Abstract
In 2001, the first author of this paper reported that schizophrenia is associated with an increased frequency of the haptoglobin (Hp)-2 gene. The precursor of Hp-2 is zonulin, a molecule that affects intercellular tight junction integrity. Recently, we reported increased plasma IgA/IgM responses to Gram-negative bacteria in deficit schizophrenia indicating leaky gut and gut dysbiosis. The current study was performed to examine the integrity of the paracellular (tight and adherens junctions) and transcellular (cytoskeletal proteins) pathways in deficit versus non-deficit schizophrenia. We measured IgM responses to zonulin, occludin, E-cadherin, talin, actin, and vinculin in association with IgA responses to Gram-negative bacteria, CCL-11, IgA responses to tryptophan catabolites (TRYCATs), immune activation and IgM to malondialdehyde (MDA), and NO-cysteinyl in 78 schizophrenia patients and 40 controls. We found that the ratio of IgM to zonulin + occludin/talin + actin + viculin (PARA/TRANS) was significantly greater in deficit than those in non-deficit schizophrenia and higher in schizophrenia than those in controls and was significantly associated with increased IgA responses to Gram-negative bacteria. IgM responses to zonulin were positively associated with schizophrenia (versus controls), while IgM to occludin was significantly associated with deficit schizophrenia (versus non-deficit schizophrenia and controls). A large part of the variance (90.8%) in negative and PHEM (psychosis, hostility, excitation, and mannerism) symptoms was explained by PARA/TRANS ratio, IgA to Gram-negative bacteria, IgM to E-cadherin and MDA, and memory dysfunctions, while 53.3% of the variance in the latter was explained by PARA/TRANS ratio, IgA to Gram-negative bacteria, CCL-11, TRYCATs, and immune activation. The results show an upregulated paracellular pathway with breakdown of the tight and adherens junctions and increased bacterial translocation in deficit schizophrenia. These dysfunctions in the intestinal paracellular route together with lowered natural IgM, immune activation, and production of CCL-11 and TRYCATs contribute to the phenomenology of deficit schizophrenia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In 2001, the first author of this paper reported that the Hp-2 gene frequency was significantly higher in schizophrenia patients as compared with the observed frequency in the population, indicating that genetic variation on chromosome 16 is associated with schizophrenia [1]. Hp is an acute phase protein, which is increased together with other acute phase reactants and complement factors, including C3C, during immune-inflammatory responses and schizophrenia [1]. Hp shows a polymorphism with three common phenotypes, namely Hp 1-1, Hp 2-1, and Hp 2-2, which are determined by two autosomal codominant alleles, i.e., Hp-1 and Hp-2 [2]. This is important as the Hp 2-2 phenotype is significantly associated with increased oxidative burden, low-grade inflammation, and increased levels of pro-inflammatory cytokines, including interleukin (IL)-6 and tumor necrosis factor (TNF)-α [3,4,5]. Increased Hp 2-2 genotypic distribution plays a role not only in schizophrenia [1] but also in other medical illness, including Crohn’s disease, celiac disease, and chronic kidney failure [6, 7]. We have argued that the increased frequency of the Hp 2-2 phenotype could play a role in the immune-inflammatory pathophysiology of schizophrenia [1].
There is now indeed evidence that schizophrenia and its different phenotypes, including first episode psychosis, chronic schizophrenia, treatment resistant schizophrenia, and deficit schizophrenia, are accompanied by activation of the immune-inflammatory response system (IRS) with an acute phase response, activated macrophagic M1, T helper (Th)-1, and Th-17 phenotypes [8,9,10]. Moreover, the different schizophrenia phenotypes and manifestations are accompanied by simultaneous signs of immune regulatory or anti-inflammatory processes, including activated Th-2 and tregulatory (Treg) phenotypes and increased levels of cytokine receptors, such as soluble IL-2 receptor, sTNF-R1 and sTNFR2, and sIL-1R antagonist (sIL-1RA) [10]. As such, the different phenotypes of schizophrenia are accompanied by activated IRS pathways and an activated compensatory immune-regulatory system (CIRS), which tends to downregulate the primary IRS [10, 11]. Different IRS and CIRS products have neurotoxic and excitotoxic effects and may deleteriously impact neuroplastic mechanisms and neurogenesis thereby causing neurocognitive impairments and symptoms of schizophrenia, including negative symptoms, and psychosis, hostility, excitation, and mannerism (PHEM) [9, 10, 12,13,14,15,16]. These neurotoxic substances belong to the IRS as well as CIRS and include increased levels of IL-1β, IL-6, TNF-α, interferon-γ, IL-4, IL-13, CCL-11 (eotaxin), and cytokine-induced production of neurotoxic tryptophan catabolites (TRYCATs), including xanthurenic acid (XA), picolinic acid (PA), and 3-OH-kynurenine (3HK) [9, 10, 14,15,16].
