Abstract
We investigated whether cornin, an iridoid glycoside isolated from fruits of Verbena officinalis L., regulated angiogenesis and thereby improved functional outcomes after stroke and discovered a potential mechanism. The effects of cornin on proliferation of rat artery smooth muscle cell (RASMC) and signalling was investigated in vitro. Adult male rats were subjected to 1 h of middle cerebral artery occlusion (MCAO) and reperfusion and treated with or without 25 mg/kg of cornin, starting 24 h after ischemia and reperfusion, by continuous intravenous injection daily for 14 days. Neurological functional tests were performed and cerebral Evans blue extravasation was measured. Angiogenesis and angiogenic factor expressions were measured by immunohistochemistry and Western blotting, respectively. Cornin increased the proliferation of RASMC and enhanced the expression of Wnt5a, β-catenin, cyclin D1 and angiopoietin-1 (Ang1). Cornin treatment promoted angiogenesis in the ischemic brain core and improved functional outcomes after stroke. Cornin-treated MCAO rats showed significant increase in vascularization and expression of vascular endothelial growth factor and Ang1 and phosphorylation of Tie2 and Akt compared with vehicle-treated MCAO rats. The Ang1/Tie2 axis and Wnt/β-catenin pathways appear to mediate cornin-induced angiogenesis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Stroke is a global health problem and is the second most common cause of death and a leading cause of disability worldwide (Bonita et al. 2004; Kim and Johnston 2011). To date, tissue plasminogen activator is the only approved treatment for stroke. Angiogenesis plays a vital role in striatal neurogenesis after stroke. The administration of various growth factors in the early post-ischemic phase stimulates both angiogenesis and neurogenesis and leads to improved functional recovery after stroke (Talwar and Srivastava 2014).
Vascular endothelial growth factor (VEGF) is the most potent angiogenic factor for neurovascularization and neurogenesis in ischemic injury; it can be modulated in different ways and thus can be used as therapy in stroke. In response to ischemic injury, VEGF is released by endothelial cells via a natural mechanism, leading to angiogenesis and vascularization (Talwar and Srivastava 2014). Angiopoietin-1 (Ang1) is an endogenous ligand for the vascular endothelial receptor tyrosine kinase Tie2. Signalling by Ang1 promotes vascular endothelial cell survival and the sprouting and reorganisation of blood vessels as well as inhibiting activation of the vascular endothelial barrier to reduce leakage and leukocyte migration into tissues (Moss 2013), cooperates with VEGF to establish dynamic blood vessel structures and improves functional outcomes after stroke(Chen et al. 2007; Satchell et al. 2004).
Wnts are a family of secreted glycoproteins with varying expression patterns and functions. They control a broad variety of biological processes, including cell-fate specification, polarity, migration and proliferation (Tetsu and Mccormick 1999). Wnts trigger intracellular responses via various signalling pathways, including the β-catenin-dependent canonical pathway. Among them, the cyclin D1 protein plays a key role in cell cycle control and cell proliferation (Vlad et al. 2008).
Cornin is an iridoid glycoside isolated from the fruit of Verbena officinalis L. that has protective potential against cerebral ischemia injury and induces angiogenesis in vitro (Jiang et al. 2010; Kang et al. 2013). The present study was designed to investigate the effects of cornin on angiogenesis after cerebral ischemia and reperfusion (I/R) injury and on rat artery smooth muscle cell (RASMC) proliferation and its potential mechanism.
Materials and methods
Materials
Evans blue was purchased from Urchem (Shanghai, P.R. China); VEGF antibody, phosphor-Akt antibody, phosphor-Tie2, Ang1 antibody, β-actin antibody and Von Willebrand factor (VWF) antibody were purchased from Abcam (Shanghai, P.R. China); cornin (purity >99.0 %, CAS NO.: 548-37-8, molecular formula C17H24O10: 388.37). It was dissolved in sterilized saline to make a stock solution and dilutions were prepared according to the doses.
Rat aortic smooth muscle cell (RASMC) culture
RASMCs were obtained by the enzymatic dissociation of aortas obtained from Sprague–Dawley (SD) rats. The aortic fragments were cleaned to be freed of the adventitia layer and the endothelium was removed by gently rubbing the lumen of the vessel. The fragments were cut into small pieces and digested by incubation for 90 min in Dulbecco’s Modified Eagle Medium (DMEM) containing 0.1 % BSA (Sigma Chemical Co., St. Louis, Missouri, U.S.A) and 4 mg/mL type II collagenase. After washing twice with fresh DMEM, the resulting cell suspension was plated onto 25-cm2 culture flasks in DMEM containing 10 % FBS, 100 U/mL streptomycin, 100 U/mL penicillin and 2.5 mg/mL amphotericin B. Cell characterization was performed based on both cell morphology and indirect immunohistochemisty staining of α-smooth muscle actin. Tightly confluent monolayers of RASMC from passages 2–10 were used for all experiments.
