Abstract
3-Nitropropionic acid (3-NP) is a fungal toxin well established model used for inducing symptoms of Huntington’s disease. Curcumin a natural polyphenol has been reported to possess neuroprotective activity by decreasing oxidative stress. The aim of present study was to investigate neuroprotective effect of curcumin with piperine (bioavailability enhancer) against 3-NP induced neurotoxicity in rats. Administration of 3-NP (10 mg/kg for 21 days) showed loss in body weight, declined motor function and changes in biochemical (LPO, nitrite and glutathione level), neuroinflammatory (TNF-α and IL-1β level) and neurochemical (DA, NE, 5-HT, DOPAC, 5-HIAA and HVA). Chronic treatment with curcumin (25 and 50 mg/kg) and curcumin (25 mg/kg) with piperine (2.5 mg/kg) once daily for 21 days prior to 3-NP administration. All the behavioral parameters were studied at 1st, 7th, 14th, and 21st day. On 22nd day all the animals was scarified and striatum was separated. Curcumin alone and combination (25 mg/kg) with piperine (2.5 mg/kg) showed beneficial effect against 3-NP induced motor deficit, biochemical and neurochemical abnormalities in rats. Piperine (2.5 mg/kg) with curcumin (25 mg/kg) significantly enhances its protective effect as compared with curcumin alone treated group. The results of the present study indicate that protective effect of curcumin potentiated in the presence of piperine (bioavailability enhancer) against 3-NP–induced behavioral and molecular alteration.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
3-Nitropropionic acid (3-NP) is a neurotoxin that irreversibly inhibits the succinate dehydrogenase (SDH) an enzyme involved in both the TCA and the electron transport chain in mitochondria [1]. The inhibition of complex II of mitochondrial respiratory chain interrupts oxidative phosphorylation leads to impaired glucose metabolism in brain cells cause enzyme deficiencies and ultimately inhibit production of ATP. The 3-NP model cause bilateral striatal lesion that develops spontaneously [2]. In addition striatal damage has also been seen consistently in HD patients.
Huntington disease (HD) is a rare neurodegenerative disorder of the CNS characterized by choreatic movements, behavioral, psychiatric disturbances and dementia. The presence of hyperkinesias and hypokiesia results difficulties in walking and standing, frequently leads to an ataxic gait and frequent falls [3]. The number of theories and animal models are used now a day to understand the pathogenesis of HD. The increase in oxidative stress and N-methyl-d-aspartate (NMDA) receptors over activity is directly related to impairment of motor performance, decrease oxidative defence, increase level of inflammatory markers and alterations in neurochemical level in the brain [4]. Accumulating data indicate that 3-NP by its number of molecular mechanism produces free radicals generation with consequent increase oxidative stress and ultimately behavioral and psychological disturbances in animals and humans. The 3-NP model provides a good correlation of the various behavioral, biochemical and cellular parameters which are altered significantly in HD patients [5].
Curcumin a natural (polyphenol) also called “Indian Saffron” obtained from turmeric is a non-nutritive, non toxic chemical that has been used worldwide as a dietary spice, food colouring agent and an herbal medicine [6]. Curcumin has an outstanding safety profile and a number of pleiotropic actions with potential for neuroprotective efficacy, including anti-inflammatory and antioxidant [7]. The main active constituent of curcumin is curcuminoid, chemically known as diarylheptanoids. Curcumin represents a class of anti-inflammatory, antioxidant and neuroprotective reported to be a scavanger of formed reactive oxygen species (ROS) probably more than vitamin E and A.
The major problem with curcumin is its very less oral bioavailability because on oral administration in rats its 75 % excreted in feces and only traces amount is appeared in blood [8]. However curcumin is implicated to be neuroprotective and antioxidant in a variety of neurodegenerative disorders like Huntinton’s (HD) [9]. The bioavailability enhancer piperine is a major alkaloidal constituent of black pepper. It is a powerful inhibitor of hepatic and intestinal glucuronidation, and increases the bioavailability of many drugs including curcumin. Since curcumin has a poor absorption rate and undergoes rapid metabolism which severely curtail its bioavailability, thus piperine has been tried as drug strategy with curcumin in the present investigation [10]. This effect of piperine on the pharmacokinetics of curcumin has been shown to be much greater in humans than in rats. In humans, curcumin bioavailability was increased by 2000 % at 45 min after co-administering curcumin orally with piperine.
