Abstract
Research into the neurotoxic activity of venoms from species within the snake family Viperidae is relatively neglected compared with snakes in the Elapidae family. Previous studies into venoms from the Bitis genus of vipers have identified the presence of presynaptic phospholipase A2 neurotoxins in B. atropos and B. caudalis, as well as a postsynaptic phospholipase A2 in B. arietans. Yet, no studies have investigated how widespread neurotoxicity is across the Bitis genus or if they exhibit prey selectivity of their neurotoxins. Utilising a biolayer interferometry assay, we were able to assess the binding of crude venom from 14 species of Bitis to the neuromuscular α-1 nAChR orthosteric site across a wide range of vertebrate taxa mimotopes. Postsynaptic binding was seen for venoms from B. arietans, B. armata, B. atropos, B. caudalis, B. cornuta, B. peringueyi and B. rubida. To further explore the types of neurotoxins present, venoms from the representatives B. armata, B. caudalis, B. cornuta and B. rubida were additionally tested in the chick biventer cervicis nerve muscle preparation, which showed presynaptic and postsynaptic activity for B. caudalis and only presynaptic neurotoxicity for B. cornuta and B. rubida, with myotoxicity also evident for some species. These results, combined with the biolayer interferometry results, indicate complex neurotoxicity exerted by Bitis species, which varies dramatically by lineage tested upon. Our data also further support the importance of sampling across geographical localities, as significant intraspecific variation of postsynaptic neurotoxicity was reported across the different localities.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Snake venom toxins exhibit a variety of pathophysiological activities which act synergistically, targeting key physiological systems to immobilise prey (Fry et al. 2012). Species within the family Viperidae possess highly complex venoms, with coagulotoxic effects being the most prominent and investigated activity (Serrano et al. 2005). Although neurotoxicity is a main pathophysiology of venom from most species within the family Elapidae, it has been relatively understudied within Viperidae (Barber et al. 2013; Harris et al. 2020b; Nirthanan and Gwee 2004).
The most common function of snake venom neurotoxins is to cause flaccid paralysis via inhibition of the orthosteric site (acetylcholine binding region) of postsynaptic muscle-type α-1 nicotinic acetylcholine receptors (nAChRs). This postsynaptic neurotoxicity is primarily seen in venoms which possess three finger toxins (3FTxs) which are a non-enzymatic toxin class ubiquitous to the Elapidae family along with some species of Colubridae and Lamprophiidae (Barber et al. 2013; Fry et al. 2003, 2008; Nirthanan and Gwee 2004; Pawlak et al. 2009). Snake toxins that target postsynaptic nAChRs are known as α-neurotoxins (Barber et al. 2013; Nirthanan and Gwee 2004).
Conversely, only some species of vipers have been shown to have neurotoxic activities. A toxin isolated from Crotalus durissus terrificus, known as crotoxin, is a snake venom phospholipase A2 (PLA2) (Chang and Lee 1977a). Crotoxin blocks neuromuscular transmission at the presynapse by binding to the motor nerve terminal to deplete synaptic acetylcholine (ACh) vesicles, thus impairing the release of ACh into the synaptic cleft. Toxins that target the presynapse to impair ACh vesicle release are known as β-neurotoxins. This toxin complex is widespread in Crotalus, and the relative presence may vary dramatically between populations, as has been shown for Crotalus scutatus (Dobson et al. 2018). Other neurotoxic peptides from vipers include the Azemiopsin peptides from Azemiops species and the waglerin peptides from Tropidolaemus species, which are two types of post-synaptic neurotoxins that are derived from the propeptide region of the C-type natriuretic gene (Brust et al. 2013; Debono et al. 2017; Harris et al. 2020b; Utkin et al. 2012).
Neurotoxic effects have also been identified in some African vipers within the genus Bitis. Neurotoxicity is a common pathophysiology from Bitis atropos envenomings (Van Der Walt and Muller 2018; Wium et al. 2017), with two neurotoxic PLA2 toxins having been isolated from the venom and tested for their LD50 in vivo (Van Zyl et al. 2001). Caudoxin, a presynaptic PLA2 neurotoxin, isolated from the venom of B. caudalis has been investigated using both chick biventer cervices nerve muscle (CBCNM) and mouse phrenic nerve diaphragm (MPND) (Lee et al. 1982). Further, the crude venom of B. arietans has shown postsynaptic neurotoxic activity (Fernandez et al. 2014). Additionally, a PLA2 toxin named Bitanarin has been isolated that competed with α-bungarotoxin for binding to α-7 nAChRs suggesting postsynaptic activity (Vulfius et al. 2011). However, unlike α-1, α-7 is not a physiologically relevant target, and the evolutionary implications of this activity remain to be elucidated. Regardless, these studies show that both post- and presynaptic acting PLA2 neurotoxins are prevalent within Bitis venoms.