RASMC proliferation assay
For in vitro proliferation assay, RASMCs were seeded into 96-well (1 × 105 cells/well) flat-bottom plates in triplicate with medium alone (control) or medium containing different concentrations of cornin (0, 1, 3 and 10 μM). Cell proliferation was tested by MTT assay. In brief, serum-starved cells were treated with cornin for 48 h and absorbance was recorded at 490 nm (Spectramax/M5 multi-detection reader, Molecular devices, USA) and calculated as a ratio to that of untreated cells.
Western blot analyses of wnt5a, β-catenin, cyclin D1, Ang1
The in vitro Western blot assay included two parts: (1) RASMCs were seeded into 6-well flat-bottom plates with medium alone (control) or medium containing different concentrations of cornin (0, 1, 3 and 10 μM); (2) RASMCs were seeded into 6-well flat-bottom plates with medium alone (control) or medium containing 3 μM cornin, 10 μM IWR-1-endo, or 3 μM cornin + 10 μM IWR-1-endo. IWR-1-endo was incubated for only 60 min and then discarded. The RASMCs were all cultured for 48 h, then washed twice with ice cold PBS on ice and lysed in NP40 lysis buffer supplemented with 1 mM PMSF and 1× protease inhibitor cocktail (Sigma, St. Louis, MO, USA). Equal amounts of cell protein (50 μg) were separated by SDS-PAGE and analysed by Western blotting using specific antibodies to wnt5a, β-catenin, cyclin D1, Ang1 and β-actin. Optical densities of the bands were scanned and quantified with a Gel Doc 2000 (Bio-Rad Laboratories Ltd). Data were normalized against those of the corresponding β-actin bands. Results were expressed as fold increase over the control.
Animals
Adult male SD rats were obtained from Shandong Luye Pharmaceutical Company (P.R. China). All animals were housed individually at 22 ± 2 °C and a relative humidity of 50 ± 10 % under a 12-h light/dark cycle and had free access to chow and water. All animal experimental procedures in this study were performed in accordance with the Institutional Animal Care and followed the National Institutes of Health Guide for the Care and Use of Laboratory Animals (Maryland, USA). The protocol was approved by the Committee on the Ethics of Animal Experiments of Binzhou Medical University (Permit Number: SCXK 2009 0009).
I/R procedure to induce cerebral ischemia
The body weight of rats was 280–320 g. After 1 week of acclimatization, the rats were anaesthetized with chloral hydrate (350 mg/kg, i.p.). The middle cerebral artery occlusion (MCAO) operation was conducted according to previous procedures (Jiang et al. 2011). In brief, the left common carotid artery was occluded and the branches of the external carotid artery were dissected and divided. The internal carotid artery was followed rostrally and a 4-0 filament (the diameter of the filament is 0.25, but the diameter of the tip is 0.34 mm to create a globular stopper) purchased from Beijing Shadong Biology Company, P.R. China) was introduced into the internal carotid artery and advanced until resistance was felt. The filament was removed after 1 h. Core body temperature was maintained at 37 ± 0.5 °C with a heating pad.
A pilot study was conducted with three different doses of cornin (12.5, 25 and 50 mg/kg) to determine the dose dependence effect in subacute I/R-treated rats. Cornin post-treatment at doses of 25 and 50 mg/kg significantly (P < 0.05) lowered the lesion volume by NeuN staining in ischemic brain cores of I/R rats. Accordingly, 25 mg/kg of cornin was chosen for this study.
In total, 90 rats were divided into three subgroups of 30 rats each: a non-I/R (sham) group, a vehicle-treated group and a 25 mg/kg-cornin group. After 23 h of reperfusion, all animals were administered an intravenous bolus injection (i.v. via the tail) of the corresponding drug daily for 14 days. Sham and vehicle-treated rats received saline. At 7 and 14 days, a subgroup of 8 animals in each group was used to evaluate nerve behaviour and measure the permeability of blood–brain barrier (BBB). At 14 days, the remaining animals were used for Western blotting and analysis of microvasculature density.