Therefore the present study has been designed to explore the comparative neuroprotective profile of curcumin and the bioavailability enhancing effect of piperine on 3-NP model of Huntington’s disease.
Materials and Methods
Experimental Animals
The experiment was carried out on male Wistar rats (250–280 g) obtained from central animal house of I.S.F. College of Pharmacy, Moga, Punjab (India). The animals were kept in polyacrylic cages and maintained under standard laboratory conditions (room temperature 22 ± 1 °C and relative humidity of 60 %) with 12 h light/dark cycle The food and water were made available ad libitum. All the behavioral assessments were carried out between 9:00 and 17:00 hours. The experimental protocol was approved by the Institutional Animal Ethics Committee (IAEC) and experiments were carried out in accordance with the guidelines of the Indian National Science Academy (INSA) for the use and care of experimental animals. All experiments for a given treatment were performed using age-matched animals in an attempt to avoid variability between experimental groups.
Drugs and Chemicals
3-NP (Sigma Aldrich, St. Louis, MO, USA); Curcumin (Sigma Aldrich, St. Louis, MO, USA); and piperine (Sigma Aldrich, St. Louis, MO, USA); IL-1β and TNF-α ELISA Kits (Krishgen Bio. Sys.). Unless stated, all other chemicals and biochemical reagents of highest analytical grade were used in the study. The experimental protocol was divided into five groups and each treatment group consisted of nine animals (Total no of animals i.e. n = 45).
Treatment Schedule
3-NP was dissolved in normal saline (pH 7.4) and administered intraperitoneally (i.p) at a dose of 10 mg/kg for 21 days. Curcumin was dissolved in CMC (sodium carboxy methyl cellulose) and administered at a dose of 25, 50 mg/kg and the combination of low dose of curcumin (25 mg/kg) and piperine (2.5 mg/kg) 1 h prior to 3-NP treatment. Further all the drugs or vehicles were administered once daily for 21 days by i.p route (3-NP) and oral route (curcumin). On day 1st, 7th, 14th, and 21st day behavioral parameters like grip strength, narrow beam, rota-rod and locomotor activity were assessed. Terminally on day 21st animals were sacrificed and striatum was separated, homogenized, centrifuged and clear supernatant was used to estimate biochemical parameters (LPO, nitrite, reduced GSH, and protein level) and neurochemical analysis (dopamine, nor-epinephrine, serotonin, DOPAC, HVA, 5-HIAA). The levels of pro-inflammatory cytokines (IL-1β and TNF-α) were estimated using ELISA kits. The experimental procedure is summarized in Table 1.
Measurement of Body Weight
The body weight of animals was recorded on the first and last day of experiment. Percentage change in body weight was calculated using formula:
Behavioral Assessments
Assessment of Gross Behavioral Activity (Locomotor Activity)
Open-Field Test
Open field test is used to monitor spontaneous locomotor activity using wooden, rectangular, light brown coloured open field apparatus measuring 100 × 100 × 40 cm. The floor of the apparatus was divided into 25 rectangular squares by pencil lines. The experimental room was illuminated by 40 W white bulb located 150 cm above the test apparatus. After 2 h of single exposure of apparatus, the animal was placed in the centre and number of squares cross/10 min by animal was noticed. Each crossing was considered only when all four paws were in another square. After each trial apparatus was cleaned properly and readings were taken [11].
Rotarod Activity
The motor coordination and grip performance of the animals were evaluated using the rotarod apparatus. The rats were exposed to a prior training session to acclimatize them to rotarod performance. Rats were placed on a rotating rod having a diameter of 7 cm (speed 25 rpm). The cut off time was 180 s and the average time of the fall was recorded [4].