Thus, neurotoxicity in African vipers seems to be a neglected area, especially considering the overwhelming amount of neurotoxic research conducted on elapids (Barber et al. 2013; Nirthanan and Gwee 2004). This is potentially due to the lack of neurotoxic symptoms observed in human envenoming reports from African viper species. However, since humans are not natural prey items of viperid snakes, and given the potential for both α- and β-neurotoxins to exhibit prey-specific targeting (Chang and Lee 1977b; Chang et al. 1977; Harris et al. 2020c; Heyborne and Mackessy 2013; Pawlak et al. 2006, 2009; Su and Chang 1984), the absence of neurotoxic effects in human bite victims is not evidence of the absence of neurotoxins. Therefore, the exploration of prey-selective neurotoxins in viper venoms is a rich but neglected area of research.
Bitis continues to be one of the most medically significant and widespread genera across Africa (Kasturiratne et al. 2008). Despite some evidence for neurotoxicity within the genus (Fernandez et al. 2014; Van Zyl et al. 2001; Viljoen et al. 1982), research has primarily focussed on the coagulotoxic effects of their venom (Mackay et al. 1970; Marsh and Whaler 1974; Morné et al. 2016; Pirkle et al. 1986; Sánchez et al. 2011; Viljoen et al. 1979; Youngman et al. 2019, 2020). This lack of research leaves a major gap in our understanding of the evolutionary history and presence of neurotoxicity throughout the genus. Thus, we aimed to assess the neurotoxicity of Bitis venoms utilising a well-established biolayer interferometry (BLI) assay designed to test the binding to orthosteric site (ACh binding region) of postsynaptic α-1 nAChRs to a plethora of taxon-specific mimotopes (Harris et al. 2020a, 2020b, 2020c; Zdenek et al. 2019a). We set out to determine what other members of the Bitis genus utilised postsynaptic nAChR targeting toxins and then further assess if they exhibit prey-specific targeting which is known to occur with some α-neurotoxins (Heyborne and Mackessy 2013; Pawlak et al. 2006, 2009). Bitis are also the most geographically widespread viper genus across Africa, with some species having extremely wide distributions (e.g. B. arietans) and others having multiple allopatric populations (e.g. B. atropos, B. caudalis and B. cornuta) (Barlow et al. 2019; Spawls and Branch 2020). Sampling across multiple populations is critically important to determine evolutionary facets about the venom activity. Thus, different locations of some Bitis species were also tested to determine if geographical variation of neurotoxicity occurs which has been revealed in other venomous snakes (Dobson et al. 2018; Forstner et al. 1997; Glenn and Straight 1978; Glenn et al. 1983). To further support our BLI results, representative Bitis species which exhibited postsynaptic binding were additionally tested on a CBCNM assay to further confirm the presence of neurotoxic activity.
Methods and Materials
Venoms and Reagents
All venom work was undertaken under University of Queensland IBSC Approval #IBC134BSBS2015. All venoms were sourced from captive snakes: Bitis arietans (Kenya, Mali, Saudi Arabia, Tanzania and West Africa locales) and B. nasicornis (Burundi) venoms were purchased from Latoxan (Portes-les-Valence, France); B. atropos unicolor (Limpopo province), B. cornuta (Kleinzee and Springbok) and B. parviocula (Ethiopia) were supplied by the Universeum, Gothenburg; B. armata (Cape Arguilas), B. peringueyi (UL) and B. schneideri (UL) were supplied by the Serpentarium Calden, Germany. All other venom samples were sourced from the Toxin Evolution Lab long-term cryogenic research collection. For samples from species where the geographical locality of the founding stock was unknown, the abbreviation UL (unknown locality) was used. Venoms were lyophilised and stored at − 80 °C. Working stock solutions of venom (50% glycerol and 50% double-deionised water (ddH2O) to preserve enzymatic activity) were made at a concentration of 1 mg/ml and stored at − 20 °C until required. Working stock concentrations were determined using a NanoDrop 2000 UV–Vis Spectrophotometer in triplicate.
Mimotope Production and Preparation
Extending previous research (Harris et al. 2020b, 2020c; Zdenek et al. 2019a), a 13–14 amino acid mimotope of the nAChR orthosteric site of vertebrate α-1 nAChR subunit was developed by GenicBio Ltd. (Shanghai, China) designed upon specification and adapted from publicly available sequences of cholinergic receptors (Chrna1) from Genbank and UniProt. For each taxon, the α-1 orthosteric site amino acid sequences were obtained using the following accession codes: amphibian α1 (uniprot F6RLA9), lizard α1 (Genbank XM_015426640), avian α1 (uniprot E1BT92), rodent α-1 (uniprot P25108) and human α-1 (uniprot G5E9G9). The only exception was the α-1 sequence for the snake α-1 (Coelognathus radiatus), which was Sanger sequenced in a previous study (Zdenek et al. 2019a). During peptide synthesis, the Cys-Cys of the native mimotope is replaced with Ser-Ser to avoid uncontrolled postsynthetic thiol oxidation. Replacement with Ser-Ser is not expected to have any effect upon the analyte-ligand complex formation as the Cys-Cys bond in the nAChR binding region does not participate directly in analyte-ligand binding (McLane et al. 1994, 1991; Tzartos and Remoundos 1990). However, the Cys-Cys bond is important in the conformation of the interaction site of whole receptors. Thus, direct comparisons between nAChR mimotopes and whole receptor testing using kinetics data should be avoided or approached with caution.