Behavioral tests
The forepaw placing test was performed as per the previously described method (De Ryck et al. 1989; Liu et al. 2001). Visual forepaw placing was tested first by lowering the rat toward a tabletop and then contacting the table edge with the dorsal or lateral aspect of the rat’s forepaw. Non-visual forepaw placing was also tested by lowering the rat toward the table top and by contacting the table edge. Cephalic contact stimuli were eliminated by elevating the rat’s head by 45 °C. For each test, limb placing scores were as follows: (0) immediate and complete placing; (1) delayed and/or incomplete placing (>2 s) and (2) no placing.
The modified beam balance test was performed as per the previously described method (Feeney et al. 1982; Liu et al. 2001). The rat was placed on a narrow beam (40 × 1.3 × 1.3 cm; 30 cm above a tabletop) for 60 s. At least five training scores were recorded before the MCAO. The scale was as follows: (1) steady posture with paws on top of the beam; (2) paws on the side of the beam or wavering; (3) one or two limbs slip off; (4) three limbs slip off; (5) attempts with paws on the beam, but falls and (6) drops over the beam, then falls or falls with no attempt.
The adhesive tape test was performed as per the previously described method (Feeney et al. 1982; Schallert et al. 1982). Somatosensory deficits were measured both pre- and post-operatively. All rats were familiarized with the testing environment. In an initial test, two small pieces of adhesive-backed paper dots (of equal area, 113 mm2) were used as bilateral tactile stimuli occupying the ventral side of each forepaw. The latencies for contact and removal of each stimulus from the paw were recorded for five trials per day. Individual trials were separated by at least 10 min. The average time of the five trials was used for each day’s record. The animals were trained 2 days prior to surgery. Once the rats were able to remove the paper dots within 10 s, they were subjected to the MCAO operation. The scale was evaluated as follows: (1) <10 s; (2) <10–19 s; (3) <20–29 s; (4) <30–39 s; (5) <40–49 s; (6) <50–59 s; (7) <60 s.
Measurement of Evans blue extravasation
A quantitative assay of Evans blue was performed as per the previously described method (Vakili et al. 2007). At 6 and 13 days, 0.1 ml of 4 % Evans blue in 0.9 % saline was intravenously administered. At 24 h after injection, the rat brains were perfused with heparinized saline (10 U/mL heparin in 0.9 % saline) to wash out the blood and were isolated. The brain samples were weighed and homogenized in 50 % trichloroacetic acid solution. After centrifugation at 15,000 rpm for 20 min, the absorption was spectrophotometrically measured at 610 nm. Cerebral Evans blue was quantified as micrograms of dye per gram of wet weight.
Microvessel density and histological assessment
Rats were killed 14 days after I/R. The brains were fixed by transcardial perfusion with saline, followed by perfusion and immersion in 4 % paraformaldehyde before embedding in paraffin. Seven coronal sections of tissue were processed and stained with NeuN instead of hematoxylin–eosin for calculation of volume of cerebral infarction (Swanson et al. 1990).
Following tissue infiltration, a 2 × 2 mm2 ischemic brain core (length × width) of cortical tissue was cut from the left hemisphere (the hemispheric site of I/R). The microvessel density was estimated by visualization of VWF, a specific marker of endothelial cells. For NeuN and VWF immunohistochemical staining, the sliced sections were incubated with an antibody against NeuN and VWF overnight at 4 °C and the sections were incubated with a biotinylated secondary antibody (Beyotime BIO) for 30 min and avidin–biotin–peroxidase complex (Beyotime BIO) for 45 min at 37 °C. Immunoreactive signals were developed with 0.05 % diaminobenzidine. Protein-positive cells were stained brown in the cytoplasm. Sections were then mounted and examined under a high-power microscope (200×). In each of the specimens, 3 vision test areas were randomly selected as the total area. The positive expression of VWF was analysed by the Image-Pro Plus 6.0 analysis system. The positive area of VWF was defined as g % = positive area/total area. Results were expressed as percentage increase over the sham group.
