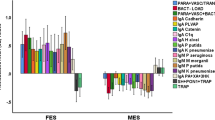
Abstract
Increased gut permeability (leaky gut) with increased translocation of Gram-negative bacteria plays a role in the gut-brain axis through effects on systemic immune-inflammatory processes. Deficit schizophrenia is characterized by an immune-inflammatory response combined with a deficit in natural IgM antibodies to oxidative-specific epitopes (OSEs), which are a first-line defense against bacterial infections. This study measured plasma IgA/IgM responses to 5 Gram-negative bacteria in association with IgM responses to malondialdehyde (MDA) and azelaic acid in 80 schizophrenia patients (40 with the deficit syndrome and 40 without) and in 38 healthy controls. Deficit schizophrenia was characterized by significantly increased IgA responses to Hafnei alvei, Pseudomonas aeruginosa, Morganella morganii, and Klebsiella pneumoniae as compared with non-deficit schizophrenia. The presence of deficit schizophrenia was highly predicted by increased IgA responses to Pseudomonas putida and IgM responses to all five Gram-negative bacteria and lowered natural IgM to MDA and azelaic acid with a bootstrap area under the receiver operating characteristic curve of 0.960 (2000 random curves). A large proportion of the variance (41.5%) in the negative subscale score of the Positive and Negative Syndrome Scale was explained by the regression on IgA responses to K. pneumoniae and IgM responses to the five enterobacteria coupled with lowered IgM antibodies to azelaic acid. There were significant associations between IgA levels to Gram-negative bacteria and Mini-Mental State Examination, Boston naming test, Verbal Fluency, and Word List Memory test scores. These findings provide further evidence that deficit schizophrenia is a distinct phenotype of schizophrenia, which is characterized by an increased impact of Gram-negative commensal bacteria coupled with a deficit in natural IgM, pointing to aberrations in B1 cells. It is concluded that increased bacterial translocation and deficits in the compensatory immune-regulatory system (CIRS) may drive negative symptoms and neurocognitive impairments, which are hallmarks of deficit schizophrenia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
There is now evidence that first episode psychosis (FEP) and acute psychotic relapses as well as chronic schizophrenia, treatment-resistant and stable-phase schizophrenia are characterized by activation of the immune-inflammatory response system (IRS) (Smith and Maes 1995; Anderson and Maes 2013; Roomruangwong et al. 2018b; Miller et al. 2011). The findings indicate an acute phase response, with increased levels of positive acute phase proteins, increased complement factors, and activation of M1 macrophagic, T helper (Th)-1, and Th-17 immune cell phenotypes (Maes et al. 1997b; Roomruangwong et al. 2018b).
These schizophrenia phenotypes are not only accompanied by an activated IRS but also by a concomitant activation of the compensatory immune-regulatory system (CIRS) including activated Th-2 and T regulatory (Treg) immune phenotypes and increased levels of soluble cytokine receptors, which have immune-regulatory effects, such as soluble interleukin-2 receptor (sIL-2R), soluble tumor necrosis factor receptor (sTNF-R)1 and sTNF-R2, and sIL-1R antagonist (sIL-1RA) (Roomruangwong et al. 2018b). As such, the CIRS exerts many immune-regulatory and anti-inflammatory activities, which tend to downregulate the IRS (Roomruangwong et al. 2018b). This is important since immune products of the IRS (IL-6, IL-1, TNF-α) as well as the CIRS (IL-4, IL-13, eotaxin) may exert cytotoxic and neurotoxic effects thereby driving neuroprogression (Davis et al. 2014, 2016; Noto et al. 2018; Roomruangwong et al. 2018b; Maes and Carvalho 2018).
Distinct schizophrenia-related phenotypes including FEP are characterized by a significantly elevated IRS/CIRS ratio, indicating a net immune-inflammatory response that does not appear to be sufficiently attenuated by an activated CIRS (Noto et al. 2018; Roomruangwong et al. 2018b). Moreover, schizophrenia is accompanied by deficits in the CIRS and hence more prominent IRS responses after immune challenges (Noto et al. 2018; Roomruangwong et al. 2018b). For example, the levels of plasma Clara cell secretory protein (CC16), an endogenous disulfide-bridged protein with protective and anti-inflammatory properties, are significantly decreased in schizophrenia patients (Maes et al. 1996, 1997a). Moreover, relatively lowered levels of plasma sTNF-R1, sTNF-R2, sIL-2R, and sIL-1RA in FEP have been reported, which may worsen the clinical outcomes in FEP (Noto et al. 2018).
There is now accumulating evidence that deficit schizophrenia is a distinct nosological category, which is significantly discriminated from non-deficit schizophrenia by more severe neuro-immune aberrations, memory impairments, negative symptoms, as well as psychotic, hostility, excitation, and mannerism (PHEM) symptoms (Kanchanatawan et al. 2018a, b). Increased IgA levels directed against tryptophan catabolites (TRYCATs) (indicating an overactivation of the TRYCAT pathway) are other biomarkers of deficit schizophrenia and negative and neurocognitive symptoms (Kanchanatawan et al. 2018a, b). The cytotoxic and neurotoxic properties of increased TRYCATs such as picolinic acid (PA), xanthurenic acid (XA), and quinolinic acid (QA) are further augmented by increased levels of eotaxin (CLL-11), a Th-2-related product that may contribute to neurocognitive deficits and negative symptoms in schizophrenia (Sirivichayakul et al. 2018a, b). However, a significant deficit in the CIRS, as indicated by lowered levels of IgM against TRYCATs and oxidative specific epitopes (OSEs), including malondialdehyde (MDA) and azelaic acid, appears to be the most prominent immune abnormality in deficit schizophrenia (Kanchanatawan et al. 2018a; Maes et al. 2018). These IgM antibodies have specificity to self-antigens and OSEs and are present even without antigenic contact, although increased surface expression of these neoepitopes following lipid peroxidation and aldehyde formation may generate adaptive IgM responses toward the same neoepitopes (Binder 2012; Weismann and Binder 2012; Díaz-Zaragoza et al. 2015; Thiagarajan et al. 2016; McMahon and Skaggs 2016). Natural IgM, especially those directed to MDA, have strong immune-regulatory effects and in fact are an integral component of the innate first-line defense against microorganisms, including Gram-negative bacteria (Binder 2012; Weismann and Binder 2012; Díaz-Zaragoza et al. 2015). Therefore, we hypothesized that lowered natural IgM responses to MDA and azelaic acid in deficit schizophrenia could be accompanied by a greater impact of Gram-negative commensal bacteria affecting schizophrenia phenomenology including negative symptoms and neurocognitive impairments.
Hence, the present study was performed to examine whether (a) deficit schizophrenia is accompanied by increased IgA/IgM levels to Gram-negative bacteria and whether these responses are positively associated with negative symptoms and neurocognitive deficits; and (b) the deficits in IgM isotype antibodies to OSEs coupled with increased IgA/IgM responses to Gram-negative bacteria have cumulative effects on negative symptoms and neurocognitive impairments above and beyond the effects of each factor alone.
Methods
Participants
This study enrolled 38 healthy controls and 80 patients with schizophrenia. All patients were in a stable phase of schizophrenia without acute episodes for at least 1 year and they complied with the diagnostic criteria for schizophrenia according to the Diagnostic and Statistical Manual of Mental Disorders-Fourth Edition-Text Revision (DSM-IV-TR) criteria. Using the Schedule for Deficit syndrome (SDS) (Kirkpatrick et al. 1989), schizophrenia patients were allocated to two groups, namely those with and those without deficit schizophrenia. All patients were outpatients at the Department of Psychiatry at the King Chulalongkorn Memorial Hospital, Bangkok, Thailand. Healthy volunteers were recruited from the same catchment area.