Grip Strength Measurement
Grip strength of the fore limbs was measured using digital grip force meter (DFIS series, Chatillon, Greensboro, NC, USA). The rat was positioned to grab the grid with the fore limbs and was gently pulled to record the grip strength. The grip strength was recorded in Kgf [4].
Beam-Crossing Task
This task requires an animal to walk on across a narrow wooden beam, measuring its motor Coordination ability. The beam consisted of two platforms (8 cm in diameter) connected by a wooden beam (0.5 mm in thickness, 2.0 cm in width, and 120 cm in length). The beam was elevated 50 cm above ground. A box filled with sawdust was placed below the beam, serving as protection for a falling rat. In order to adapt to the elevated beam, a rat was allowed to explore it for 5 min before training. A training trial started by placing the rat on the platform at one end. When a rat walked across the beam from one end to the other end, slipping of its feet occurred. Number of slips and time taken to cross in each trial was recorded [4].
Dissection and Homogenization
On day 21, all the animals were divided into three group’s one group for biochemical estimations, second for neuroinflammatory markers estimations and third for neurochemicals were sacrificed by decapitation immediately 24 h after behavioral assessments. The brains were removed, fore-brain was dissected out and cerebellum was discarded. Brains were put on the ice and the cortex and striatum regions were separated and weighed. A 10 % (w/v) tissue homogenate was prepared in 0.1 mol/1 phosphate buffer (pH 7.4). Homogenate was centrifuged for 15 min at 15,000 rpm and supernatant was stored in 80 °C for assessing the biochemical parameters.
Measurement of Oxidative Stress Parameters
Measurement of Lipid Peroxidation
The quantitative measurement of lipid peroxidation in the brain striatum was performed according to the method of Wills (1966). The amount of malondialdehyde (MDA), a measure of lipid peroxidation, was measured by reaction with thiobarbituric acid at 532 nm using a Perkin Elmer lambda 20 spectrophotometer. The values were calculated using the molar extinction coefficient of the chromophore (1.56 × 105 M/cm) and expressed as a percentage of the vehicle-treated group [12].
Estimation of Nitrite
The accumulation of nitrite in the striatum supernatant, an indicator of the production of nitric oxide (NO), was determined by a colorimetric assay with Greiss reagent (0.1 % N-(1-naphthyl) ethylenediamine dihydrochloride, 1 % sulphanilamide and 2.5 % phosphoric acid) as described by Green et al. (1982). Equal volumes of supernatant and Greiss reagent were mixed, and this mixture was incubated for 10 min at room temperature in the dark. Absorbance at 540 nm was measured with a Perkin Elmer lambda 20 spectrophotometer. The concentration of nitrite in the supernatant was determined from the standard curve and expressed as a percentage of the vehicle-treated group [13].
Estimation of Reduced Glutathione Levels
Reduced glutathione was estimated according to the method described by Ellman.34 Supernatant (1 ml) was precipitated with 4 % sulphosalicylic acid (1 ml) and cold digested for 1 h at 4 °C. The samples were then centrifuged at 1200g for 15 min at 4 °C. To 1 ml of the supernatant obtained, 2.7 ml of phosphate buffer (0.1 mmol/l, pH 8) and 0.2 ml of 5, 5′ dithio-bis (2-nitrobenzoic acid) (DTNB) was added. The yellow color developed was measured at 412 nm using a Perkin Elmer Lambda 20 spectrophotometer. Results were calculated using molar extinction coefficient of chromophore (1.36 × 104/M/cm) and expressed as percentage of vehicle [14].
Protein Estimation
The protein was measured by the Lowry method using Folin phenol reagent [15].
Estimation of Tumor Necrosis Factor-Alpha (TNF-α) and IL-1β in Striatum
The quantifications of TNF-α and IL-1β were done by rat TNF-α and IL-1β immunoassay kit (KRISHGEN BioSystem, USA). The quantikine rat TNF-α and IL-1β immunoassay is a 4.5 h solid phase ELISA designed to measure rat TNF-α and IL-1β levels. It is a solid- phase sandwich enzyme linked immunosorbent assay (ELISA) using a microtitre plate reader. Concentrations of TNF-α were calculated from the plotted standard curves.