Mimotopes were further synthesised to a biotin linker bound to two aminohexanoic acid (Ahx) spacers, forming a 30 Å linker. Dimethyl sulfoxide (DMSO) was used to solubilise mimotope dry stocks, then diluted in 1:10 dilution of double deionised water to create a working stock of 50 µg/ml and stored at − 80 °C until use.
Biolayer Interferometry
The biolayer interferometry (BLI) assay was performed on an Octet HTX system (ForteBio™, Fremont, CA, USA). All methodology followed previous research that developed this nAChR binding assay (Harris et al. 2020c; Zdenek et al. 2019a). In brief, Streptavidin biosensors were hydrated in assay running buffer for 30–60 min and agitated at 2.0 revolutions per minute (RPM) on a shaker, prior to experimentation. Venom (analyte) samples were diluted to make a final experimental concentration of 50 µg/ml per well and mimotope aliquots were diluted to an experimental concentration of 1 µg/ml per well. 1× DPBS with 0.1% BSA and 0.05% Tween-20 was used for the assay running buffer. Analyte dissociation occurred using a standard acidic solution (glycine buffer), made up of 10 mM glycine (pH 1.5–1.7) in ddH2O. Raw data is provided in supplementary file 1. All experiments were conducted in triplicate across the mimotopes. Due to limitations in venom supply, B. worthingtoni was unable to be tested upon the human mimotope.
All data obtained from the BLI assay was processed in exact accordance to the validation of this assay (Zdenek et al. 2019a). The association step data was obtained in an excel.csv file extracted from raw outputs of the Octet HTX system and then imported into Prism 8.0 software (GraphPad Software Inc., La Jolla, CA, USA) for analysis and graphing. Raw data is provided in supplementary file 1.
Chick Biventer Cervicis Nerve Muscle Preparation
The chick biventer work was undertaken under Monash University Animal Ethics Committee (MARP2 committee) approval # 22575. Chicks aged 4 to 10 days were euthanized with CO2. After dissection, the chick biventer cervicis nerve muscle (CBCNM) preparations were mounted under 1 g tension in 5-ml organ baths containing physiological salt solution (NaCl, 118.4 mM; KCl, 4.5 mM; MgSO4, 1.2 mM; KH2PO4, 1.2 mM; CaCl2, 2.5 mM; NaHCO3, 25 mM; and glucose, 11.1 mM). Organ baths were maintained at 34 °C and bubbled with carbogen (95% O2; 5% CO2). Electrodes were placed around the tendon of the biventer muscle and the motor nerve stimulated (0.2 ms duration, 0.1 Hz, supramaximal V), using a Grass S88 stimulator (Grass Instruments, Quincy, MA, USA), to evoke indirect twitches. Selective stimulation of the nerve was confirmed by the abolition of twitches with d-tubocurarine (10 µM), a nAChR competitive antagonist. Tissues were then washed repeatedly with physiological salt solution to restore twitch responses to nerve stimulation. The stimulation was ceased, and the contractile responses to acetylcholine (ACh, 1 mM for 30 s), carbachol (CCh, 20 µM for 60 s) and potassium chloride (KCl, 40 mM for 30 s) were obtained and recorded. The organ bath was then washed, and electrical stimulation was resumed and maintained for 30 min to allow the preparation to equilibrate. Venom (10 µg/ml) was added to the organ bath and the twitch height was recorded until the abolition of twitch responses or after a 1 h period. The stimulator was turned off again and the bath was washed. Contractile responses to ACh, CCh and KCl were obtained again to compare with responses prior to venom addition. The twitch responses to electrical stimulations and contractile responses to agonists (ACh, CCh and KCI) were measured using a Grass FT03 force displacement transducer (Grass Instruments, Quincy, MA, USA) and recorded on a PowerLab system (ADInstruments Pty Ltd., Bella Vista, NSW, Australia). To compare the responses to exogenous agonists, one-way ANOVAs were used followed by Tukey’s multiple comparison post hoc tests. Graphs were prepared using Prism8.0 software (GraphPad Software Inc., La Jolla, CA, USA). Raw data is provided in supplementary file 2.
Results and Discussion
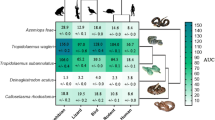
Previous research investigating the crude venom of B. arietans and isolated PLA2 toxins has been the only indication of postsynaptic neurotoxicity within Bitis venom to date (Fernandez et al. 2014; Vulfius et al. 2011). Our investigation into the postsynaptic binding activity of the crude venom from 14 species across the Bitis genus revealed that postsynaptic neurotoxicity is more common throughout the genus than previously realised, and in particular is a trait of many dwarf Bitis species. However, no significant postsynaptic binding was observed for the dwarf species B. worthingtoni which is the most basal of the genus, or in any of the giant species tested which make up the Macrocerastes clade (i.e. B. gabonica, B. nasicornis, B. parviocula, B. rhinoceros) (Fig. 1). Intraspecific venom variation was also seen for multiple Bitis sp., further supporting the occurrence of geographical variation of venom (Fig. 1).