Exclusion criteria for patients were (a) acute episodes of schizophrenia the year prior to inclusion; (b) axis-1 DSM-IV-TR psychiatric disorders such as major depression, bipolar disorder, schizoaffective disorder, substance-use disorders, and psycho-organic disorders; (c) medical illness such as psoriasis, rheumatoid arthritis, chronic obstructive pulmonary disease, diabetes (type 1 and 2), and inflammatory bowel disease; (d) neurological disorders including stroke, Parkinson’s disease, multiple sclerosis, and Alzheimer’s disease; and (e) use of immunomodulatory drugs and antioxidant supplements and ω3-polyunsaturated fatty acids. Exclusion criteria for controls were (a) lifetime or current axis I diagnosis according to DSM-IV-TR criteria; and (b) a positive family history of schizophrenia.
All controls and patients as well as the guardians of patients, namely parents or other close family members, provided written informed consent prior to participation in this study. The study was conducted according to International and Thai ethics and privacy laws. Approval for the study was obtained from the Institutional Review Board of the Faculty of Medicine, Chulalongkorn University, Bangkok, Thailand (#298/57), which is in compliance with the International Guidelines for Human Research protection as required by the Declaration of Helsinki, The Belmont Report, CIOMS Guideline, and International Conference on Harmonization in Good Clinical Practice (ICH-GCP).
Measurements
Clinical Assessments
The authors used a semistructured interview applied by the same senior psychiatrist, specialized in the treatment of schizophrenia (BK), in order to collect sociodemographic and clinical data. The Mini-International Neuropsychiatric Interview (M.I.N.I.) was used in a validated Thai translation (Kittirathanapaiboon and Khamwongpin 2005) to make the diagnosis of schizophrenia, while the SDS was used to make the diagnosis of primary deficit schizophrenia. BK also scored the Positive and Negative Syndrome Scale (PANSS), with scores on the negative (PANSSneg) and positive (PANSSpos) subdomains (Kay et al. 1987). We computed (Kanchanatawan et al. 2018c) four z-unit-weighted composite scores reflecting four different symptom dimension scores using items of the PANSS and the Brief Psychiatric Rating Scale (BPRS) (Overall and Gorham 1962), namely (a) the psychotic dimension as sum of z score of PANSS P1 (delusion) (zP1) + zP3 (hallucinations) + zP6 (suspiciousness) + zBPRS11 (suspiciousness) + zBPRS12 (hallucinatory behavior) + BPRS15 (unusual thought content); (b) the hostility dimension as sum of zP7 (hostility) + zPANSS general14 (zG14, poor impulse control) + zBPRS10 (hostility) + zBPRS14 (uncooperativeness); (c) the excitement dimension as zP14 (excitement) + zP5 (grandiosity) + zBPRS8 (grandiosity) + zBPRS17 (excitement); and (d) the mannerism dimension as zG5 + zBPRS7 (both mannerism and posturing). The diagnosis of tobacco use disorder (TUD) was made using DSM-IV-TR criteria. Body mass index (BMI) was assessed the same day as the clinical interview and rating scale scoring as body weight (kg)/length (m2).
The authors also measured different Consortium to Establish a Registry for Alzheimer’s disease (CERAD)-Neuropsychological tests (CERAD 1986). These probes were scored by a trained research assistant who was blinded to the clinical diagnosis. The CERAD tests were carried out the same day we completed the semistructured interview and clinical scorings. The CERAD probes used in the current study were (a) the Mini-Mental State Examination (MMSE), which probes a more general neuropsychological defect including naming, orientation, memory, concentration, and constructional praxis; (b) the Boston naming test to probe naming; (c) Verbal Fluency Test (VFT) to probe fluency and semantic memory; and (d) Word List Memory (WLM) to probe learning ability and verbal episodic memory.
Assays
In patients and controls, fasting blood was sampled at 8.00 a.m. for the assay of IgA and IgM responses to five Gram-negative bacteria and IgM-mediated autoimmune responses directed against MDA and azelaic acid. A description of the measurements of IgM or IgA isotypes directed against the Gram-negative bacteria is provided elsewhere (Roomruangwong et al. 2017). Antigens derived from five Gram-negative bacteria were assayed after sonication, namely Hafnia alvei, Klebsiella pneumonia, Morganella morganii, Pseudomonas aeruginosa, and Pseudomonas putida. Polystyrene 96-well plates (NUNC) were coated with 200 μl solution containing bacterial components at 4 μg/ml in 0.05 M carbonate buffer at pH 9.6. Well plates were incubated at 4 °C for 16 h under agitation. Then, we added 200 μl blocking solution (PBS, Tween 20 0.05%, 5 g/l BSA) for 1 h and placed at 37 °C. Following two washes with PBS, plates were filled up with 100 μl of sera diluted at 1:1000 in the blocking buffer A (PBS, 0.05% Tween 20, 2.5 g/l BSA) and incubated at 37 °C for 105 min. After three washes with PBS-0.05% Tween 20, plates were incubated at 37 °C for 1 h with peroxidase-labeled anti-human IgM or IgA secondary antibodies diluted respectively at 1: 15,000 and 1: 10,000 in the blocking buffer (PBS, 0.05% Tween 20, 2.5 g/l BSA). Afterwards, plates were washed three times with PBS-0.05% Tween 20, and incubated with the detection solution for 10 min in the dark. Chromogen detection solution (tetramethylbenzedine) was used for the peroxidase assay at 16.6 ml per liter in 0.11 M sodium acetate trihydrate buffer (pH 5.5) containing 0.01% H2O2. The reaction was stopped with 25 μl 2-N HCl. After addition of stop solution (H2SO4 or HCl), the obtained, proportional absorbance in the tested sample (compared to established concentration of respective antibodies) was measured at 450 nm with one alpha of correction at 660 nm.
ELISA methods were used to measure IgM levels directed against conjugated azelaic acid and MDA (Maes et al. 2018). Azelaic acid and MDA were linked to fatty acid free-BSA according to previously described methods. The detection of IgM autoantibodies to the conjugates was performed by an indirect ELISA tests (Maes et al. 2018). Briefly, polystyrene 96-well plates (NUNC) were coated with 200 μl solution containing the conjugates or BSA in 0.05 M carbonate buffer at pH 9.6. Well plates were incubated at 4 °C for 16 h under agitation. Then, a 200 μl of blocking solution (PBS, 2.5 g/l BSA) was added for 1 h and placed at 37 °C. Following three washes with PBS, plates were filled up with 100 μl of sera diluted at 1:1000 in the blocking buffer A (PBS, 0.05% Tween 20, 10% Glycerol, 2.5 g/l BSA, 1 g/l BSA-G) and incubated at 37 °C for 2 h. After three washes with PBS-0.05% Tween 20, plates were incubated at 37 °C for 1 h with peroxidase-labeled anti-human IgM secondary antibodies diluted respectively at 1: 15,000, in the blocking buffer (PBS, 0.05% Tween 20, 2.5 g/l BSA). They were then washed three times with PBS-0.05% Tween 20, and incubated with the detection solution for 10 min in the dark. Chromogen detection solution was used for the peroxidase assay at 8% in 0.1 M acetate and 0.01 M phosphate buffer (pH 5.0) containing 0.01% H2O2. The reaction was stopped with 25 μl 2-N HCl. ODs were measured at 492 nm using a multiscan spectrophotometer. All assays were carried out in duplicate. The intra-assay coefficients of variation (CV) were < 6% (Maes et al. 2018).