Recently, we discovered a deficit in the CIRS that is strongly associated with deficit schizophrenia, namely lowered IgM antibody levels to conjugated oxidative specific epitopes (OSEs), including malondialdehyde (MDA) [17]. Moreover, lowered levels of IgM to MDA were highly significantly associated with the negative symptoms of schizophrenia and excitation and impairments in semantic and episodic memory, including direct and delayed recall [17]. Those antibodies directed against conjugated OSEs are part of the innate immune system and may be present even without antigenic contact [18,19,20,21,22]. These natural antibodies are produced by B1 cells and have housekeeping, immune-regulatory, anti-inflammatory, and anti-oxidant properties, thereby protecting against uncontrolled inflammation [23, 24].
Importantly, we discovered that deficit schizophrenia is accompanied by increased IgA responses to gut commensal bacteria, including Hafnia alvei, Pseudomonas aeruginosa, Morganella morganii, and Klebsiella pneumoniae as compared with non-deficit schizophrenia, and that increased IgA directed to LPS of Pseudomonas putida, IgM responses to all abovementioned Gram-negative bacteria coupled with lowered natural IgM to OSEs predict deficit schizophrenia with a bootstrapped area under the ROC curve of 0.960 [25]. Natural IgM to OSEs is an integral pre-existing part of the innate first-line defense system protecting against microorganisms, including Gram-negative bacteria, through early recognition and neutralization of the invading pathogens “in the lag time for adaptive antibody production” [18,19,20, 23, 26]. The LPS of translocated bacteria in turn may activate receptors of the innate immune system, including the Toll-like receptor (TLR)-4 complex, thereby activating intracellular signaling networks (e.g., nuclear factor (NF)-κB and MAPK) and stimulating the production of inducible nitric oxide (NO) synthase, reactive oxygen species, and pro-inflammatory cytokines [27]. Moreover, LPS and other bacterial antigens may exert profound neurotoxic effects [28] by accessing the brain via different routes, including the blood-brain-barrier, the circumventricular organs and area postrema, or via outer membrane vesicles [28,29,30,31,32,33]. Based on this knowledge, we concluded that schizophrenia patients with lowered natural IgM are more prone to the detrimental effects of LPS and other bacterial neurotoxic antigens, which in turn may drive schizophrenia symptoms, including memory impairments [25].
The Hp-2 gene and increased bacterial translocation are both related to pre-Hp-2, namely zonulin [6]. The latter increases the permeability of the tight junctions (TJs) composed of occludin and claudin, which in the gut join epithelial cells together thereby protecting against entrance of unwanted enterobacteria and food molecules [6]. Besides, TJs and E-cadherin binds epithelial cells together via adherens junctions (AJs), thereby increasing stability of the gut barrier [34]. Gut dysbiosis and gliadin may cause loosening of the TJ barrier and therefore increased translocation of bacterial and other antigens (e.g., food) into the blood stream via the paracellular route [34,35,36]. Loss of intracellular cytoskeletal components, including actin, vinculin, and talin, may allow entrance of antigens via the transcellular route [34]. However, there are no data whether (deficit) schizophrenia is accompanied by upregulated paracellular or transcellular pathways, leading to increased translocation of gut commensal Gram-negative bacteria.
Hence, the current study examined whether (deficit) schizophrenia and its phenomenological characteristics (i.e., negative and PHEM symptoms and memory deficits) are accompanied by dysfunctions in the paracellular or transcellular pathways as measured using IgM responses to zonulin, occludin, E-cadherin, talin, actin, and vinculin.