Western blot analysis of VEGF, phosphorylation of Akt, Ang1 and Tie2
Ischemic brain core (at bregma levels approximating −1.3 to −3.3 mm and −3.3 to −5.3 mm) was used for the Western blot assays. Fourteen days after I/R, the samples were homogenized and lysed in lysis buffer containing 50 mM Tris–HCl (pH 8.0), 150 mM NaCl, 0.02 % sodium azide, 100 µg/ml phenylmethylsulfonyl fluoride, 1 µg/ml aprotinin and 1 % Triton X-100. After centrifugation at 12,000 rpm, 50 µg of total protein of each sample was separated on a 12 % SDS-PAGE gel and transferred to a nitrocellulose membrane. Protein concentrations were determined with the BCA protein assay kit (Merck). The membranes were incubated with specific antibodies against VEGF, Ang1, phosphor-Akt and phosphor-Tie2. β-actin was used as a loading control. Optical densities of the bands were scanned and quantified with Gel Doc 2000 (Bio-Rad). Results were expressed as fold increase over the sham rats.
Statistical analysis
Behavioral test scores between groups were compared by nonparametric test. Quantitative data from experiments were expressed as mean ± SD. Statistical significance was determined by one-way analysis of variance (ANOVA) followed by Dunnett’s test. In all cases, differences were considered significant if P < 0.05.
Results
Effects of cornin on RASMC proliferation and Wnt signalling
RASMC proliferation plays a key role in angiogenesis and enhances vascular maturation. RASMC proliferation was tested by the MTT assay. It had an apparent effect on RASMC proliferation in the presence of cornin at 1, 3 and 10 μM (Fig. 1a).
Effects of Cornin on RASMC proliferation and the Wnt signaling. a Effects of Cornin on cell proliferation was tested by MTT. b, c RASMC were incubated with Cornin for 48 h, Wnt5a, β-catenin, cyclin D1 and Ang1 expression were analyzed by western blotting. All data were shown as mean ± SD, n = 5. *P < 0.015, *P < 0.01 vs. Normal group. Significance was determined by one-way analysis of ANOVA followed by Dunnett’s test
To elucidate the role of cornin in RASMC proliferation, the key protein of Wnt signalling Wnt5a, β-catenin and cyclin D1 expression were investigated in cornin-treated RASMCs. RASMCs incubated with 3 μM and 10 μM cornin for 48 h showed robustly activated wnt signalling leading to a rapid increase in wnt5a levels (Fig. 1b). The activation of this pathway was significantly inhibited by incubation with the specific wnt inhibitor IWR-1-endo at 10 μM for 60 min (compared with cornin at 3 μM).
Considering that the wnt pathway is known to be associated with β-catenin signalling, we next investigated the role of β-catenin in cornin-induced RASMC proliferation. The incubation of RASMCs with 3 and 10 μM cornin for 48 h rapidly increased β-catenin, as shown in Figs. 1b and 2a. The activation of β-catenin occurs downstream of cyclin D1 signalling, considering that blocking the pathway with IWR-1-endo 10 μM for 60 min significantly decreased cornin-induced cyclin D1 levels (compared with cornin at 3 μM), as shown in Figs. 1b and 2a. This observation suggested that cornin enhanced RASMC proliferation via Wnt signalling.
Effects of Cornin plus IWR-1-endo on the Wnt signaling. a–b RASMC in the presence or absence of IWR-1-endo 10 μM for 1 h before incubation with Cornin at 3 μM for 48 h, Wnt5a, β-catenin and cyclin D1 expressions were analyzed by western blotting. All data were shown as mean ± SD, n = 5. #P < 0.01, vs. Normal group, *P < 0.01, versus Normal group, aP < 0.01, versus Cornin 3 μM. Significance was determined by one-way analysis of ANOVA followed by Dunnett’s test
Effects of cornin on blood–brain barrier leakage, neurological outcome and lesion volume
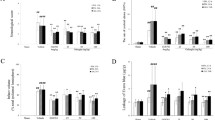
BBB permeability was significantly increased in MCAO rats, because of ischemia-induced damage to BBB function and/or the immature nature of the newly formed vessel. Vascular permeability was quantitatively evaluated by the detection of extravasated Evans blue in ischemic brain, as shown in Fig. 3. The results indicated that 25 mg/kg cornin treatment in I/R rats at 7 and 14 days decreased BBB leakage compared with that invehicle-treated rats.
Behavioral evaluation is an important indicator of neurological recovery. The combination of the forepaw placing test, modified beam balance test and adhesive tape test was used to evaluate the sensorimotor function of the stroke rats. The scores of these tests were higher in vehicle-treated rats, whereas on days 3, 7 and 14, in I/R rats treated with 25 mg/kg of cornin, the scores of the three tests were all reduced (Table 1).