Statistical Analysis
Analysis of variance (ANOVAs) was used to assess differences in continuous variables between groups, while analysis of contingency tables (Χ2 test) was used to check associations between categorical variables. Multinomial logistic regression analysis was employed to assess associations between IgA/IgM levels to Gram-negative bacteria and diagnosis (deficit versus non-deficit schizophrenia versus normal controls). We employed binary regression analysis to assess the most significant predictors (IgM/IgA responses to Gram-negative bacteria) of deficit schizophrenia versus non-deficit schizophrenia with or without normal controls. Moreover, we computed odds ratios (OR) and 95% confidence intervals. In the current study, we used multiple regression analysis to assess the best prediction of negative symptoms and neurocognitive test results (entered as dependent variables), using IgA/IgM responses to Gram-negative bacteria and IgM levels to MDA/azelaic acid as explanatory variables. In order to examine the effects of extraneous variables on the IgM/IgA responses to bacteria, we employed multivariate general linear model (GLM) analysis with the IgM/IgA responses as dependent variables and age, sex, BMI, TUD, and the drug state of the patients as explanatory variables. Tests for between-subject effects were consequently employed to delineate the effects of significant independent variables on IgM/IgA responses. We used receiver operating characteristics (ROC) analysis to compute the area under the ROC curve (including after 2000 bootstraps). The MDA and azelaic acid OD data were Ln transformed in order to normalize the data distribution of the IgM responses and all OD data were processed in z transformations. We interpreted the bootstrapped (n = 1000) results of regression analyses and report if there are differences between results with and without bootstrapping. Results of univariate GLM analysis with multiple comparisons were p-corrected for false discovery rate (FDR) (Benjamini and Hochberg 1995). All results of regression analyses were also checked for collinearity using tolerance and VIF values. Since the IgM responses to Gram-negative bacteria were highly correlated (yielding significant collinearity in regression analysis), we used a z-unit-weighted composite score reflecting total IgM responses to the five bacteria (zsumIgM), which was computed as the z transformation of the sum of all five z-transformed IgM OD bacterial data. We also computed a zsumzIgA composite score (based on the IgA responses to the five bacteria) and divided the study group according to two subgroups based on the median-split method. Statistical analyses were performed using IBM SPSS windows version 22. Tests were two-tailed and a p value of 0.05 was used for statistical significance.
In order to examine the causal links among IgM/IgA responses to Gram-negative bacteria, IgM antibodies to MDA and azelaic acid, cognitive tests, and symptoms, we employed partial least squares (PLS) analysis (SmartPLS; Ringle et al. 2014). PLS is a structural equation modeling technique that uses path modeling performed on latent vectors (LV) extracted from indicator variables (Ringle et al. 2014). A LV extracted from the six items of the SDS was the final output variable (reflecting SDS score or negative symptoms) and the direct explanatory variables were a LV extracted from three CERAD tests, namely True Recall, WLM, and VFT (reflecting “memory impairments”); an LV extracted from IgA responses to Gram-negative bacteria; zsumzIgM (one indicator variable); and a LV extracted from both OSEs (reflecting natural IgM). Moreover, the model examines whether the effects of bacteria and IgM directed against OSEs are mediated by the memory LV (see Fig. 2). As such, we use a multistep path mediated model (Cepeda-Carrion et al. 2018), namely from IgM/IgA to Gram-negative bacteria and IgM to OSEs ➔ cognitive impairments (possible mediator) ➔ negative symptoms. The quality of the model was assessed using SRMR < 0.08 as an overall model fit criterion. New constructs were accepted only when they showed a good reliability and discriminant validity, including Cronbach’s alpha > 0.7, composite reliability > 0.7, and average variance extracted (AVE) > 0.500. Moreover, indicators of the latent constructs should have factor loadings > 0.5 and p values < 0.001 (Ringle et al. 2014). We used consistent PLS bootstrapping (2000 bootstraps) to compute path coefficients with exact p value, total effects, total indirect, and specific indirect effects.
Results
Sociodemographic Data
Table 1 displays the demographic, clinical, and biomarker data in two subgroups divided according to the zsumzIgA composite score using the median split method (median = − 0.0772019), yielding two study groups, a first with lower and a second with higher zsumIgA values, reflecting a lower versus higher bacterial load. The results of these multiple comparisons were not p-corrected for false discovery rate (and not adjusted for relevant confounding variables) because these results together with the results of intercorrelation matrices were employed to delineate the explanatory variables to be entered in the ultimate regression and PLS analyses. Table 1 shows no significant differences in age, gender, marital status, education, TUD, BMI, number of psychotic episodes, PANSS positive subscale score, psychosis, hostility, excitement, mannerism, IgM levels to MDA and azelaic acid, and MMSE among these two study groups. Subjects in the high zsumzIgA composite score showed significantly higher SDS and PANSS negative subscale scores, lower BNT, VFT, WLM, and True Recall values.
Associations Between IgA/IgM Responses to Enterobacteria and (Deficit) Schizophrenia
Table 2 shows the associations between the IgA responses to Gram-negative bacteria and diagnosis into three classes, namely deficit versus non-deficit schizophrenia versus controls. Diagnostic categories were the dependent variables and biomarkers the explanatory variables. Entry of the separate IgA and IgM values showed that the IgA values directed to H. alvei, P. aeruginosa, M. morganii, and K. pneumonia were significantly associated with the deficit phenotype (versus the non-deficit phenotype) and also separated the deficit phenotype from controls (H. alvei and K. pneumoniae). There were no significant associations between the diagnostic groups and either IgA responses to P. putida and IgM responses to the five Gram-negative bacteria. After considering the effects of IgM responses to MDA and azelaic acid in those multinomial regression analyses, we found that IgM responses to MDA (Χ2 = 32.90, df = 2, p < 0.001), and azelaic acid (Χ2 = 11.00, df = 2, p = 0.004), IgA responses to P. putida (Χ2 = 14.52, df = 2, p = 0.001) and zsumIgM (Χ2 = 22.79, df = 2, p < 0.001) were significantly associated with diagnostic classes. Deficit schizophrenia was significantly separated from non-deficit schizophrenia and controls by higher IgA responses to P. putida and zsumIgM and lower IgM antibodies to MDA and azelaic acid.
Subsequently, we have also examined the discrimination of deficit schizophrenia from all other subjects or from non-deficit schizophrenia using binary logistic regression analysis. Table 3 shows the results of these two regression analysis with diagnosis as dependent variable and biomarkers (together with age, sex, and BMI) as explanatory variables. The first analysis shows that deficit schizophrenia (versus all other participants) was significantly associated with higher zsumIgM levels and IgA levels to P. putida and lower IgM levels to MDA and azelaic acid. 83.1% of all cases were correctly classified with a sensitivity of 70.0% (to detect deficit schizophrenia), a specificity of 89.7% and an area under the ROC curve of 0.927 (± 0.024). Figure 1 shows the measurements of the IgA/IgM responses to Gram-negative bacteria and IgM responses to MDA and azelaic acid in patients with deficit schizophrenia versus all other subjects (all in z transformations of the OD values). Natural IgM to MDA (F = 35.42, df = 1/116, p < 0.001; partial eta squared = 0.234) and azelaic acid (F = 23.77, df = 1/116, p < 0.001; partial eta squared = 0.170) were significantly lower in deficit schizophrenia as compared with all other subjects.