Comparison of wavelength (nm) curves of the association step (ka binding step) for Bitis, conducted over a 120-s assay period. Venoms were tested against amphibian (green), lizard (red), snake (aqua), bird (blue), rodent (brown) and human (purple) mimotopes in triplicate (n = 3). Crotalus horridus was included as a negative control to represent a venom rich in non-binding toxin types. The dots surrounding the curve lines represent the standard error of the mean (SEM). Due to the high precision of the BLI assay, the SEMs are too small to be viewed for some curves
Significant geographical variation was found within B. arietans, with only the venoms from the Saudi Arabia and Eastern Cape localities possessing notable neurotoxic activity out of seven localities tested (Fig. 1). Geographical variation in venom composition and function has been documented within many snake species (Forstner et al. 1997; Glenn et al. 1983; Jayanthi and Veerabasappa Gowda 1988; Zdenek et al. 2019b), including previously for B. arietans venom (Currier et al. 2010; Youngman et al. 2019). Interestingly, the two B. arietans populations which showed postsynaptic binding are not located close to each other, being highly separated at the north-eastern and south-eastern extents of B. arietans’ range (Barlow et al. 2019; Spawls and Branch 2020). Analysis of mitochondrial data across B. arietans has also shown that the Arabian population is allopatric and has diverged into its own clade, separate from other B. arietans populations including that of the Eastern Cape (Barlow et al. 2013). Since nothing is known that distinctively links these two populations over other populations of B. arietans, either ecologically or geographically, it is possible that this postsynaptic neurotoxic activity was amplified independently twice within B. arietans or that these are the only two populations to retain this activity while all other sampled populations have secondarily lost this activity.
Although the basal dwarf species B. worthingtoni did not show binding to the nAChR mimotopes, several species of dwarf Bitis from the subgenus Calechidna displayed binding to the nAChR mimotopes (Fig. 1). These results therefore indicate that postsynaptic neurotoxicity has been amplified at the base of the Calechidna group of dwarf Bitis. Bitis atropos, B. caudalis, B. cornuta, B. peringueyi and B. rubida all showed postsynaptic binding activity to the orthosteric site of the neuromuscular α-1 nAChR mimotopes (Fig. 1). This activity was also subject to intense geographical variation among different populations of B. atropos, B. caudalis and B. cornuta, all of which have multiple allopatric populations (Fig. 1). Three localities of B. caudalis showed the highest binding affinity of all Bitis species. However, the Messina locality did not show evidence of neurotoxicity, indicating that this locality has had a secondary loss of this trait (Fig. 1). One locality of B. atropos showed binding while three localities did not. B. cornuta also showed significant variation, with two out of three localities showing evidence of neurotoxicity but the third secondarily lacking this trait. Therefore, our results indicate that within the dwarf Bitis, there has been multiple secondary losses of this postsynaptic binding activity, underscoring how extremely dynamic this trait is.
For most species that showed postsynaptic nAChR activity, there was no prey-specific targeting (Fig. 1). However, some species such as B. arietans (Eastern cape), B. armata and all locales of B. caudalis seemed to show some degree of preferential prey binding (Fig. 1). Bitis arietans (Eastern cape) and B. armata had their highest binding toward the amphibian mimotope. Bitis caudalis showed preferential targeting towards the bird mimotope with amphibian close behind (Fig. 1) across all localities to varying degrees of binding intensity. Although these results are not indicative of true prey selectivity, they do suggest that certain proportions of postsynaptic nAChR toxins within Bitis have the propensity to target certain prey orthosteric sites than others. This is also evident in that all venoms were either very low binding or did not bind to the human and rodent mimotopes.
Previous research utilising the BLI method has shown that the orthosteric site of the human α-1 nAChR is susceptible (albeit weak compared with taxa types tested) to other classes of α-neurotoxins (Harris et al. 2020b). This suggests that the α-neurotoxin susceptibility of Bitis venoms toward the human α-1 orthosteric site is low. This also further supports the lack of neurotoxic symptoms in Bitis envenomations, except B. atropos which is likely to be caused by presynaptic neurotoxins. These results further support the importance of using taxa which are representative of potential prey to capture the whole function of the venom when investigating activity. Numerous studies only aim to understand venom activity regarding medical significance and thus are focussed on the pathophysiology of human envenomations, which is not always an accurate reflection of the physiological system specific toxins have evolved toward natural prey. Future work should endeavour to determine the biochemical interactions associated with the human α-1 orthosteric site which prevent the binding of Bitis α-neurotoxins.
To further investigate the neurotoxic activity of Bitis venoms, we tested representative species using the in vitro CBCNM preparation. The selected species were B. armata (Cape Arguilas), B. caudalis (Namaqualand), B. cornuta (Springbok) and B. rubida. Interestingly, although B. caudalis had the strongest response on the BLI, it displayed a relatively weak neurotoxic response on the CBCNM, only reducing twitch height by approximately 50% over 60 min (Fig. 2a). However, B. caudalis venom showed the largest inhibitory effect on contractile responses to ACh and CCh, potentially indicating the highest postsynaptic activity (Fig. 2b). However, only the decrease in ACh response (F(4,14) = 6.156, p = 0.0084) was statistically significant when compared with the time control and this was accompanied by a significant drop in the contractile response to KCl (F(4,14) = 3.84, p = 0.0466) (Fig. 2b). The drop in response to KCl is suggestive that there may be myotoxic effects in addition to postsynaptic neurotoxic binding, causing the reduction in response to the exogenous agonists ACh and CCh in this tissue preparation.