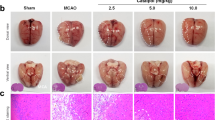
Lesion volume was quantitatively evaluated as the number of neurons by NeuN staining in ischemic brain cores of I/R rats (Fig. 4). Significant differences in ischemic lesion volume in the 25 mg/kg cornin-treated group (18.6 ± 4.2 %) were detected compared with those invehicle-treated rats (24.9 ± 5.1 %).
Effect of Cornin on infarct volume. Infarct volume was quantitatively evaluated by detecting the number of neurons (NeuN staining) at 14th after I/R. a Representative light microscopic appearance of NeuN staining (original magnification ×400) for Sham (a1), Vehicle-treated (a2), Cornin 25 mg/kg (a3). Data are mean ± SD, n = 8. #P < 0.01 versus Sham group; *P < 0.05, versus vehicle-treated group. Significance was determined by one-way analysis of ANOVA followed by Dunnett’s test
Effects of cornin on microvasculature density in the ischemic brain
VWF, a biomarker of angiogenesis, is expressed in large amounts on endothelial cells. VWF-positive cells were significantly increased in the ipsilateral hemispheres of 25 mg/kg cornin-treated I/R rats on day 14, compared with those of vehicle-treated rats (Fig. 5). These results indicated that cornin increased angiogenesis in the ischemic brain zone of stroke rats.
Effect of Cornin on microvasculature density in the ischemic brain. The microvasculature density was estimated by visualization of VWF positive cells at ×400 magnification. Sham (a1), vehicle-treated (a2), Cornin 25 mg/kg. Data are mean ± SD, n = 5. *P < 0.01 versus the vehicle-treated rats. Significance was determined by one-way analysis of ANOVA followed by Dunnett’s test
Effects of cornin on VEGF, Ang1, phospho-Akt and phospho-Tie2 expression in the ischemic brain
Ang1/Tie2 plays an important role in inducing angiogenesis. Akt is the primary mediator of Ang1-induced endothelial cell survival, whereas multiple pathways downstream of VEGF are responsible for endothelial cell survival. To examine the signalling pathways of cornin-induced angiogenesis, we measured phosphor-Akt and Tie2 in vivo and Ang 1 in RASMCs by Western blot analysis. Treatment with cornin (25 mg/kg) of I/R rats for 14 days increased phosphor-Akt and Tie2 in the ischemic core compared with vehicle-treated rats, Incubation of RASMCs with 3 μM and 10 μM cornin for 48 h robustly increased Ang 1 expression (Fig. 6).
Effect of Cornin on VEGF, Ang1, phospho-Akt and phospho-Tie2 expression in the ischemic brain core by western blotting analysis. a Representative Phospho-Akt, VEGF, phospho-Tie2 and Ang1 expression in the ischemic brain by western blotting analysis. b Effect of Cornin on Phospho-Akt, VEGF, phospho-Tie2 and Ang1 expression in the ischemic brain core. Data are mean ± SD, n = 5. *P < 0.01 versus the vehicle-treated rats. Significance was determined by one-way analysis of ANOVA followed by Dunnett’s test
Discussion
In previous studies, SD rats were subjected to MCAO for 1 h and then reperfusion for 23 h. Cornin significantly decreased neurological deficit scores and reduced cerebral infarct volume and degenerative neurons. It increased the brain ATP content, improved mitochondrial energy metabolism, inhibited the elevation of MDA content and ROS generation and attenuated the decrease of SOD and GPx activities in the brain mitochondria. The protective potential of cornin against cerebral ischemia injury and its protective effects may be because of the amelioration of cerebral mitochondrial function and to its antioxidant properties (Jiang et al. 2010).
In the present study, we showed that treatment of MCAO rats with cornin ameliorates sensorimotor function and increases microvasculature density, VEGF and Ang1/Tie2 expression and phospho-Akt and phospho-Tie2 expression after i.v. administration for 14 days. Cornin also decreased the permeability of the BBB. Incubation of RASMCs with 3 μM and 10 μM cornin for 48 h promoted proliferation by wnt signalling.
In rodents as in humans, MCAO operation produces a syndrome characterized predominantly by hemiparesis and hemisensory loss. Accordingly, we employed a battery of sensorimotor tests aimed specifically at assessing long-term functional outcomes in MCAO rats (Roof et al. 2001). Our results showed that administration of cornin 25 mg/kg daily for 3, 7 and 14 days improved the sensorimotor function of stroke rats.
