEL-positive cells in the ischemic brain of the morin-treated group, which presented a decrease in apoptotic cells. Our results also showed that morin treatment reduced brain morphological changes in the cerebral cortex. We found that the neuronal cells showed pyknotic nuclei and overall shrinkage in the vehicle group (Fig. 2). These results suggest morin can improve brain damage in the recovery phase of cerebral I/R injury by promoting angiogenesis via Wnt/β-catenin signaling pathway.
In conclusion, our study provided evidence that morin has a neuroprotective effect on cerebral ischemia/reperfusion at 14 days after I/R injury. It enhanced angiogenic factors through the Ang1/Tie-2 pathway and Wnt/β-catenin signaling and also improved functional outcomes. We found that morin promoted angiogenesis in the recovery phase after I/R injury.
Availability of Data and Material
Data available on request from the corresponding author.
Abbreviations
- Ang1:
-
Angiopoietin 1
- BBB:
-
Blood-brain barrier
- HIF-1α:
-
Hypoxia-inducible factor 1-alpha
- I/R:
-
Ischemia/reperfusion
- MCAO:
-
Middle cerebral artery occlusion
- NRP-1:
-
Neuropilin-1
- ROS:
-
Reactive oxygen species
- t-PA:
-
Tissue-plasminogen activator
- TTC:
-
2,3,5-Triphenyltetrazolium chloride
- VEGF:
-
Vascular endothelial growth factor
- VEGFR:
-
Vascular endothelial growth factor receptor
- vWF:
-
Von Willebrand factor
References
Ansari S, Azani H, McConnell DJ, Afzal A, Mocco J (2011) Intraluminal middle cerebral artery occlusion (MCAO) model for ischemic stroke with laser doppler flowmetry guidance in mice. JoVE 8:2879. https://doi.org/10.3791/2879
Ashwal S, Tone B, Tian HR, Cole DJ, Liwnicz BH et al (1999) Core and penumbral nitric oxide synthase activity during cerebral ischemia and reperfusion in the rat pup. Pediatr Res 46:390–400. https://doi.org/10.1203/00006450-199910000-00006
Beck H, Acker T, Wiessner C, Allegrini PR, Plate KH (2000) Expression of angiopoietin-1, angiopoietin-2, and Tie receptors after middle cerebral artery occlusion in the rat. Am J Pathol 157:1473–1483. https://doi.org/10.1016/S0002-9440(10)64786-4
Chen J, Cui X, Zacharek A, Chopp M (2009) Increasing Ang1/Tie-2 expression by simvastatin treatment induces vascular stabilization and neuroblast migration after stroke. J Cell Mol Med 13:1348–1357. https://doi.org/10.1111/j.1582-4934.2008.00380.x
Chen JY, Yu Y, Yuan Y, Zhang YJ, Fan XP et al (2017) Enriched housing promotes post-stroke functional recovery through astrocytic HMGB1-IL-6-mediated angiogenesis. Cell Death Dis 3:17054. https://doi.org/10.1038/cddiscovery.2017.54
Croll SD, Wiegand SJ (2001) Vascular growth factors in cerebral ischemia. Mol Neurobiol 23:121–135. https://doi.org/10.1385/MN:23:2-3:121
Donnan GA, Fischer M, Maeleod M, Davis SM (2008) Stroke Lancet 371:1612–1623. https://doi.org/10.1016/s0140-6736(08)60694-7
Gandin C, Widemann C, Lazdunski M, Heruteaux C (2016) MLC901 favors angiogenesis and associated recovery after ischemic stroke in mice. Cerebrovasc Dis 42:139–154. https://doi.org/10.1159/000444810
Hayashi T, Noshita N, Sugawara T, Chan PH (2003) Temporal profile of angiogenesis and expression of related genes in the brain after ischemia. J Cereb Blood Flow Metab 23:166–180. https://doi.org/10.1097/01.WCB.0000041283.53351.CB
Janyou A, Wicha P, Jittiwat J, Suksamrarn A, Tocharus C et al (2017) Dihydrocapsaicin attenuates blood brain barrier and cerebral damage in focal cerebral ischemia/reperfusion via oxidative stress and inflammatory. Sci Rep 7:10556. https://doi.org/10.1038/s41598-017-11181-5
Janyou A, Wicha P, Seechamnanturakit V, Bumroongkit K, Tocharus C et al (2020) Dihydrocapsaicin-induced angiogenesis and improved functional recovery after cerebral ischemia and reperfusion in a rat model. J Pharmacol Sci 143:9–16. https://doi.org/10.1016/j.jphs.2020.02.001
Johnston SC, Mendis S, Mathers CD (2009) Global variation in stroke burden and mortality: estimates from monitoring, surveillance, and modeling. Lancet Neurol 8:345–354. https://doi.org/10.1016/S1474-4422(09)70023-7
Khamchai S, Chumboatong W, Hata J, Tocharus C, Suksamran A et al (2020) Morin protects the blood-brain barrier integrity against cerebral ischemia reperfusion through anti-inflammatory actions in rats. Sci Rep 10:13379. https://doi.org/10.1038/s41598-020-70214-8
Kim HK, Cheon BS, Kim YH, Kim SY, Kim HP (1999) Effects of naturally occurring flavonoids on nitric oxide production in the macrophage cell line RAW 264.7 and their structure-activity relationships. Biochem Pharmacol 58:759–765. https://doi.org/10.1016/s0006-2952(99)00160-4
Krupinski J, Kaluza J, Kumar P, Kumar S, Wang JM (2007) Role of angiogenesis in patients with cerebral ischemic stroke. Curr Treat Options Cardiovasc Med 9:205–212. https://doi.org/10.1161/01.str.25.9.1794