Chick biventer cervicis nerve muscle preparation showing the neurotoxic activity of venoms at 10 µg/ml from B. armata, B. rubida, B. caudalis and B. cornuta and relative agonist blockage. a Inhibition of indirect twitches by the crude venom of B. armata (blue), B. rubida (red), B. caudalis (green) and B. cornuta (purple). Vehicle (white) represents the time control. b Effect of the venoms on contractile response to exogenous acetylcholine (ACh), carbachol (CCh) and potassium chloride (KCI). All venoms were tested in quadruplicate (n = 4), except B. armata which could only be tested in triplicate (n = 3) due to a limited amount of venom. Error bars represent the SEM
The neurotoxic activity of B. armata, B. cornuta (Springbok) and B. rubida venom all showed varying levels of binding to the nAChR mimotopes. Bitis armata venom displayed almost no neurotoxicity on the CBCNM assay (Fig. 2a) which is also consistent with the lack of binding toward the bird mimotope (Fig. 1). However, the level of binding which occurred to the amphibian mimotope by B. armata venom in the BLI assay is suggestive that there may be a small proportion of postsynaptic neurotoxins in the venom which specifically target amphibian postsynaptically (Fig. 1). However, more research is needed to confirm the level of specificity which these kinds of toxins might exhibit, such as using amphibian neuromuscular tissue preparations. Venoms from B. cornuta (Springbok) and B. rubida showed binding in the BLI assay to various nAChR mimotopes tested including the bird mimotope. Testing upon the CBCNM assay showed potent neurotoxic activity from B. cornuta (Springbok) and B. rubida venom, both venoms causing 100% inhibition of twitch height (Fig. 2a). These venoms also partially reduced contractile responses to ACh and CCh indicating that this neurotoxic effect may be due to the activity of both presynaptic and postsynaptic neurotoxins, although this was also accompanied by a decrease in response to KCl which likely indicates a myotoxic in addition to postsynaptic neurotoxic effect similar to the results for the venom of B. caudalis (Fig. 2b).
A potential scenario for the neurotoxic activity of B. caudalis, B. cornuta and B. rubida is that the binding seen towards the nAChR mimotopes in the BLI assays is due to the crude venoms possessing a low proportion of neurotoxins which target the postsynaptic nAChR orthosteric site. However, this activity is not discernible in the CBCNM due to the venom being proportionally higher in presynaptic acting neurotoxins (Lee et al. 1982; Van Zyl et al. 2001; Viljoen et al. 1982) as well as, potentially, myotoxins which obscures any potential postsynaptic activity. PLA2 toxins possessing both presynaptic neurotoxic activity as well as myotoxic activity have indeed been described, such as notexin from the venom of the elapid snake Notechis scutatus (Dixon and Harris 1996; Harris and Johnson 1978; Harris et al. 1973). Due to the propensity for PLA2 toxins to have multiple functions, it is possible that some of the α-neurotoxins present in B. caudalis, B. cornuta, B. rubida which bind to the postsynaptic mimotopes in the BLI assay are also displaying presynaptic or myotoxic activity on the CBCNM causing our observed results. This is thus a rich area for future research.
Conclusions
Our study identifies for the first time the wide prevalence of postsynaptic α-1 nAChR orthosteric targeting venoms across the genus Bitis. Postsynaptic neurotoxicity was present in the venom of B. arietans as well as numerous dwarf Bitis species within the Calechidna clade. This suggests that this form of neurotoxicity may be a basal trait that has been independently amplified on multiple occasions within Bitis. Bitis caudalis, B. cornuta and B. rubida all showed evidence for possessing both presynaptic and postsynaptic neurotoxicity, in addition to myotoxicity. These results therefore suggest that neurotoxic venom activity is more widespread throughout the Bitis genus than previously known. Significant intraspecific geographical variation was also revealed for the postsynaptic neurotoxic activity of B. arietans, B. atropos, B. caudalis and B. cornuta. Thus, these results further support the growing body of literature which establishes the importance in assessing geographical variation in venom activity, particularly for species with extensive or isolated ranges. Future work should investigate additional species across a wider range of concentrations and with more replicates in order to more fully investigate this neglected area of research. The isolation and characterisation of neurotoxins from the venoms would also be beneficial to elucidate their site of action. This is particularly important for B. atropos which is well-characterised as producing potent human effects yet was not very strong in this assay. Thus, the human medicine site of action appears to lie outside the orthosteric site, and thus, follow-up studies should investigate using the chick biventer assay and also ascertain the efficacy of the available South African antivenom. This and other neglected aspects of Bitis venom neurotoxicity is a rich area of future research.