VWF is a specific marker of endothelial cells and is expressed in large amounts on endothelial cells, which control and regulate angiogenesis (Starke et al. 2011; Randi et al. 2013). In the central nervous system, angiogenesis results in the restoration of cerebral blood flow (CBF) in the ischemia penumbra, contributing to long-term functional recovery after ischemic stroke (Jaquet et al. 2002). The newly formed microvessels improve tissue perfusion within the ischemic penumbra, thereby promoting the recovery of neurological function following ischemia (Zhang et al. 2002). A correlation between angiogenesis and stroke has been shown on the basis of the observations of decreased morbidity and mortality rates in stroke patients because they display increased CBF and microvessel density. The promotion of angiogenesis is beneficial to patients suffering from a stroke. Our results indicated that cerebral I/R rats administered 25 mg/kg of cornin daily for 14 days showed increased angiogenesis in the ischemic brain zone.
VEGF induces neuroprotection, neurogenesis and angiogenesis after stroke (Sun et al. 2003). However, VEGF is notorious for causing increased vascular permeability, inflammation and vasodilation (Croll et al. 2004). VEGF-induced angiogenic vessels, particularly in the formative stages, are leaky (Schoch et al. 2002). These effects of VEGF lead to BBB leakage and edema and damage the nervous tissue (Pettersson et al. 2000). A moderate up-regulation of VEGF might offer benefit in improving motion and sensation function. Our results showed that 25 mg/kg cornin treatment for 7 and 14 days increased VEGF but reduced BBB leakage in cerebral I/R rats.
Ang1 is a family of endothelial growth factors and functions as the ligand for the endothelial-specific receptor tyrosine kinase Tie2. Ang1-induced Tie2 phosphorylation is essential to vasculogenesis and the maintenance of vascular endothelial integrity (Chen et al. 2009). Inducing Ang1/Tie2 amplifies angiogenesis and vascular stabilization after stroke (Mammoto et al. 2013). Tie2 activation leads to Akt activation, and Akt is necessary and sufficient for increasing endothelial cell sprouting stimulated by both Ang1 and VEGF. The Tie2 receptor and Akt are essential elements in the signal transduction pathway leading to endothelial cell survival induced by the paracrine activity of Ang1 (Yu et al. 2001). Cornin treatment in stroke rats for 14 days increased Ang1 expression and upregulated phosphorylation of Tie2 and Akt activity in the ischemic border.
Endothelial progenitor cells (EPCs) have robust therapeutic angiogenic potential, yet are limited in the capacity to develop into fully mature vasculature. Mature vasculature requires the presence of supporting elements, such as smooth muscle cells (SMCs), which are essentially vascular pericytes that enhance the angiogenic performance of EPCs (Yang and Proweller 2011; Shudo et al. 2013). Vascular development and the process of angiogenesis depend on evaluating the function of novel regulatory pathways such as Wnt/β-catenin signalling (Parmalee and Kitajewski 2008). The Wnt/β-catenin pathway is beneficial to cerebral ischemia and angiogenesis and plays a key role in SMC proliferation (Zerlin et al. 2008). In brief, β-catenin activates and up-regulates pro-proliferative genes including cyclin D1 (Lyon et al. 2011; Xing et al. 2012). Our previous results showed that cornin induces angiogenesis in vitro via a programmed PI3K/Akt/eNOS/VEGF signalling axis in a brain microvascular endothelial cell line (HBMEC) (Kang et al. 2013). Consistent with this result, cornin increased RASMC proliferation and increased Wnt5a, β-catenin, cyclin D1 and Ang1 expression in RASMC. In particular, cornin increases Ang1 expression in RASMC, assisting in the maturation of new blood vessels. This observation suggests that cornin induces RASMC proliferation, which helps to enhance vasculature maturation. Wnt/β-catenin pathways mediates cornin-induced RASMC proliferation; however, whether this is via PI3 K/Akt/eNOS signalling remain to be determined.
In summary, we have shown that the treatment of experimental stroke rats with cornin for 14 days significantly improved functional outcomes and increased angiogenesis. Cornin decreased BBB leakage, increased proliferation and increased Wnt5a, β-catenin, cyclin D1 and Ang1 expression in RASMC. These findings showed that cornin can promote angiogenesis, leading to an improvement of functional outcomes after stroke. The Ang1/Tie2 and Wnt/β-catenin pathways appear to contribute to cornin-induced angiogenesis.
References
Bonita, R., S. Mendis, T. Truelsen, J. Bogousslavsky, J. Toole, and F. Yatsu. 2004. The global stroke initiative. Lancet Neurology 3(7): 391–393.