Neurochemical Analysis
Catecholamines (DA, Serotonin and NE) and their metabolites (DOPAC, 5-HIAA, HVA) levels were estimated by HPLC using electrochemical detector. Waters standard system consisting of a high pressure isocratic pump, a 20 μl manual sample injector valve, C18 reverse phase column and electrochemical detector were used in the study. Mobile phase consisted of sodium citrate buffer (pH 4.5)—acetonitrile (87:13 v/v). Sodium citrate buffer consisted of 10 mM citric acid, 25 mM NaH2HPO4, 25 mM EDTA, and 2 mM of 1-heptane sulfonic acid. Electrochemical conditions for the experiment were +0.75 V, sensitivity ranges from 5 to 50 nA. Separation was carried out at a flow rate of 0.8 ml/min. Samples (20 μl) were injected manually. On the day of experiment frozen brain samples were thawed and they were homogenized in homogenizing solution containing 0.2 M perchloric acid. After that samples were centrifuged at 12,000g for 5 min. The supernatant was filtered through 0.22 mm nylon filters before injecting in the HPLC sample injector. Data were recorded and analyzed with the help of breeze software. Concentrations of neurotransmitter and their metabolites were calculated from the standard curve generated by using standard in a concentration range of 10–100 ng/ml [16].
Statistical Analysis
The data obtained is expressed as Mean ± SEM. The behavioral data was analyzed using two way analysis of variance (ANOVA) followed by Bonferroni Post-hoc test for multiple comparison. P < 0.05 was considered statistically significant. For biochemical parameters one way analysis of variance (ANOVA) followed by Tukey’s post hoc test is used for comparison. P < 0.05 was considered statistically significant.
Results
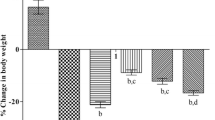
Effect of Curcumin on 3-NP Induced Decrease in Body Weight of Rats
There was no significant change in initial and final body weight of normal control animals. However, 3-NP treated animals showed significant decrease in body weight on last day (21st day), as compared to normal control group. Curcumin pre-treatment (25 and 50 mg/kg/day orally) significantly restored the body weight as compared to the 3-NP treated groups. However co-administration of piperine (2.5 mg/kg/day) with curcumin (25 mg/kg/day) showed significant improvement in the body weight as compared to curcumin alone treated group (Fig. 1).
Effect of Curcumin on 3-NP Induced Changes in Locomotor Activity, Rotarod and Grip Strength Performance of Rats
The systemic administration of 3-NP (10 mg/kg/day) significantly decreased grip strength, locomotor activity and rotarod activity (day 7th, 14th and 21st) as compared to normal control group. Curcumin pre-treatment (25 and 50 mg/kg/day) significantly ameliorated the impairment in grip strength and locomotor activity as compared to 3-NP group. However co-administration of piperine (2.5 mg/kg/day) with curcumin (25 mg/kg/day) synergistically attentuate the impaired grip strength as compared to curcumin alone treated group (Figs. 2, 3, 4).
Effect of Curcumin on Narrow Beam Walks Parameter in 3-NP Treated Rats
The 3-NP administration significantly increased transfer latency to cross narrow beam runway and foot errors on narrow beam walk on day 21st as compared to normal control group. Curcumin pre-treatment (25 and 50 mg/kg/day) significantly improved the latency and decreased foot errors on narrow beam walk apparatus as compared to 3-NP alone treated rats. Moreover co-administration of piperine (2.5 mg/kg/day) with curcumin (25 mg/kg/day) effect significantly as compared to curcumin alone treated group (Fig. 5).