References
Barber CM, Isbister GK, Hodgson WC (2013) Alpha neurotoxins. Toxicon 66:47–58
Barlow A, Baker K, Hendry CR, Peppin L, Phelps T, Tolley KA, Wüster CE, Wüster W (2013) Phylogeography of the widespread African puff adder (Bitis arietans) reveals multiple Pleistocene refugia in southern Africa. Mol Ecol 22:1134–1157
Barlow A, Wüster W, Kelly CMR, Branch WR, Phelps T, Tolley KA (2019) Ancient habitat shifts and organismal diversification are decoupled in the African viper genus Bitis (Serpentes: Viperidae). J Biogeogr 46:1234–1248. https://doi.org/10.1111/jbi.13578
Brust A, Sunagar K, Undheim EA, Vetter I, Yang DC, Casewell NR, Jackson TN, Koludarov I, Alewood PF, Hodgson WC (2013) Differential evolution and neofunctionalization of snake venom metalloprotease domains. Mol Cell Proteomics 12:651–663
Chang C, Lee J (1977) Crotoxin, the neurotoxin of South American rattlesnake venom, is a presynaptic toxin acting like β-bungarotoxin. Naunyn Schmiedeberg’s Arch Pharmacol 296:159–168. https://doi.org/10.1007/BF00508469
Chang CC, Lee JD, Eaker D, Fohlman J (1977) The presynaptic neuromuscular blocking action of taipoxin. A comparison with β-bungarotoxin and crotoxin. Toxicon 15:571–576
Currier RB, Harrison RA, Rowley PD, Laing GD, Wagstaff SC (2010) Intra-specific variation in venom of the African Puff Adder (Bitis arietans): differential expression and activity of snake venom metalloproteinases (SVMPs). Toxicon 55:864–873. https://doi.org/10.1016/j.toxicon.2009.12.009
Debono J, Xie B, Violette A, Fourmy R, Jaeger M, Fry BG (2017) Viper venom botox: the molecular origin and evolution of the waglerin peptides used in anti-wrinkle skin cream. J Mol Evol 84:8–11
Dixon RW, Harris JB (1996) Myotoxic activity of the toxic phospholipase, notexin, from the venom of the Australian tiger snake. J Neuropathol Exp Neurol 55:1230–1237
Dobson J, Yang DC, Op den Brouw B, Cochran C, Huynh T, Kurrupu S, Sánchez EE, Massey DJ, Baumann K, Jackson TN (2018) Rattling the border wall: pathophysiological implications of functional and proteomic venom variation between Mexican and US subspecies of the desert rattlesnake Crotalus scutulatus. Comp Biochem Physiol C: Toxicol Pharmacol 205:62–69
Fernandez S, Hodgson W, Chaisakul J, Kornhauser R, Konstantakopoulos N, Smith AI, Kuruppu S (2014) In vitro toxic effects of puff adder (Bitis arietans) venom, and their neutralization by antivenom. Toxins 6:1586. https://doi.org/10.3390/toxins6051586
Forstner MRJ, Hilsenbeck RA, Scudday JF (1997) Geographic variation in whole venom profiles from the mottled rock rattlesnake (Crotalus lepidus lepidus) in Texas. J Herpetol 31:277–287. https://doi.org/10.2307/1565397
Fry B, Lumsden N, Wüster W, Wickramaratna J, Hodgson W, Manjunatha Kini R (2003) Isolation of a neurotoxin (α-colubritoxin) from a nonvenomous colubrid: evidence for early origin of venom in snakes. J Mol Evol 57:446–452. https://doi.org/10.1007/s00239-003-2497-3
Fry BG, Casewell NR, Wüster W, Vidal N, Young B, Jackson TNW (2012) The structural and functional diversification of the Toxicofera reptile venom system. Toxicon 60:434–448. https://doi.org/10.1016/j.toxicon.2012.02.013
Fry BG, Scheib H, van der Weerd L, Young B, McNaughtan J, Ramjan SR, Vidal N, Poelmann RE, Norman JA (2008) Evolution of an arsenal: structural and functional diversification of the venom system in the advanced snakes (Caenophidia). Mol Cell Proteomics 7:215–246
Glenn JL, Straight R (1978) Mojave rattlesnake Crotalus scutulatus scutulatus venom: variation in toxicity with geographical origin. Toxicon (Oxford) 16:81–84. https://doi.org/10.1016/0041-0101(78)90065-X
Glenn JL, Straight RC, Wolfe MC, Hardy DL (1983) Geographical variation in Crotalus scutulatus scutulatus (Mojave rattlesnake) venom properties. Toxicon (Oxford) 21:119–130. https://doi.org/10.1016/0041-0101(83)90055-7
Harris J, Johnson MA (1978) Further observations on the pathological responses of rat skeletal muscle to toxins isolated from the venom of the Australian tiger snake, Notechis scutatus scutatus. Clin Exp Pharmacol Physiol 5:587–600
Harris J, Karlsson E, Thesleff S (1973) Effects of an isolated toxin from Australian tiger snake (Notechis scutatus scutatus) venom at the mammalian neuromuscular junction. Br J Pharmacol 47:141
Harris RJ, Youngman NJ, Zdenek CN, Huynh TM, Nouwens A, Hodgson WC, Harrich D, Dunstan N, Portes-Junior JA, Fry BG (2020) Assessing the binding of venoms from aquatic Elapids to the nicotinic acetylcholine receptor orthosteric site of different prey models. Int J Mol Sci 21:7377