Lee HS, Jung KH, Hong SW, Park IS, Lee C et al (2008) Morin protects acute liver damage by carbon tetrachloride (CCl(4)) in rat. Arch Pharm Res 31:1160–1165. https://doi.org/10.1007/s12272-001-1283-5
Longa EZ, Weinstein PR, Carlson S, Commin R (1989) Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke 1:84–91. https://doi.org/10.1161/01.str.20.1.84
Marti HJ, Bernaudin M, Bellail A, Schoch H, Euler M et al (2000) Hypoxia-induced vascular endothelial growth factor expression precedes neovascularization after cerebral ischemia. Am J Pathol 156:965–976. https://doi.org/10.1016/S0002-9440(10)64964-4
Martowicz A, Trusohamn M, Jensen N, Wisniewska-Kruk J, Corada M et al (2019) Endothelial β-catenin signaling supports postnatal brain and retinal angiogenesis by promoting sprouting, tip cell formation, and VEGFR (vascular endothelial growth factor receptor) 2 expression. Arterioscler Thromb Vasc Biol 39:2273–2288. https://doi.org/10.1161/ATVBAHA.119.312749
Mey L, Hormann M, Schleicher N, Reuter P, Donges S (2013) Neuropilin-1 modulates vascular endothelial growth factor-induced poly (ADP ribose)-polymerase leading to reduced cerebrovascular apoptosis. Neurobiol Dis 59:111–125. https://doi.org/10.1016/j.nbd.2013.06.009
Moss A (2013) The angiopoietin: Tie 2 interaction: a potential target for future therapies in human vascular disease. Cytokine Growth Factor Rev 24:579–592. https://doi.org/10.1016/j.cytogfr.2013.05.009
Oh WK, Lee CH, Lee MS, Bae EY, Sohn CB et al (2004) Antidiabetic effects of extracts from Psidium guajava. J Ethnopharmacol 96:411–415. https://doi.org/10.1016/j.jep.2004.09.041
Pettersson A, Nagy JA, Brown LF (2000) Heterogeneity of the angiogenic response induced in different normal adult tissues by vascular permeability factor/vascular endothelial growth factor. Lab Invest 80:99–115. https://doi.org/10.1038/labinvest.3780013
Pundik S, Xu K, Sundararajan S (2012) Reperfusion brain injury: focus on cellular bioenergetics. Neurology 79:44–51
Talwar T, Srivastava MV (2014) Role of vascular endothelial growth factor and other growth factors in post-stroke recovery. Ann Indian Acad Neurol 17:1–6. https://doi.org/10.4103/0972-2327.128519
Uyar Z, Boke N, Turkay E, Koz O, Yasa I et al (2006) Flavonoid glycosides and methylinositol from Ebenus haussknechtii. Nat Prod Res 20:999–1007. https://doi.org/10.1080/14786410600921516
Widgerow AD (2014) Ischemia-reperfusion injury: influencing the microcirculatory and cellular environment. Ann Plast Surg 72:253–260. https://doi.org/10.1097/SAP.013e31852c089c
Wong KS, Chen C, Ng PW, Tsoi TH, Li HL et al (2007) Low-molecular-weight heparin compared with aspirin for the treatment of acute ischaemic stroke in Asian patients with large artery occlusive disease: a randomized study. Lancet Neurol 6:407–413. https://doi.org/10.1016/S1474-4422(07)70079-0
Xu Y, Zhang G, Kang Z, Xu Y, Jiang W et al (2016) Cornin increases angiogenesis and improves functional recovery after stroke via the Ang1/Tie2 axis and the Wnt/b-catenin pathway. Arch Pharm Res 39:133–142. https://doi.org/10.1007/s12272-015-0652-1
Yang Y, Rosenberg GA (2011) Blood-brain barrier breakdown in acute and chronic cerebrovascular disease. Stroke 42:3323–3328. https://doi.org/10.1161/STROKEAHA.110.608257
Zhang R, Kang KA, Piao MJ, Maeng YH, Lee KH et al (2008) Cellular protection of morin against the oxidative stress induced by hydrogen peroxide. Chem Biol Interact 177:21–27. https://doi.org/10.1016/j.cbi.2008.08.009
Zhang Z, Chop M (2002) Vascular endothelial growth factor and angiopoeitins in focal cerebral ischemia. Tradit Chin Med 12:62–66. https://doi.org/10.1016/s1050-1738(01)00149-9
Acknowledgements
This study was supported by Chiang Mai University, Center for Research and Development of Natural Products for Health, Faculty of Medicine, Chiang Mai University grant no. 39/2564, The Thailand Research Fund (DBG6180030), the Center of Excellence for Innovation in Chemistry, Ministry of Higher Education, Science, Research and Innovation, and the Royal Golden Jubilee Ph.D. Program (PHD/0011/2558 SK).
Author information
Authors and Affiliations
Contributions
Conceptualization: Satchakorn Khamchai, Jiraporn Tocharus; Methodology: Satchakorn Khamchai, Wijitra Chumboatong, Janejira Hata, Chainarong Tocharus, Apichart Suksamrarn; Formal analysis and investigation: Satchakorn Khamchai, Jiraporn Tocharus; Writing-original draft preparation: Satchakorn Khamchai; Writing-review and editing: Jiraporn Tocharus, Apichart Suksamrarn; Funding acquisition: Jiraporn Tocharus, Apichart Suksamrarn; Supervision: Jiraporn Tocharus.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Khamchai, S., Chumboatong, W., Hata, J. et al. Morin Attenuated Cerebral Ischemia/Reperfusion Injury Through Promoting Angiogenesis Mediated by Angiopoietin-1-Tie-2 Axis and Wnt/β-Catenin Pathway. Neurotox Res 40, 14–25 (2022). https://doi.org/10.1007/s12640-021-00470-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12640-021-00470-7