Effect of Curcumin on Lipid Peroxidation and Nitrite Levels in Rats Administered with 3-NP
Systemic administration of 3-NP significantly increased oxido-nitrosative stress parameters, i.e., MDA and nitrite level in the striatum were significantly higher in 3-NP group as compared to the normal control group. Curcumin pre-treatment (25 and 50 mg/kg/day) significantly restored the altered levels of oxido-nitrosative stress in 3-NP administered rats as compared to the 3-NP group. In addition, co-administration of piperine (2.5 mg/kg/day) with curcumin (25 mg/kg/day) significantly decreases oxido-nitrosative stress as compared to curcumin (25 mg/kg/day) and curcumin (50 mg/kg/day) treatment alone as shown in (Table 2).
Effect of Curcumin on Reduced Glutathione in 3-NP Treated Rats
Administration of 3-NP for 21 days significantly depleted reduced glutathione (GSH) enzyme concentration in brain striatum. The treatment with curcumin (25 and 50 mg/kg/day) significantly restored the levels of GSH as compared to the 3-NP treated groups. Further, co-administration of piperine (2.5 mg/kg/day) with curcumin (25 mg/kg/day) significantly enhances its protective effect than alone treated group (Table 2).
Effect of Curcumin on TNF-α and IL-1β Levels in 3-NP Treated Rats
TNF-α and IL-1β are important pro-inflammatory markers in HD and other neuro- degenerative disorders. Systemic administration of 3-NP significantly increased the level of TNF-α and IL-1β levels in brain striatum as compared to normal control group. Curcumin pre-treatment (25 and 50 mg/kg/day) significantly reduced the levels of TNF-α and IL-1β as compared to the 3-NP alone treated groups. In addition, co-administration of piperine (2.5 mg/kg/day) with curcumin (25 mg/kg/day) significantly decreases TNF-α and IL-1β levels in brain striatum as compared to curcumin alone treated group (Fig. 6).
Effect of Curcumin on Catecholamines Levels in the Striatum in 3-NP Treated Rats
In the brain, dopamine is metabolized enzymatically by monoamine oxidase-B (MAO-B) giving rise to 3, 4-dihydroxyphenylacetic acid (DOPAC), which is further metabolized to homovanillic acid (HVA). The levels of catecholamine’s (nor-epinephrine, DA and serotonin) in striatum were found to be significantly decreased with 3-NP treatment. Pre-treatment with curcumin (25 and 50 mg/kg/day) and treatment with co-administration of curcumin (25 mg/kg/day) and piperine (2.5 mg/kg/day) significantly improves the catecholamines levels in striatum as compared to curcumin treatment alone. Also, treatment with 3-NP results in significant increase in DOPAC and HVA levels whereas decrease in levels of 5-HIAA in striatum. Pre-treatment with curcumin (25 and 50 mg/kg/day) significantly attenuated the increased levels of DOAPC and HVA and decreased levels of 5-HIAA when compared to 3-NP alone group. However, co-administration of curcumin (25 mg/kg/day) and piperine (2.5 mg/kg/day) significantly prevented the increase in DOPAC and HVA levels whereas increase in levels of 5-HIAA levels in striatum as compared to curcumin alone treated group (Figs. 7, 8, 9).
Discussion
The present study revealed ameliorative effect of curcumin along with piperine in 3-NP model of Huntington’s disease. The systemic administration of 3-NP cause motor changes and cognitive dysfunction but the mechanism how these changes occur is still unclear [17, 18]. 3-NP (fungal toxin) irreversibly inhibits the mitochondrial enzyme SDH and invariably causes cell death in the caudate, putamen resulting in severe dystonia in humans and animals [19]. Intraperitoneal administration of 3-NP in rats produces striatal lesions with neurobehavioral changes like impairment in locomotor activity, rotarod performance, grip strength and narrow beam walk performance in rats, mimicking those observed in HD. The observed effects are in line with previous reported studies. The pathogenesis of this disease is now a day is burdensome problem but the recent hypothesis explains the etiology of HD that increase in oxidative stress, decrease mitochondrial function and over glutaminergic discharge increase neuronal excitotoxocity, all these factors leads to neurodegeneration [20]. Systemic administration of 3-NP induced alterations in electron transport chain and glucose metabolism results in decreased ATP and increases level of reactive oxygen species (ROS) [21]. These ROS cause ca2+ dependent exitotoxicity and production of NO by nitric oxide synthase type I (nNOS). In turn NO is transformed into peroxynitrite (ONOO−) after reacting with superoxide anion (O2 −) from electron transport chain (ETC). These events create imbalance between oxidant and antioxidant systems ultimately promote typical oxidative stress cascades, such as oxidation of proteins and DNA [22]. Excessive production of ROS cause biochemical alterations like increase lipid peroxidation, nitrite level, glutathione (GSH) depletion and enhanced superoxide activity with subsequent cellular apoptosis are considered leading factors observed in HD pathology.