Harris RJ, Zdenek CN, Debono J, Harrich D, Fry BG (2020) Evolutionary interpretations of nicotinic acetylcholine receptor targeting venom effects by a clade of Asian Viperidae snakes. Neurotox Res. https://doi.org/10.1007/s12640-020-00211-2
Harris RJ, Zdenek CN, Harrich D, Frank N, Fry BG (2020c) An appetite for destruction: detecting prey-selective binding of α-neurotoxins in the venom of Afro-Asian Elapids Toxins 12 https://doi.org/10.3390/toxins12030205
Heyborne WH, Mackessy SP (2013) Identification and characterization of a taxon-specific three-finger toxin from the venom of the Green Vinesnake (Oxybelis fulgidus family Colubridae). Biochimie 95:1923–1932. https://doi.org/10.1016/j.biochi.2013.06.025
Jayanthi GP, Veerabasappa Gowda T (1988) Geographical variation in India in the composition and lethal potency of Russell’s viper (Vipera russelli) venom. Toxicon (Oxford) 26:257–264. https://doi.org/10.1016/0041-0101(88)90216-4
Kasturiratne A, Wickremasinghe AR, de Silva N, Gunawardena NK, Pathmeswaran A, Premaratna R, Savioli L, Lalloo DG, de Silva HJ (2008) The global burden of snakebite: a literature analysis and modelling based on regional estimates of envenoming and deaths (Global Burden of Snakebite). PLoS Medicine 5:e218. https://doi.org/10.1371/journal.pmed.0050218
Lee CY, Ho CL, Botes DP (1982) Site of action of caudoxin, a neurotoxic phospholipase A2 from the horned puff adder (Bitis caudalis) venom. Toxicon 20:637–647. https://doi.org/10.1016/0041-0101(82)90057-5
Mackay N, Ferguson JC, McNicol GP (1970) Effects of the venom of the rhinoceros horned viper (Bitis nasicornis) on blood coagulation, platelet aggregation, and fibrinolysis. J Clin Pathol 23:789. https://doi.org/10.1136/jcp.23.9.789
Marsh NA, Whaler BC (1974) Separation and partial characterization of a coagulant enzyme from Bitis gabonica venom. Br J Haematol 26:295–306. https://doi.org/10.1111/j.1365-2141.1974.tb00474.x
McLane KE, Wu X, Conti-Tronconi BM (1994) An alpha-bungarotoxin-binding sequence on the torpedo nicotinic acetylcholine receptor alpha-subunit: conservative amino acid substitutions reveal side-chain specific interactions. Biochemistry 33:2576–2585
McLane KE, Wu XD, Diethelm B, Conti-Tronconi BM (1991) Structural determinants of alpha-bungarotoxin binding to the sequence segment 181–200 of the muscle nicotinic acetylcholine receptor alpha subunit: effects of cysteine/cystine modification and species-specific amino acid substitutions. Biochemistry 30:4925–4934. https://doi.org/10.1021/bi00234a013
Morné AS, Janette B, Sthembile M, Etheresia P (2016) The effect of physiological levels of South African puff adder (Bitis arietans) snake venom on blood cells: an in vitro model Scientific Reports 6 https://doi.org/10.1038/srep35988
Nirthanan S, Gwee MC (2004) Three-finger α-neurotoxins and the nicotinic acetylcholine receptor, forty years on. J Pharmacol Sci 94:1–17
Pawlak J, Mackessy SP, Fry BG, Bhatia M, Mourier G, Fruchart-Gaillard C, Servent D, Ménez R, Stura E, Ménez A, Kini RM (2006) Denmotoxin, a three-finger toxin from the colubrid snake Boiga dendrophila (Mangrove Catsnake) with bird-specific activity. J Biol Chem 281:29030. https://doi.org/10.1074/jbc.M605850200
Pawlak J, Mackessy SP, Sixberry NM, Stura EA, Le Du MH, Ménez R, Foo CS, Ménez A, Nirthanan S, Kini RM (2009) Irditoxin, a novel covalently linked heterodimeric three-finger toxin with high taxon-specific neurotoxicity. FASEB journal : official publication of the Federation of American Societies for Experimental Biology 23:534. https://doi.org/10.1096/fj.08-113555
Pirkle H, Theodor I, Miyada D, Simmons G (1986) Thrombin-like enzyme from the venom of Bitis gabonica. Purification, properties, and coagulant actions. J Biol Chem 261:8830
Sánchez EE, Hotle D, Rodríguez-Acosta A (2011) Neutralization of Bitis parviocula (Ethiopian mountain adder) venom by the South African Institute of Medical Research (SAIMR) antivenom. Rev Inst Med Trop Sao Paulo 53:213. https://doi.org/10.1590/S0036-46652011000400007
Serrano SMT, Shannon JD, Wang D, Camargo ACM, Fox JW (2005) A multifaceted analysis of viperid snake venoms by two-dimensional gel electrophoresis: an approach to understanding venom proteomics. Proteomics 5:501–510. https://doi.org/10.1002/pmic.200400931