The second regression analysis shows that deficit schizophrenia versus non-deficit schizophrenia was significantly associated with higher zsumIgM levels and IgA levels to H. alvei and lower IgM levels directed to MDA and azelaic acid. Then, 91.3% of all cases were correctly classified with a sensitivity of 92.5% (to detect deficit schizophrenia), a specificity of 90.0%, and an area under the ROC curve of 0.963 (± 0.020). The bootstrapped AUC ROC estimation (n = 2000 random curves) was 0.960 with 95% confidence intervals of 0.915–0.992. Age, sex, and BMI were not significant in this regression analysis.
Impact of Extraneous Variables
To delineate possible effects of age, sex, BMI, education, and medications on the IgA/IgM responses to Gram-negative bacteria, multivariate GLM analysis was performed with the biomarkers (IgA and IgM levels to the five Gram-negative bacteria) and diagnosis (in three groups) as dependent variables. There were significant effects of sex (F = 2.02, df = 10/98, p = 0.039) and age (F = 3.87, df = 10/98, p < 0.001), but not BMI (F = 0.37, df = 10/98, p = 0.957) on the IgA/IgM responses. After p-correction for FDR, the IgA responses to P. aeruginosa (p = 0.020), M. morganii (p = 0.028), and P. putida (p = 0.010) and IgM responses to H. alvei (p = 0.033), P. aeruginosa (p = 0.02), M. morganii (p = 0.02), P. putida (p = 0.02), and K. pneumoniae (p = 0.033) were higher in females than in males. After p-correction, there were significant and positive correlations between age and IgA responses to all five bacteria except P. putida (all p < 0.011), and negative correlations between age and IgM responses to all five bacteria (all < 0.010). In any case, all regression analyses used in this study were adjusted for possible effects of age and sex but those variables did not affect the association between biomarkers and diagnosis. There were no significant effects of education (F = 1.75, df = 10/97, p = 0.080) and TUD (F = 0.32, df = 10/97, p = 0.976) on the biomarkers. Multivariate GLM analysis showed no significant effects of the drug state of the patients on the biomarkers, namely risperidone (n = 33, F = 0.54, df = 10/88, p = 0.860), clozapine (n = 10, F = 1.58, df = 10/88, p = 0.127), haloperidol (n = 8, F = 0.99, df = 10/88, p = 0.457), perphenazine (n = 20, F = 0.60, df = 10/88, p = 0.810), antidepressants (n = 26, F = 0.54, df = 10/88, p = 0.859), mood stabilizers (n = 12, F = 1.07, df = 10/88, p = 0.398), and anxiolytics/hypnotics (n = 27, F = 1.02, df = 10/88, p = 0.437).
Associations Between IgA/IgM Responses to Bacteria and Schizophrenia Phenomenology
In order to examine the associations between schizophrenia symptomatology and biomarkers, we have entered the total SDS score and its six-item scores, the PANSS positive and negative subscale scores, and four PHEM composite scores as dependent variables and IgA responses to five Gram-negative bacteria, zsumIgM, and IgM antibody levels against MDA and azelaic acid as explanatory variables in multiple regression analyses. Table 4 shows that 48.2% of the variance in the total SDS score was explained by the regression on IgM azelaic acid and education (inversely) and IgA to K. pneumoniae and zsumIgM (both positively). Also the six items of the SDS scale were significantly predicted (29.0–40.3% of the variance) by IgM to OSEs combined with zsumIgM and IgA responses to one of the five bacteria (most often K. pneumonia) and education. The PANSS positive subscale was not associated with any of the biomarkers, whereas 41.5% of the variance in the PANSS negative subscore was explained by the regression on IgM azelaic acid (inversely) and IgA to K. pneumoniae and zsumIgM (both positively). Then, 16.8% of the variance in psychotic symptoms was explained by increased IgA levels to K. pneumoniae and education. Further, 26.6% of the variance in the excitation composite score was explained by IgM to azelaic acid and education (inversely) and IgA to K. pneumoniae (positively). There were no significant associations between hostility or mannerism and any of the biomarkers. Age and sex were not significant in the analyses shown in Table 4.
Associations Among IgA/IgM Responses to Gram-Negative Bacteria and Neurocognitive Tests
In order to assess the associations between IgA/IgM responses to Gram-negative bacteria and OSEs and neurocognitive tests, we employed multiple regression analyses with the neurocognitive CERAD tests as dependent variables and the biomarkers, age, sex, and education as independent variables. We found that 21.1% of the variance in the BNT test results was explained by education (inversely) and IgA responses to K. pneumoniae. Then, 40.6% of the variance in the MMSE was explained by the regression on education, IgM against MDA (both negatively) and IgA to P. putida (the bootstrapped p values were significant for all three variables, including IgA against P. putida, namely p = 0.043). Moreover, 11.7% of the variance in the VFT was explained by IgA levels to K. pneumoniae. And 33.7% of the variance in WLM test was explained by the regression on education, IgM levels against MDA (inversely) and IgA levels to K. pneumoniae. Table 5 shows also that there are positive associations between IgM against MDA or azelaic acid and zsumIgM composite score.
Results of PLS Analysis
Figure 2 shows the outcome of the multistep PLS path analysis with path coefficients and exact p values of the model presented in the “Methods” section. We found that 73.4% of the variance in the LV extracted from the six SDS items was explained by memory LV, natural IgM LV, zsumIgM, and IgA Gram-negative LV. Then, 29.3% of the variance in the memory LV was explained by the regression on natural IgM LV, zsumIgM, and IgA Gram-negative LV. There were significant total and specific indirect effects of IgA Gram-negative bacteria LV (t = + 2.44, p = 0.015) and IgM against OSEs (t = 2.15, p = 0.032). There were total effects of IgA to Gram-LV on memory (t = + 3.25, p = 0.001) and SDS LV (t = + 4.24, p < 0.001) and of zsumIgM on SDS LV (t = 3.50, p = 0.001) and of natural IgM to OSEs on memory (t = + 2.68, p = 0.008) and SDS LV (t = − 5.94, p < 0.001) (Fig. 3).
Results of PLS path modeling with a latent vector (LV) extracted from the six SDS symptoms as outcome variable and as explanatory variables: a LV extracted from verbal fluency test (VFT), word list memory (WLM), and true recall (named “memory”). b LV extracted from IgA to Gram-negative bacteria (named: “IgA Gram-“). c IgM to sum of five Gram-negative bacteria (named “IgM Gram-“). d LV extracted from IgM to malondialdehyde (MDA) and azelaic acid (named “Natural IgM). Shown are p coefficients (with exact p values) for the inner model, and t values for the outer model
Discussion
The first major finding of this study is that deficit schizophrenia is accompanied by (a) significantly increased IgA responses to four Gram-negative bacteria, namely H. alvei, P. aeruginosa, M. morganii, and K. pneumoniae; and (b) and increased IgA responses to P. putida coupled with increased IgM responses to all five Gram-negative bacteria and lowered IgM responses to MDA and azelaic acid. These five bacteria, including K. pneumoniae, belong to the normal intestinal flora (Wiest 2005; Todar 2018), although the latter also belongs to the lung microbiome (O'Dwyer et al. 2016). Increased serum IgA levels directed against sonicated samples of Gram-negative bacteria (thus including LPSs) reflect LPS exposure (Pasternak et al. 2010) and, therefore, constitute an indirect assay of increased bacterial translocation into the blood or mesenteric lymph nodes (MLNs) with or without systemic spreading of the bacteria. Translocated bacteria into the MLNs may prime and activate immune cells in the MLNs thereby inducing an immune response (Kwa et al. 2006). Therefore, our results indicate that in deficit, but non non-deficit, schizophrenia, Gram-negative bacteria may have translocated from the gut (and/or lung) thereby increasing bacterial load and inducing a systemic immune response.