3-NP also promotes neuroinflammation by activation of oxidative stress (OS) pathway induce activation of NF-kB and due to over expression of lactate dehydrogenase which is a marker of neuronal cell death occur by necrosis [23]. Pro-inflammatory cytokines such as IL-1β & TNF-α directly activates NF-κB, which in turn leads to increased production of NO. These cytokines can directly activate inflammatory and apoptotic pathway, resulting in loss of neuronal activity. TNF-α by binding to TNFr1-type receptor on neurons leading to activation of apoptotic cascade and neurodegeneration in striatum [24]. It is reported that ROS and apoptosis induced neurodegeneration decreased the level of catecholamines (NE, dopamine and serotonin) and 5-HIAA and increased the level of DOAPC and HVA in the striatum nuclei [25]. Further the administration of neurotoxin like 3-NP also alter the neurochemical level in the striatum by increasing dopamine turnover the data is supported by important finding that dopamine transporter knock-out mice are more susceptible to systemic 3-NP administration [26].
Curcumin is polyphenol obtained from curcuma longa rhizome commonly known as turmeric (Indian saffron), is used as a spice worldwide. It has reported neuroprotective and anti-inflammatory activity but has less oral bioavailability that’s why in the present study piperine is co-administered with curcumin [27]. Investigated the pharmacokinetic properties of curcumin administered either orally or intraperitoneal (i.p.) in mice. With oral administration of 1.0 g/kg of curcumin, low plasma levels of 0.13 µg/ml appeared in plasma after 15 min, while a maximum plasma level of 0.22 µg/ml was obtained at 1 h. Piperine is used in combination with curcumin, which is a bioavailability enhancer to increase drug concentration in the brain [28]. Piperine is a major alkaloidal constituent of black pepper. It is a powerful inhibitor of hepatic and intestinal glucuronidation, and increases the bioavailability of curcumin [29].
In the present study pre-treatment with curcumin at dose of 25 and 50 mg/kg significantly attenuated hypolocomotion in an open field test, fair grip strength and reduced narrow beam walk performance. Further co-administration of piperine (2.5 mg/kg, po) with curcumin (25 mg/kg, po) significantly improve the motor coordination than the treatment with curcumin alone. Curcumin at the dose of 25 and 50 mg/kg act as potent antioxidant inhibit lipid peroxidation (LPO) and lower the nitrite level and restore the depleted endogenous antioxidant defense enzyme (reduced GSH) but the treatment with combination of curcumin (25 mg/kg, po) and piperine (2.5 mg/kg, po) shows more significant effect than the treatment with curcumin alone. The treatment with 3-NP shows increase in the level of inflammatory cytokines but pre-treatment with curcumin at low dose of (25 mg/kg, po) and high dose (50 mg/kg, po) shows significant decrease in TNF-α and IL-1β levels. Further co-administration of piperine (2.5 mg/kg, po) with curcumin (25 mg/kg, po) shows significant reduction in cytokines levels than curcumin treatment alone. Administration of 3-NP for 21 days significantly decreased the level of catecholamines (NE, dopamine and serotonin) and 5-HIAA but the Pre-treatment with curcumin at low dose of (25 mg/kg, po) and high dose (50 mg/kg, po) shows significant improvement in the neurochemical levels in the striatum of animal was seen. Further co-administration of curcumin (25 mg/kg, po) and piperine (2.5 mg/kg, po) shows more significant results than curcumin treatment alone.