Spawls S, Branch B (2020) The dangerous snakes of Africa. Bloomsbury Publishing, London, UK
Su M, Chang C (1984) Presynaptic effects of snake venom toxins which have phospholipase A2 activity (β-bungarotoxin, taipoxin, crotoxin). Toxicon 22:631–640
Tzartos SJ, Remoundos MS (1990) Fine localization of the major alpha-bungarotoxin binding site to residues alpha 189–195 of the Torpedo acetylcholine receptor. Residues 189, 190, and 195 are indispensable for binding. J Biol Chem 265:21462–21467
Utkin YN, Weise C, Kasheverov IE, Andreeva TV, Kryukova EV, Zhmak MN, Starkov VG, Hoang NA, Bertrand D, Ramerstorfer J (2012) Azemiopsin from Azemiops feae viper venom, a novel polypeptide ligand of nicotinic acetylcholine receptor. J Biol Chem 287:27079–27086
Van Der Walt AJ, Muller GJ (2018) Berg adder (Bitis atropos) envenoming: an analysis of 14 cases. Clin Toxicol 1–6 https://doi.org/10.1080/15563650.2018.1499931
Van Zyl JM, Müller GJ, Van der Merwe MJ (2001) Purification and properties of two phospholipase A2 enzymes from berg adder (Bitis atropos) venom. S Afr J Sci 97:437–444
Viljoen CC, Botes DP, Kruger H (1982) Isolation and amino acid sequence of caudoxin, a presynaptic acting toxic phospholipase A2 from the venom of the horned puff adder (Bitis caudalis). Toxicon 20:715–737. https://doi.org/10.1016/0041-0101(82)90120-9
Viljoen CC, Meehan CM, Botes DP (1979) Separation of Bitis gabonica (Gaboon adder) venom arginine esterases into kinin-releasing, clotting and fibrinolytic factors. Toxicon 17:145–154. https://doi.org/10.1016/0041-0101(79)90293-9
Vulfius CA, Gorbacheva EV, Starkov VG, Osipov AV, Kasheverov IE, Andreeva TV, Astashev ME, Tsetlin VI, Utkin YN (2011) An unusual phospholipase A2 from puff adder Bitis arietans venom – a novel blocker of nicotinic acetylcholine receptors. Toxicon 57:787–793. https://doi.org/10.1016/j.toxicon.2011.02.013
Wium C, Marks C, Du Plessis C, Müller G (2017) Berg adder (Bitis atropos): an unusual case of acute poisoning. S Afr Med J 107:1075–1077
Youngman NJ, Debono J, Dobson JS, Zdenek CN, Harris R, J, Brouw BOD, Coimbra FCP, Naude A, Coster K, Sundman E, Braun R, Hendrikx I, Fry BG, (2019) Venomous landmines: clinical implications of extreme coagulotoxic diversification and differential neutralization by antivenom of venoms within the Viperid snake genus Bitis. Toxins 11:422. https://doi.org/10.3390/toxins11070422
Youngman NJ, Walker A, Naude A, Coster K, Sundman E, Fry BG (2020) Varespladib (LY315920) neutralises phospholipase A2 mediated prothrombinase-inhibition induced by Bitis snake venoms Comparative Biochemistry and Physiology Toxicology & Pharmacology 236 https://doi.org/10.1016/j.cbpc.2020.108818
Zdenek CN, Harris RJ, Kuruppu S, Youngman NJ, Dobson JS, Debono J, Khan M, Smith I, Yarski M, Harrich D, Sweeney C, Dunstan N, Allen L, Fry BG (2019a) A taxon-specific and high-throughput method for measuring ligand binding to Nicotinic Acetylcholine Receptors Toxins 11 https://doi.org/10.3390/toxins11100600
Zdenek CN, Hay C, Arbuckle K, Jackson TNW, Bos MHA, Op Den Brouw B, Debono J, Allen L, Dunstan N, Morley T, Herrera M, Gutiérrez JM, Williams DJ, Fry BG (2019) Coagulotoxic effects by brown snake (Pseudonaja) and taipan (Oxyuranus) venoms, and the efficacy of a new antivenom. Toxicol In Vitro 58:97–109. https://doi.org/10.1016/j.tiv.2019.03.031
Acknowledgements
This research was supported by Australian Research Council Discovery Project DP210102406. N.J.Y. and R.J.H were supported by PhD scholarships funded from the University of Queensland. T.M.H. was supported by PhD scholarship funded from an Australian National Health and Medical Research Council (NHMRC) Centres for Research Excellence Grant (ID:1110343).
Author information
Authors and Affiliations
Contributions
Conceptualisation, B.G.F.; data acquisition, N.J.Y., R.J.H., T.M.H.; funding acquisition, B.G.F.; investigation, N.J.Y., R.J.H., T.M.H.; methodology, N.J.Y., R.J.H., T.M.H., W.C.H., B.G.F.; resources, K.C., E.S., R.B., A.N., W.C.H., B.G.F.; writing—original manuscript, N.J.Y.; writing—review and editing, N.J.Y., R.J.H., B.G.F.; supervision, B.G.F.
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Youngman, N.J., Harris, R.J., Huynh, T.M. et al. Widespread and Differential Neurotoxicity in Venoms from the Bitis Genus of Viperid Snakes. Neurotox Res 39, 697–704 (2021). https://doi.org/10.1007/s12640-021-00330-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12640-021-00330-4