Previously, we have shown that major depression, bipolar disorder, and chronic fatigue syndrome are accompanied by increased bacterial translocation (Maes et al. 2007, 2008, 2018, 2012, 2013a, in preparation). In patients with major depression, significant associations were found between IgA against Gram-negative bacteria and IgG responses to oxidized low-density lipoprotein cholesterol, serum lysozyme levels, and IgM responses to MDA and azelaic acid, indicating that bacterial translocation is associated with peripheral immune-inflammatory and autoimmune processes (Maes et al. 2013a). Moreover, in CFS, it was reported that increased IgA to Gram-negative bacteria are significantly associated with increased serum levels of IL-1, TNF-α, neopterin, elastase, and autoantibodies directed to serotonin (Maes et al. 2012, 2013b), indicating that increased bacterial translocation may cause peripheral activation of M1 macrophagic, Th-1, neutrophil, and autoimmune pathways.
Apart from LPSs, bacteria contain many immunogenic proteins and pathogen-associated molecular patterns (PAMPs), which may induce immune-inflammatory responses (Hoppe et al. 2012). Nevertheless, best documented are the effects of LPSs triggering the Toll-like receptor 4 (TLR4) complex, a receptor of the innate immune system. Following TLR4 activation, an intracellular cascade is induced leading to activated cell signaling networks, including nuclear factor (NF)-κB and MAPK, which in turn may induce the production of pro-inflammatory cytokines and inducible nitric oxide synthase (iNOS) (Lucas and Maes 2013; Chan and Riches 2001). Moreover, LPS may stimulate the production of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, with consequent activation of superoxide and hydrogen peroxides, and cyclooxygenase-2 and lysozyme by mononuclear phagocytes (Lin et al. 2011; Check et al. 2010; Peng et al. 2005; Pai and Sodhi 1991; Lewis et al. 1990). Moreover, the increased IgA levels against Gram-negative bacteria measured here are significantly associated with TRYCAT pathway activation, which may be explained by effects of LPSs or its downstream products, including pro-inflammatory cytokines, inducing the TRYCAT pathway (Maes et al. in preparation). LPSs have profound neurotoxic effects leading to neurodegenerative processes which are mediated by microglial activation (Arai et al. 2004). Gut microbiota may increase the permeability of the blood-brain barrier (BBB) (Braniste et al. 2014), while LPS can access the brain via different routes, including circumventricular organs and area postrema as well as via peripheral nerves signal transduction (Zakaria et al. 2017). Moreover, Gram-negative bacteria encountering environmental stress may produce outer membrane vesicles (OMVs), which contain antigens, PAMPs, and virulence factors (Muraca et al. 2015; Anand and Chaudhuri 2016; Ellis and Kuehn 2010; Ellis et al. 2010). OMVs may increase the permeability of the blood-brain barrier and may, subsequently, be delivered to the brain (Muraca et al. 2015). Interestingly, some OMVs are pathogenic and have pro-inflammatory effects, while other OMVs may have immune-regulatory functions, for example by inducing immune tolerance (Kaparakis-Liaskos and Ferrero 2015; Shen et al. 2012). As such, increased bacterial translocation could contribute to the immune-inflammatory pathophysiology of mental disorders, including deficit schizophrenia.
The second major finding of this study is that increased IgA and IgM responses to Gram-negative bacteria are significantly associated with negative, but not positive, symptoms. Recently, we have shown that neuro-immune biomarkers to a large extent determine the variance in negative symptoms as measured with the SDS (and its six items) and the PANSS negative subscale, including increased levels of eotaxin (CCL-11), a Th-2-related product, and IL-10 (a Treg cytokine) and increased production of neurotoxic TRYCATs (Kanchanatawan et al. 2018a; Sirivichayakul et al. 2018a, b). Thus, one possible explanation is that bacterial translocation, as established in the present study, may play a role by inducing different neuro-immune pathways that lead to increased neurotoxic effects and thus neuroprogression. Our negative findings on possible associations between Gram-negative bacteria and positive symptoms should be explained by the lack of specificity of the latter (Kanchanatawan et al. 2018c). Indeed, we have shown that “positive symptomatology” is not a viable concept as these symptoms should be “dissected” into more relevant symptoms dimensions, including psychosis (delusions, hallucinations), hostility, excitation, and mannerism. As such, the current study detected significant associations between IgA/IgM responses to Gram-negative bacteria and psychosis and excitation. Again, psychosis and excitation are significantly associated with increased IL-10 levels (reflecting immune activation) and increased neurotoxic TRYCATs, which may be induced by increased bacterial translocation (Kanchanatawan et al. 2018c; Sirivichayakul et al. 2018b). Future research should address the question whether the effects of bacterial translocation on negative symptoms, psychosis, and excitation are mediated by changes in the immune system and TRYCAT patterning.
The third major finding of this study is that IgA and IgM responses to Gram-negative bacteria explain part of the variance in neurocognitive deficits, including a more general impairment in neurocognitive functioning (MMSE), naming ability (BNT), and semantic (VFT) and episodic memory (WLM). Previously, it was shown that not only activation of immune-inflammatory pathways and oxidative stress but also gut microbiota are involved in the cognitive decline seen in patients with diabetic encephalopathy (Xu et al. 2017). Gut microbiota increase the permeability of tight-junctions of the BBB, which in turn allow induction of cognitive impairments (Braniste et al. 2014). In humans, administration of low-dose LPS affects long-term memory performance for emotional stimuli, but not accuracy in working memory (Grigoleit et al. 2011). In rodent models, acute, subchronic, and chronic administration of LPSs significantly induces diverse cognitive dysfunctions in learning and (working and spatial) memory, which are associated with increased expression of M1 macrophagic cytokines, IL-1β, amyloid-β, induced-apoptosis in the brain, and/or attenuated hippocampal neurogenesis (Hauss-Wegrzyniak et al. 1998; Zhu et al. 2014; Skelly et al. 2018; Valero et al. 2014). Based on these findings, some authors have proposed LPS-induced memory impairments in rats as a model of Alzheimer’s disease (Zakaria et al. 2017) and LPS exposure in the prenatal period in the rodent as a model of schizophrenia (Meyer 2014). Interestingly, based on a new cognitive model of schizophrenia (Sirivichayakul et al. 2018a), the current study found that the effects of IgA (but not IgM) responses to Gram-negative bacteria on negative symptoms are in part mediated by memory deficits, indicating that more direct pathways not related to memory impairments are involved. As such, increased bacterial translocation in deficit schizophrenia may aggravate neurocognitive impairments and negative symptoms associated with immune activation, increased eotaxin levels, and neurotoxic TRYCATs.