Subjects and Methods
Participants
The current study recruited 79 patients with schizophrenia and 40 healthy controls. All participants were recruited from the same catchment area, namely province Bangkok, Thailand. Patients attended the Department of Psychiatry, King Chulalongkorn Memorial Hospital, Bangkok, Thailand and fulfilled all DSM-IV-TR diagnostic criteria. They were stabilized patients who did not suffer from acute psychotic episodes the year prior to the study. Patients were divided into two groups, namely those with and without deficit schizophrenia [37]. Exclusion criteria for patients were axis-1 DSM-IV-TR disorders other than schizophrenia, including bipolar disorder, schizoaffective disorder, major depression, substance use disorders, and psycho-organic disorders. Exclusion criteria for healthy controls were lifetime and current diagnoses of axis I, DSM-IV-TR diagnoses, and a positive family history of schizophrenia. Exclusion criteria for both patients and controls were (a) medical illnesses, such as inflammatory bowel disease, psoriasis, chronic obstructive pulmonary disease, rheumatoid arthritis, lupus erythematosus, and diabetes types 1 and 2; (b) neuro-inflammatory and neurodegenerative disorders, including multiple sclerosis, stroke, Parkinson’s disease, and Alzheimer’s disease; (c) use of medications known to suppress immune functions, such as glucocorticoids or immunosuppresiva; and (d) use of supplements with ω3-polyunsaturated fatty acids or antioxidants the months prior to the study. All patients and controls as well as the guardians (parents or close family members) gave written informed consent prior to participation in our study. The study was conducted according to International and Thai ethics and privacy laws. Approval for the study (298/57) was obtained from the Institutional Review Board of the Faculty of Medicine, Chulalongkorn University, Bangkok, Thailand, which is in compliance with the International Guidelines for Human Research protection as required by the Declaration of Helsinki, The Belmont Report, CIOMS Guideline and International Conference on Harmonization in Good Clinical Practice.
Measurements
Clinical Assessments
A senior psychiatrist (BK) specialized in schizophrenia made the diagnosis of schizophrenia using DSM-IV-TR diagnostic criteria and the Mini-International Neuropsychiatric Interview (MINI) in a validated Thai translation [38]. On the same day, the same senior psychiatrist used a semi-structured interview to assess clinical and socio-demographic data in patients and controls. Patients were divided into those with and without primary deficit schizophrenia using the Schedule for the Deficit Syndrome [37]. We also made the diagnosis of first episode of psychosis versus multiple psychotic episodes using DSM-5 criteria. On the same day, BK assessed the Positive and Negative Syndrome Scale (PANSS) [39], the Scale for the Assessment of Negative Symptoms (SANS) [40], the Brief Psychiatric Rating Scale (BPRS) [41], and the Hamilton Depression (HAM-D) and Anxiety (HAM-A) Rating Scales [42, 43] and the Fibromyalgia and Chronic Fatigue Syndrome Rating scale (FF) [44].
Consequently, we have computed indices reflecting different symptoms clusters of schizophrenia pathology as explained previously [15,16,17, 25, 45]. A psychomotor retardation index (PMRI) was constructed based on items of the BPRS, HDRS, and PANSS as z score of HDRS item 8 (HDRS8: psychomotor retardation: slowness of thought and speech, decreased motor activity, impaired inability to concentrate) plus z score of the general psychopathology scale of the PANSS, item G7 (zPANSSG7; reduction in motor activity as reflected in slowing or lessening of movements and speech, diminished responsiveness to stimuli, and reduced body tone) plus z score of item 13 of the BPRS (zBPRS13; reduction in energy level evidenced in slowed movements). Formal thought disorders (FTD) were computed as z value of PANNS P2 (item P2 of the PANNS scale or conceptual disorganization, zP2) plus zN5 (item N5 of the PANNS or difficulty in abstract thinking) plus zBPRS4 (item 4 of the BPRS or conceptual disorganization). The severity of psychotic symptoms was computed as sum of zPANSS (positive subscale item 1) P1 (delusion) plus zPANSSP3 (hallucinations) + zPANNSP6 (suspiciousness) plus zBPRS11 (suspiciousness) plus zBPRS12 (hallucinatory behavior) plus zBPRS15 (unusual thought content). The severity of hostility was computed as sum of zPANSS7 (hostility) plus zPANSSG14 (poor impulse control) plus zBPRS10 (hostility) plus zBPRS14 (uncooperativeness). The excitement dimension score was computed as zP14 (excitement) plus zP5 (grandiosity) plus zBPRS8 (grandiosity) plus zBPRS17 (excitement). Mannerism was computed as zG5 plus zBPRS7 (both mannerism and posturing). Severity of gastro-intestinal symptoms (GIS) was computed as: HAM-A11 plus FF10 (both GIS).