In summery the present study demonstrates that co-administration of curcumin (25 mg/kg) and piperine (2.5 mg/kg) synergistically effective in ameliorating 3-NP induced cognitive, motor deficit abnormalities and oxidative stress. These outlines are supported by results obtained from the whole study and previous finding that curcumin act as potent antioxidant, inhibit cyclooxygenase and decrease levels of various inflammatory cytokines, inhibit xanthine oxidase, decrease iNOS expression there by suppressing ROS induced neurodegeneration.
Conclusion
In conclusion, the study demonstrated multifaceted effects of curcumin and piperine on functional recovery in 3-NP induced motor, behavioral, biochemical, neuroinflammatory and neurochemical alterations. The study indicated that antioxidant property and blockade inflammatory mediators production by curcumin treatment displayed potent neuroprotective effect against 3-NP-induced neurotoxicity in rats. It is further recommended that targeting potential neuroprotective pathways could be a useful approach in the management of HD-like symptoms.
Abbreviations
- 3-NP:
-
3-Nitropropionic acid
- HD:
-
Huntington’s disease
- LPO:
-
Lipid peroxidation
- IL:
-
Interlukin
- ROS:
-
Reactive oxygen species
- TNF-α:
-
Tumour necrosis factor-alpha
References
Kumar P, Kalonia H, Kumar A (2010) Cyclosporine a attenuates 3-nitropropionic acid-induced Huntington-like symptoms in rats: possible nitric oxide mechanism. Int J Toxicol 29(3):318–325
Chakraborty J, Pandey M, Navneet A, Appukuttan T, Varghese M, Sreetama S et al (2014) Profilin-2 increased expression and its altered interaction with β-actin in the striatum of 3-nitropropionic acid-induced Huntington’s disease in rats. Neuroscience 281:216–228
Chen JY, Wang EA, Cepeda C, Levine MS (2013) Dopamine imbalance in Huntington’s disease: a mechanism for the lack of behavioral flexibility. Front Neurosci 7:114
Khan A, Jamwal S, Bijjem K, Prakash A, Kumar P (2015) Neuroprotective effect of hemeoxygenase-1/glycogen synthase kinase-3β modulators in 3-nitropropionic acid-induced neurotoxicity in rats. Neuroscience 287:66–77
Colle D, Hartwig JM, Soares FAA, Farina M (2012) Probucol modulates oxidative stress and excitotoxicity in Huntington’s disease models in vitro. Brain Res Bull 87(4):397–405
Aggarwal BB, Deb L, Prasad S (2014) Curcumin differs from tetrahydrocurcumin for molecular targets. Signal Pathw Cell Responses Mol 20(1):185–205
Kumar P, Padi S et al (2007) Possible neuroprotective mechanisms of curcumin in attenuating 3-nitropropionic acid-induced neurotoxicity. Methods Find Exp Clin Pharmacol 29(1):19–26
Ghalandarlaki N, Alizadeh AM et al (2014) Nanotechnology-applied curcumin for different diseases therapy. Biomed Res Int 2014:1–23
Sandhir R, Yadav A et al (2014) Curcumin nanoparticles attenuate neurochemical and neurobehavioral deficits in experimental model of Huntington’s disease. NeuroMol Med 16(1):106–118
Patil UK, Singh A et al (2011) Role of piperine as a bioavailability enhancer. Int J Recent Adv Pharm Res 4:16–23
Thangarajan S, Deivasigamani A, Natarajan SS, Krishnan P, Mohanan SK (2014) Neuroprotective activity of L-theanine on 3-nitropropionic acid-induced neurotoxicity in rat striatum. Int J Neurosci 124(9):673–684
Plaa GL, Witschi H (1976) Chemicals, drugs, and lipid peroxidation. Annu Rev Pharmacol Toxicol 16(1):125–142
Daniel WL, Han MS, Lee J-S, Mirkin CA (2009) Colorimetric nitrite and nitrate detection with gold nanoparticle probes and kinetic end points. J Am Chem Soc 131(18):6362–6363