Chen, J., X. Cui, A. Zacharek, and M. Chopp. 2009. Increasing Ang1/Tie2 expression by simvastatin treatment induces vascular stabilization and neuroblast migration after stroke. Journal of Cellular and Molecular Medicine 13(7): 1348–1357.
Chen, J., X. Cui, A. Zacharek, H. Jiang, C. Roberts, C. Zhang, M. Lu, A. Kapke, C.S. Feldkamp, and M. Chopp. 2007. Niaspan increases angiogenesis and improves functional recovery after stroke. Ann Neurol 62(1): 49–58.
Croll, S.D., J.H. Goodman, and H.E. Scharfman. 2004. Vascular endothelial growth factor (VEGF) in seizures: A double-edged sword. Advances in Experimental Medicine and Biology 548: 57–68.
De Ryck, M., J. Van Reempts, M. Borgers, A. Wauquier, and P.A. Janssen. 1989. Photochemical stroke model: Flunarizine prevents sensorimotor deficits after neocortical infarcts in rats. Stroke 20(10): 1383–1390.
Feeney, D.M., A. Gonzalez, and W.A. Law. 1982. Amphetamine, haloperidol, and experience interact to affect rate of recovery after motor cortex injury. Science 217(4562): 855–857.
Jaquet, K., K. Krause, M. Tawakol-Khodai, S. Geidel, and K.H. Kuck. 2002. Erythropoietin and VEGF exhibit equal angiogenic potential. Microvascular Research 64(2): 326–333.
Jiang, W.L., S.P. Zhang, H.B. Zhu, and J. Hou. 2011. Effect of 8-O-acetyl shanzhiside methylester increases angiogenesis and improves functional recovery after stroke. Basic & Clinical Pharmacology & Toxicology 108(1): 21–27.
Jiang, W.L., S.P. Zhang, H.B. Zhu, H. Jian, and J.W. Tian. 2010. Cornin ameliorates cerebral infarction in rats by antioxidant action and stabilization of mitochondrial function. Phytotherapy Research 24(4): 547–552.
Kang, Z., W. Jiang, H. Luan, F. Zhao, and S. Zhang. 2013. Cornin induces angiogenesis through PI3K-Akt-eNOS-VEGF signaling pathway. Food and Chemical Toxicology 58: 340–346.
Kim, A.S., and S.C. Johnston. 2011. Global variation in the relative burden of stroke and ischemic heart disease. Circulation 124(3): 314–323.
Liu, X.F., J.R. Fawcett, R.G. Thorne, T.A. DeFor, and W.H. Frey. 2001. Intranasal administration of insulin-like growth factor-I bypasses the blood–brain barrier and protects against focal cerebral ischemic damage. Journal of the Neurological Sciences 187(1–2): 91–97.
Lyon, C., C. Mill, A. Tsaousi, H. Williams, and S. George. 2011. Regulation of VSMC behavior by the cadherin-catenin complex. Frontiers in Bioscience (Landmark Ed) 16: 644–657.
Mammoto, T., A. Jiang, E. Jiang, and A. Mammoto. 2013. Platelet rich plasma extract promotes angiogenesis through the angiopoietin1-Tie2 pathway. Microvascular Research 89: 15–24.
Moss, A. 2013. The angiopoietin: Tie 2 interaction: A potential target for future therapies in human vascular disease. Cytokine & Growth Factor Reviews 24(6): 579–592.
Parmalee, N.L., and J. Kitajewski. 2008. Wnt signaling in angiogenesis. Current Drug Targets 9(7): 558–564.
Pettersson, A., J.A. Nagy, L.F. Brown, C. Sundberg, E. Morgan, S. Jungles, R. Carter, J.E. Krieger, V.S. Harvey, I.A. Eckelhoefer, D. Feng, A.M. Dvorak, R.C. Mulligan, and H.F. Dvorak. 2000. Heterogeneity of the angiogenic response induced in different normal adult tissues by vascular permeability factor/vascular endothelial growth factor. Laboratory Investigation 80(1): 99–115.
Randi, A.M., M.A. Laffan, and R.D. Starke. 2013. Von Willebrand factor, angiodysplasia and angiogenesis. Mediterranean Journal of Hematology and Infectious Diseases 5(1): e2013060.
Roof, R.L., G.P. Schielke, X. Ren, and E.D. Hall. 2001. A comparison of long-term functional outcome after 2 middle cerebral artery occlusion models in rats. Stroke 32(11): 2648–2657.
Satchell, S.C., K.L. Anderson, and P.W. Mathieson. 2004. Angiopoietin 1 and vascular endothelial growth factor modulate human glomerular endothelial cell barrier properties. Journal of the American Society of Nephrology 15(3): 566–574.