The fourth major finding of this study is that Gram-negative bacteria coupled with lower IgM to MDA and azelaic acid are, together, strong predictors of deficit schizophrenia and negative symptoms above and beyond the effects of each factor alone. Natural IgM to MDA and azelaic acid are natural antibodies, which are produced by B1 cells and play a key role in protection and remediation of infections by neutralizing invading pathogens (Rothstein et al. 2013). These natural antibodies are constitutively secreted by B1 cells even without antigen presentation and as such constitute a “pre-existing shield” protecting against infections “in the lag time for adaptive antibody production” (Rothstein et al. 2013; Aziz et al. 2015). Thus, recovery from different types of infections depends heavily upon these poly- and autoreactive B1 natural autoantibodies (Rothstein et al. 2013). Besides their key role in antimicrobial defenses, these natural IgM autoantibodies also have homeostatic properties by clearing apoptotic cells and potentially inflammatory (Aziz et al. 2015) and oxidative (Roomruangwong et al. 2018a) molecules. This may explain that mice lacking natural IgM are more prone to infectious, immune-inflammatory, and oxidative stress-related disorders, including infections with Streptococcus pneumoniae and influenza virus, arteriosclerosis, and autoimmune disorders (Aziz et al. 2015). As such, lower natural IgM to OSEs in deficit schizophrenia are accompanied by a greater impact of Gram-negative bacteria on negative symptoms, psychosis, excitation, and neurocognitive impairments. Nevertheless, it should be stressed that our study does not indicate any involvement of Gram-negative bacteria in the pathophysiology of schizophrenia per se: the effects of Gram-negative bacteria only appear in people with low natural IgM and therefore their effects are confined to the deficit phenotype.
The main limitation of the study is that we used a case-control design which does not allow to establish firm causal inferences. Secondly, it would have been even more informative if we had examined IgA/IgM responses to other Gram-negative gut commensal bacteria. The findings pertaining the ROC analysis deserve replication in an independent cohort.
Figure 2 shows a summary of the findings in deficit schizophrenia. Trigger factors including infections may activate the IRS and the CIRS (Roomruangwong et al. 2018b). Nevertheless, when there are pre-existing deficits in the CIRS, including lowered levels of Clara cell protein (CC16) and soluble interleukin-2 receptor (sIL-2R) and soluble tumor necrosis factor receptor (sTNFR1/R2), the immune-regulatory CIRS functions may be insufficient thereby increasing risk toward detrimental effects of an activated IRS (Roomruangwong et al. 2018b). The latter coupled with ensuing oxidative processes may affect long interspersed element-1 (LINE-1) partial methylation patterns, which in turn may cause more profound aberrations in neuro-oxidative and neuro-immune pathways (Kalayasiri et al. 2018). Many IRS products have cytotoxic and neurotoxic effects, which may cause neuroprogression and, consequently, schizophrenia phenomenology (Roomruangwong et al. 2018b). These products include M1macrophagic-related cytokines such as IL-1, TNF-α, and IL-6; Th-1 products such as interferon-γ, IL-12 and picolinic acid (PA), xanthurenic acid (XA), and 3-OH-kynurenine (3HK); and Th-2 products such as IL-4, IL-5, IL-13, and eotaxin (CCL-11) (Kanchanatawan et al. 2018a; Sirivichayakul et al. 2018a, b; Roomruangwong et al. 2018b). However, pre-existing deficits in B1 cell functions including lower natural IgM (Maes et al. 2018) are accompanied by increased neurotoxic effects of Gram-negative bacteria, quinolinic acid (QA), and nitrosylated and nitrated proteins (Kanchanatawan et al. 2018a; Maes et al. 2018). All those factors together may contribute to negative symptoms, psychosis, excitation, and neurocognitive deficits thereby shaping the deficit phenotype.
References
Anand D, Chaudhuri A (2016) Bacterial outer membrane vesicles: new insights and applications. Mol Membr Biol 33:125–137
Anderson G, Maes M (2013) Schizophrenia: linking prenatal infection to cytokines, the tryptophan catabolite (TRYCAT) pathway, NMDA receptor hypofunction, neurodevelopment and neuroprogression. Prog Neuro-Psychopharmacol Biol Psychiatry 42:5–19
Arai H, Furuya T, Yasuda T, Miura M, Mizuno Y, Mochizuki H (2004) Neurotoxic effects of lipopolysaccharide on nigral dopaminergic neurons are mediated by microglial activation, interleukin-1beta, and expression of caspase-11 in mice. J Biol Chem 279:51647–51653
Aziz M, Holodick NE, Rothstein TL, Wang P (2015) The role of B-1 cells in inflammation. Immunol Res 63:153–166
Benjamini Y, Hochberg Y (1995) Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B Methodol 57:289–300
Binder CJ (2012) Naturally occurring IgM antibodies to oxidation-specific epitopes. Adv Exp Med Biol 750:2–13
Braniste V, Al-Asmakh M, Kowal C, Anuar F, Abbaspour A, Tóth M, Korecka A, Bakocevic N, Ng LG, Kundu P, Gulyás B, Halldin C, Hultenby K, Nilsson H, Hebert H, Volpe BT, Diamond B, Pettersson S (2014) The gut microbiota influences blood-brain barrier permeability in mice. Sci Transl Med 6(263):263ra158
Cepeda-Carrion GA, Nitzl C, Roldan JL (2018) Mediation analyses in partial least squares structural equation modeling: guidelines and empirical examples. Chapter 9. Partial least squares structural equation modeling: basic concepts, methodological issues and applications. In: Latan H, Noonan R (eds) . Springer, Heidelberg, pp 173–195. https://doi.org/10.1007/978-3-319-64069-3_8
CERAD (1986) CERAD—an overview: the consortium to establish a registry for Alzheimer’s disease. https://sites.duke.edu/centerforaging/cerad/. Accessed 7 Dec 2018
Chan ED, Riches DW (2001) IFN-gamma + LPS induction of iNOS is modulated by ERK, JNK/SAPK, and p38(mapk) in a mouse macrophage cell line. Am J Phys Cell Physiol 280:C441–C450
Check J, Byrd CL, Menio J, Rippe RA, Hines IN, Wheeler MD (2010) Src kinase participates in LPS-induced activation of NADPH oxidase. Mol Immunol 47(4):756–762
Davis J, Moylan S, Harvey BH, Maes M, Berk M (2014) Neuroprogression in schizophrenia: pathways underpinning clinical staging and therapeutic corollaries. Aust N Z J Psychiatry 48:512–529
Davis J, Eyre H, Jacka FN, Dodd S, Dean O, McEwen S, Debnath M, McGrath J, Maes M, Amminger P, McGorry PD, Pantelis C, Berk M (2016) A review of vulnerability and risks for schizophrenia: beyond the two hit hypothesis. Neurosci Biobehav Rev 65:185–194
Díaz-Zaragoza M, Hernández-Ávila R, Viedma-Rodríguez R, Arenas-Aranda D, Ostoa-Saloma P (2015) Natural and adaptive IgM antibodies in the recognition of tumor-associated antigens of breast cancer (review). Oncol Rep 34:1106–1114