Koster J, Biemond P, Swaak A (1986) Intracellular and extracellular sulphydryl levels in rheumatoid arthritis. Ann Rheum Dis 45(1):44–46
Lowry OH, Rosebrough NJ et al (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193(1):265–275
Sharma S, Deshmukh R (2014) Vinpocetine attenuates MPTP-induced motor deficit and biochemical abnormalities in Wistar rats. Neuroscience 286:393–403
Kumar P, Kalonia H, Kumar A (2010) Cyclosporine a attenuates 3-nitropropionic acid-induced Huntington-like symptoms in rats: possible nitric oxide mechanism. Int J Toxicol 29(3):318–325
Kumar P, Kalonia H, Kumar A (2011) Novel protective mechanisms of antidepressants against 3-nitropropionic acid induced Huntington’s-like symptoms: a comparative study. J Psychopharmacol 25(10):1399–1411
Kumar P, Kalonia H, Kumar A (2012) Possible GABAergic mechanism in the neuroprotective effect of gabapentin and lamotrigine against 3-nitropropionic acid induced neurotoxicity. Eur J Pharmacol 674(2):265–274
Colle D, Santos DB, Moreira ELG, Hartwig JM, dos Santos AA, Zimmermann LT et al (2013) Probucol increases striatal glutathione peroxidase activity and protects against 3-nitropropionic acid-induced pro-oxidative damage in rats. PloS ONE 8(6):e67658
Amor S, Puentes F, Baker D, Van Der Valk P (2010) Inflammation in neurodegenerative diseases. Immunology 129(2):154–169
Im A-R, Chae S-W, jun Zhang G, Lee M-Y (2014) Neuroprotective effects of Psoralea corylifolia Linn seed extracts on mitochondrial dysfunction induced by 3-nitropropionic acid. BMC Complement Altern Med 14(1):370
Túnez I, Tasset I, Pérez-De La Cruz V, Santamaría A (2010) 3-Nitropropionic acid as a tool to study the mechanisms involved in Huntington’s disease: past, present and future. Molecules 15(2):878–916
Hsieh H-L, Yang C-M (2013) Role of redox signaling in neuroinflammation and neurodegenerative diseases. Biomed Res Int 2013:1–18
Rossi S, Motta C, Studer V, Macchiarulo G, Volpe E, Barbieri F et al (2014) Interleukin-1β causes excitotoxic neurodegeneration and multiple sclerosis disease progression by activating the apoptotic protein p53. Mol Neurodegener 9(1):56
Müller E, Parati E, Panerai A, Cocchi D, Caraceni T (1979) Growth hormone hyperresponsiveness to dopaminergic stimulation in Huntington’s chorea. Neuroendocrinology 28(5):313–319
Brouillet E, Jacquard C, Bizat N, Blum D (2005) 3-Nitropropionic acid: a mitochondrial toxin to uncover physiopathological mechanisms underlying striatal degeneration in Huntington’s disease. J Neurochem 95(6):1521–1540
Pant MK, Panthari P, Kharkwal A, Kharkwal H, Kharkwal H (2014). Curcumin: a wonder therapeutical drug. World J Pharm and Pharm Sci 3(6):374–396
Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB (2007) Bioavailability of curcumin: problems and promises. Mol Pharm 4(6):807–818
Acknowledgments
Authors are thankful to Science and Engineering Board (SERB), Department of Science and Technology, Govt. of India, New Delhi for providing financial assistance under Fast Track Scheme (DST-SERB-FTYS) to Dr. Puneet Kumar.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Singh, S., Jamwal, S. & Kumar, P. Piperine Enhances the Protective Effect of Curcumin Against 3-NP Induced Neurotoxicity: Possible Neurotransmitters Modulation Mechanism. Neurochem Res 40, 1758–1766 (2015). https://doi.org/10.1007/s11064-015-1658-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-015-1658-2