Schallert, T., M. Upchurch, N. Lobaugh, S.B. Farrar, W.W. Spirduso, P. Gilliam, D. Vaughn, and R.E. Wilcox. 1982. Tactile extinction: Distinguishing between sensorimotor and motor asymmetries in rats with unilateral nigrostriatal damage. Pharmacology, Biochemistry and Behavior 16(3): 455–462.
Schoch, H.J., S. Fischer, and H.H. Marti. 2002. Hypoxia-induced vascular endothelial growth factor expression causes vascular leakage in the brain. Brain 125(Pt 11): 2549–2557.
Shudo, Y., J.E. Cohen, J.W. Macarthur, P. Atluri, P.F. Hsiao, E.C. Yang. 2013. Spatially oriented, temporally sequential smooth muscle cell-endothelial progenitor cell bi-level cell sheet neovascularizes ischemic myocardium. Circulation 128: S59–S68.
Starke, R.D., F. Ferraro, K.E. Paschalaki, N.H. Dryden, T.A. McKinnon, R.E. Sutton, E.M. Payne, D.O. Haskard, A.D. Hughes, D.F. Cutler, M.N. Laffan, and A.M. Randi. 2011. Endothelial von Willebrand factor regulates angiogenesis. Blood 117(3): 1071–1080.
Sun, Y., K. Jin, L. Xie, J. Childs, X.O. Mao, A. Logvinova, and D.A. Greenberg. 2003. VEGF-induced neuroprotection, neurogenesis, and angiogenesis after focal cerebral ischemia. Journal of Clinical Investigation 111(12): 1843–1851.
Swanson, R.A., M.T. Morton, G. Tsao-Wu, R.A. Savalos, C. Davidson, and F.R. Sharp. 1990. A semiautomated method for measuring brain infarct volume. Journal of Cerebral Blood Flow and Metabolism 10(2): 290–293.
Talwar, T., and M.V. Srivastava. 2014. Role of vascular endothelial growth factor and other growth factors in post-stroke recovery. Annals of Indian Academy of Neurology 17(1): 1–6.
Tetsu, O., and F. McCormick. 1999. Beta-catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature 398(6726): 422–426.
Vakili, A., F. Hosseinzadeh, and T. Sadogh. 2007. Effect of aminoguanidine on post-ischemic brain edema in transient model of focal cerebral ischemia. Brain Research 1170: 97–102.
Vlad, A., S. Rohrs, L. Klein-Hitpass, and O. Muller. 2008. The first five years of the Wnt targetome. Cellular Signalling 20(5): 795–802.
Xing, Y., X. Zhang, K. Zhao, L. Cui, L. Wang, L. Dong, and X. Cao. 2012. Beneficial effects of sulindac in focal cerebral ischemia: A positive role in Wnt/beta-catenin pathway. Brain Research 1482: 71–80.
Yang, K., and A. Proweller. 2011. Vascular smooth muscle Notch signals regulate endothelial cell sensitivity to angiogenic stimulation. Journal of Biological Chemistry 286(15): 13741–13753.
Yu, Y., J. Varughese, L.F. Brown, J.B. Mulliken, and J. Bischoff. 2001. Increased Tie2 expression, enhanced response to angiopoietin-1, and dysregulated angiopoietin-2 expression in hemangioma-derived endothelial cells. American Journal of Pathology 159(6): 2271–2280.
Zerlin, M., M.A. Julius, and J. Kitajewski. 2008. Wnt/Frizzled signaling in angiogenesis. Angiogenesis 11(1): 63–69.
Zhang, Z.G., L. Zhang, Q. Jiang, and M. Chopp. 2002. Bone marrow-derived endothelial progenitor cells participate in cerebral neovascularization after focal cerebral ischemia in the adult mouse. Circulation Research 90(3): 284–288.
Acknowledgments
The study was supported by National Natural Science Foundation of China (Grant No. 31170321) and in part financially supported by National Natural Science Foundation of China (Grant No. 31270391), and in part financially supported by Taishan Scholar Project.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interests
The author(s) declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Xu, Y., Zhang, G., Kang, Z. et al. Cornin increases angiogenesis and improves functional recovery after stroke via the Ang1/Tie2 axis and the Wnt/β-catenin pathway. Arch. Pharm. Res. 39, 133–142 (2016). https://doi.org/10.1007/s12272-015-0652-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12272-015-0652-1