Ellis TN, Kuehn MJ (2010) Virulence and immunomodulatory roles of bacterial outer membrane vesicles. Microbiol Mol Biol Rev 74:81–94
Ellis TN, Leiman SA, Kuehn MJ (2010) Naturally produced outer membrane vesicles from Pseudomonas aeruginosa elicit a potent innate immune response via combined sensing of both lipopolysaccharide and protein components. Infect Immun 78:3822–3831
Grigoleit JS, Kullmann JS, Wolf OT, Hammes F, Wegner A, Jablonowski S, Engler H, Gizewski E, Oberbeck R, Schedlowski M (2011) Dose-dependent effects of endotoxin on neurobehavioral functions in humans. PLoS One 6(12):e28330
Hauss-Wegrzyniak B, Dobrzanski P, Stoehr JD, Wenk GL (1998) Chronic neuroinflammation in rats reproduces components of the neurobiology of Alzheimer's disease. Brain Res 780:294–303
Hoppe S, Bier FF, von Nickisch-Rosenegk M (2012) Microarray-based method for screening of immunogenic proteins from bacteria. J Nanobiotechnol 10:12
Kalayasiri R, Kraijak K, Mutirangura A, Maes M (2018) Paranoid schizophrenia and methamphetamine-induced paranoia are both characterized by a similar LINE-1 partial methylation profile, which is more pronounced in paranoid schizophrenia. BioRxiv 403535. https://doi.org/10.1101/403535
Kanchanatawan B, Sriswasdi S, Thika S, Sirivichayakul S, Carvalho AF, Geffard M, Kubera M, Maes M (2018a) Deficit schizophrenia is a discrete diagnostic category defined by neuro-immune and neurocognitive features: results of supervised machine learning. Metab Brain Dis 33:1053–1067
Kanchanatawan B, Sriswasdi S, Thika S, Stoyanov D, Sirivichayakul S, Carvalho AF, Geffard M, Maes M (2018b) Towards a new classification of stable phase schizophrenia into major and simple neuro-cognitive psychosis: results of unsupervised machine learning analysis. J Eval Clin Pract 24:879–891
Kanchanatawan B, Thika S, Sirivichayakul S, Carvalho AF, Geffard M, Maes M (2018c) In schizophrenia, depression, anxiety, and physiosomatic symptoms are strongly related to psychotic symptoms and excitation, impairments in episodic memory, and increased production of neurotoxic tryptophan catabolites: a multivariate and machine learning study. Neurotox Res 33:641–655
Kaparakis-Liaskos M, Ferrero RL (2015) Immune modulation by bacterial outer membrane vesicles. Nat Rev Immunol 15:375–387
Kay SR, Fiszbein A, Opler LA (1987) The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull 13:261–276
Kirkpatrick B, Buchanan RW, McKenney PD, Alphs LD, Carpenter WT Jr (1989) The schedule for the deficit syndrome: an instrument for research in schizophrenia. Psychiatry Res 30:119–123
Kittirathanapaiboon P, Khamwongpin M (2005) The validity of the mini international neuropsychiatric interview (M.I.N.I.) Thai version. J Mental Health Thail 13(3):125–135
Kwa SF, Beverley P, Smith AL (2006) Peyer's patches are required for the induction of rapid Th1 responses in the gut and mesenteric lymph nodes during an enteric infection. J Immunol 176:7533–7541
Lewis CE, McCarthy SP, Lorenzen J, McGee JO (1990) Differential effects of LPS, IFN-gamma and TNF alpha on the secretion of lysozyme by individual human mononuclear phagocytes: relationship to cell maturity. Immunology 69:402–408
Lin WN, Lin CC, Cheng HY, Yang CM (2011) Regulation of cyclooxygenase-2 and cytosolic phospholipase A2 gene expression by lipopolysaccharide through the RNA-binding protein HuR: involvement of NADPH oxidase, reactive oxygen species and mitogen-activated protein kinases. Br J Pharmacol 163(8):1691–1706
Lucas K, Maes M (2013) Role of the toll like receptor (TLR) radical cycle in chronic inflammation: possible treatments targeting the TLR4 pathway. Mol Neurobiol 48:190–204
Maes M, Carvalho AF (2018) The compensatory immune-regulatory reflex system (CIRS) in depression and bipolar disorder. Mol Neurobiol 55:8885–8903. https://doi.org/10.1007/s12035-018-1016-x
Maes M, Bosmans E, Ranjan R, Vandoolaeghe E, Meltzer HY, De Ley M, Berghmans R, Stans G, Desnyder R (1996) Lower plasma CC16, a natural anti-inflammatory protein, and increased plasma interleukin-1 receptor antagonist in schizophrenia: effects of antipsychotic drugs. Schizophr Res 21:39–50
Maes M, Bosmans E, Kenis G, De Jong R, Smith RS, Meltzer HY (1997a) In vivo immunomodulatory effects of clozapine in schizophrenia. Schizophr Res 26:221–225
Maes M, Delange J, Ranjan R, Meltzer HY, Desnyder R, Cooremans W, Scharpé S (1997b) Acute phase proteins in schizophrenia, mania and major depression: modulation by psychotropic drugs. Psychiatry Res 66:1–11
Maes M, Mihaylova I, Leunis JC (2007) Increased serum IgA and IgM against LPS of enterobacteria in chronic fatigue syndrome (CFS): indication for the involvement of gram-negative enterobacteria in the etiology of CFS and for the presence of an increased gut-intestinal permeability. J Affect Disord 99:237–240
Maes M, Kubera M, Leunis JC (2008) The gut-brain barrier in major depression: intestinal mucosal dysfunction with an increased translocation of LPS from gram negative enterobacteria (leaky gut) plays a role in the inflammatory pathophysiology of depression. Neuro Endocrinol Lett 29:117–124
Maes M, Twisk FN, Kubera M, Ringel K, Leunis JC, Geffard M (2012) Increased IgA responses to the LPS of commensal bacteria is associated with inflammation and activation of cell-mediated immunity in chronic fatigue syndrome. J Affect Disord 136:909–917
Maes M, Kubera M, Leunis JC, Berk M, Geffard M, Bosmans E (2013a) In depression, bacterial translocation may drive inflammatory responses, oxidative and nitrosative stress (O&NS), and autoimmune responses directed against O&NS-damaged neoepitopes. Acta Psychiatr Scand 127:344–354
Maes M, Ringel K, Kubera M, Anderson G, Morris G, Galecki P, Geffard M (2013b) In myalgic encephalomyelitis/chronic fatigue syndrome, increased autoimmune activity against 5-HT is associated with immuno-inflammatory pathways and bacterial translocation. J Affect Disord 150:223–230
Maes M, Kanchanatawan B, Sirivichayakul S, Carvalho AF (2018) In Schizophrenia, low natural IgM antibody titers to oxidative specific epitopes and higher IgM responses to Nitrated and nitrosylated proteins strongly predict negative symptoms, neurocognitive impairments and the deficit syndrome. Preprints 2018100083. https://doi.org/10.20944/preprints201810.0083.v2)
McMahon M, Skaggs B (2016) Autoimmunity: do IgM antibodies protect against atherosclerosis in SLE? Nat Rev Rheumatol 12:442–444
Meyer U (2014) Prenatal poly(i:C) exposure and other developmental immune activation models in rodent systems. Biol Psychiatry 75:307–315
Miller BJ, Buckley P, Seabolt W, Mellor A, Kirkpatrick B (2011) Meta-analysis of cytokine alterations in schizophrenia: clinical status and antipsychotic effects. Biol Psychiatry 70:663–671
Muraca M, Putignani L, Fierabracci A, Teti A, Perilongo G (2015) Gut microbiota-derived outer membrane vesicles: under-recognized major players in health and disease? Discov Med 19:343–348
Noto MN, Maes M, Nunes SO, Ota VK, Rossaneisf AC, Verri Jr WA, Cordeiro Q, Belangero SI, Gadelha A, Bressan RA, Noto C (2018) Activation of the immune-inflammatory response system and the compensatory immune-regulatory reflex system in antipsychotic naive first episode psychosis. Preprints Preprints2018090314.v2
O'Dwyer DN, Dickson RP, Moore BB (2016) The lung microbiome, immunity, and the pathogenesis of chronic lung disease. J Immunol 196:4839–4847
Overall JE, Gorham DR (1962) The brief psychiatric rating scale. Psychol Rep 10:799–812
Pai K, Sodhi A (1991) Effect of cisplatin, rIFN-Y, LPS and MDP on release of H2O2, O2- and lysozyme from human monocytes in vitro. Indian J Exp Biol 29:910–915
Pasternak BA, d'Mello S, Jurickova II, Han X, Willson T, Flick L, Petiniot L, Uozumi N, Divanovic S, Traurnicht A, Bonkowski E, Kugathasan S, Karp CL, Denson LA (2010) Lipopolysaccharide exposure is linked to activation of the acute phase response and growth failure in pediatric Crohn's disease and murine colitis. Inflamm Bowel Dis 16:856–869
Peng T, Lu X, Feng Q (2005) NADH oxidase signaling induces cyclooxygenase-2 expression during lipopolysaccharide stimulation in cardiomyocytes. FASEB J 19:293–295
Ringle CM, da Silva D, Bido D (2014) Structural equation modeling with the SmartPLS. Braz J Mark 13(2)
Roomruangwong C, Kanchanatawan B, Sirivichayakul S, Anderson G, Carvalho AF, Duleu S, Geffard M, Maes M (2017, 2017) IgA/IgM responses to gram-negative bacteria are not associated with perinatal depression, but with physio-somatic symptoms and activation of the tryptophan catabolite pathway at the end of term and postnatal anxiety. CNS Neurol Disord Drug Targets 16. https://doi.org/10.2174/1871527316666170407145533
Roomruangwong C, Barbosa DS, de Farias CC, Matsumoto AK, THL B, Morelli NR, Kanchanatawan B, Duleu S, Geffard M, Maes M (2018a) Natural regulatory IgM-mediated autoimmune responses directed against malondialdehyde regulate oxidative and nitrosative pathways and coupled with IgM responses to nitroso adducts attenuate depressive and physiosomatic symptoms at the end of term pregnancy. Psychiatry Clin Neurosci 72:116–130
Roomruangwong C, Noto C, Kanchanatawan B, Anderson G, Kubera M, Carvalho AF, Maes M (2018b) The role of aberrations in the immune-inflammatory response system (IRS) and the compensatory immune-regulatory reflex system (CIRS) in different phenotypes of schizophrenia: the IRS-CIRS theory of schizophrenia. Preprint. https://doi.org/10.20944/preprints201809.0289.v1
Rothstein TL, Griffin DO, Holodick NE, Quach TD, Kaku H (2013) Human B-1 cells take the stage. Ann N Y Acad Sci 1285:97–114
Shen Y, Giardino Torchia ML, Lawson GW, Karp CL, Ashwell JD, Mazmanian SK (2012) Outer membrane vesicles of a human commensal mediate immune regulation and disease protection. Cell Host Microbe 12:509–520
Sirivichayakul S, Kanchanatawan B, Thika S, Carvalho AF, Maes M (2018a) Eotaxin, an endogenous cognitive deteriorating chemokine (ECDC), is a major contributor to cognitive decline in Normal people and to executive, memory, and sustained attention deficits, formal thought disorders, and psychopathology in schizophrenia patients. Neurotox Res 18. https://doi.org/10.1007/s12640-018-9937-8
Sirivichayakul S, Kanchanatawan B, Thika S, Carvalho A, Maes M (2018b) A new schizophrenia model: immune activation is associated with induction of the tryptophan catabolite pathway and increased eotaxin levels which together determine memory impairments and schizophrenia symptom dimensions. BioRxiv 393173. https://doi.org/10.1101/393173
Skelly DT, Griffin ÉW, Murray CL, Harney S, O'Boyle C, Hennessy E, Dansereau MA, Nazmi A, Tortorelli L, Rawlins JN, Bannerman DM, Cunningham C (2018) Acute transient cognitive dysfunction and acute Brain Inj induced by systemic inflammation occur by dissociable IL-1-dependent mechanisms. Mol Psychiatry. https://doi.org/10.1038/s41380-018-0075-8
Smith RS, Maes M (1995) The macrophage-T-lymphocyte theory of schizophrenia: additional evidence. Med Hypotheses 45:135–141
Thiagarajan D, Frostegård AG, Singh S, Rahman M, Liu A, Vikström M, Leander K, Gigante B, Hellenius ML, Zhang B, Zubarev RA, de Faire U, Lundström SL, Frostegård J (2016) Human IgM antibodies to malondialdehyde conjugated with albumin are negatively associated with cardiovascular disease among 60-year-olds. J Am Heart Assoc 20(5):12
Todar K (2018) Todar’s online textbook of bacteriology. http://www.textbookofbacteriology.net/. Accessed 11 Dec 2018
Valero J, Mastrella G, Neiva I, Sánchez S, Malva JO (2014) Long-term effects of an acute and systemic administration of LPS on adult neurogenesis and spatial memory. Front Neurosci 8:83
Weismann D, Binder CJ (2012) The innate immune response to products of phospholipid peroxidation. Biochim Biophys Acta 1818:2465–2475
Wiest R (2005) Bacterial translocation. Biosci Microflora 24:61–90
Xu Y, Zhou H, Zhu Q (2017) The impact of microbiota-gut-brain Axis on diabetic cognition impairment. Front Aging Neurosci 9:106
Zakaria R, Wan Yaacob WM, Othman Z, Long I, Ahmad AH, Al-Rahbi B (2017) Lipopolysaccharide-induced memory impairment in rats: a model of Alzheimer's disease. Physiol Res 66:553–565
Zhu F, Zheng Y, Ding YQ, Liu Y, Zhang X, Wu R, Guo X, Zhao J (2014) Minocycline and risperidone prevent microglia activation and rescue behavioral deficits induced by neonatal intrahippocampal injection of lipopolysaccharide in rats. PLoS One 9(4):e93966
Acknowledgements
The study was supported by the Asahi Glass Foundation, Chulalongkorn University Centenary Academic Development Project, and Ratchadapiseksompotch Funds, Faculty of Medicine, Chulalongkorn University, grant numbers RA60/042 (to BK) and RA61/050 (to MM).
Author information
Authors and Affiliations
Contributions
All the contributing authors have participated in the manuscript. MM and BK designed the study. BK recruited patients and completed diagnostic interviews and rating scale measurements. MM carried out the statistical analyses. All authors (BK, MM, SS, and AC) contributed to interpretation of the data and writing of the manuscript. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Maes, M., Kanchanatawan, B., Sirivichayakul, S. et al. In Schizophrenia, Increased Plasma IgM/IgA Responses to Gut Commensal Bacteria Are Associated with Negative Symptoms, Neurocognitive Impairments, and the Deficit Phenotype. Neurotox Res 35, 684–698 (2019). https://doi.org/10.1007/s12640-018-9987-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12640-018-9987-y