On the same day, a well-trained research assistant (a MSc in Mental Health) assessed neuropsychological functions in all participants using the Consortium to Establish a Registry for Alzheimer’s Disease (CERAD)-neuropsychological battery [46]. Here, we assessed the (a) Verbal Fluency Test (VFT) to probe semantic memory and fluency; (b) Word List Memory (WLM) to test verbal episodic memory and learning ability; and (c) Word List Recall, true recall (True Recall) to test verbal episodic memory recall. In addition, we employed DSM-IV-TR criteria to make the diagnosis of tobacco use disorder (TUD), while we computed the body mass index (BMI) using body weight (kg)/length (m2).
Assays
In patients and controls, fasting blood was sampled at 8.00 a.m. for the assay of IgM responses to intestinal barrier; TJs (zonulin and occludin); AJs (E-cadherin) and cytoskeletal-related proteins (talin, actin, and vinculin); IgM antibody levels to MDA and NO-cysteinyl; IgA responses to TRYCATs; and IL-10, sIL-1RA, macrophage inflammatory protein (MIP)-1α, and CCL-11 (eotaxin) levels.
Zonulin, occludin, E-cadherin, talin, and vinculin were purchased from Bio-Synthesis® (Lewisville, TX, USA) and Abcam® (Cambridge, MA, USA), while actin was obtained from Sigma-Aldrich® (St. Louis, MO, USA). Proteins and peptides at a concentration of 1 mg/mL were dissolved in 0.01-M Tris buffer and diluted 1:50 in 0.1-M carbonate buffer at pH 9.5. One hundred microliters of each diluted antigen was added to each well of the microtiter plate. Plates were incubated overnight at 4 °C and then washed three times with 200-μL 0.01-M PBS containing 0.05% Tween 20 at a pH of 7.4. After washing, 200 μL of 2% bovine serum albumin (BSA) was added to each well to prevent non-specific binding of the antibody to the plate. Plates were washed, and then 100 μL of serum diluted 1:100 for IgM detection in serum diluent was added to duplicate wells coated with each antigen. Plates were incubated for an additional 1 h at room temperature. The plates were then washed five times with Tris-buffered saline (TBS)-Tween. Alkaline phosphatase-labeled IgM at dilutions of 1:400 for IgM were then added to all wells and incubated again for 1 h at room temperature. The enzyme reaction was started by adding 100 μL of paranitrophenylphosphate at a concentration of 1 mg/mL in diethanolamine buffer containing 1-mN MgCl2 and sodium azide at a pH of 9.8. The reaction was stopped 45 min later with 75 μL of 1-N NaOH, and the samples were read by an ELISA reader; the optical densities (ODs) were recorded. Several wells were coated with non-specific proteins, such as human serum albumin (HAS) and rabbit serum albumin, which were used as controls for detecting the ELISA background. Sera from healthy subjects were used as negative controls, and sera from patients with intestinal autoimmunity with moderate and high titers of IgM antibodies were used as calibrator and positive controls and for the calculation of ELISA indices using the following formula: antibody ELISA index = OD of tested specimen − OD blank / OD of calibrator − OD of blank. All OD values were then converted into z scores, which were used in the statistical analyses. Consequently, we computed z unit-weighted composite scores to obtain indices of paracellular and transcellular leaky gut. For paracellular TJ leaky gut, we computed the sum of the z scores of zonulin and occludin as z zonulin plus z occludin (PARA). Furthermore, we also computed z zonulin plus z occludin plus z E-cadherin as an index of the total paracellular pathway (PARA2). For the transcellular route, we computed the composite score of z talin plus z actin plus z vinculin (TRANS). In order to estimate which route prevails in schizophrenia, we have computed the ratio between PARA versus TRANS as z score of (z zonulin plus z occludin) − z score of z talin plus z actin plus z vinculin (PARA/TRANS). As an estimate of total leaky gut, we computed the sum of the z scores of all six proteins, namely z zonulin plus z occludin plus z talin plus z actin plus z vinculin plus z E-cadherin (named “overall LEAKY index”).
The assays of IgA responses to different conjugated TRYCATs were performed as described previously [47,48,49]. The six TRYCATs were dissolved in 200-μL dimethylsulfoxide (DMSO) (Acros). BSA (ID Bio) was dissolved in 3-mL 2-morpholino-ethanesulfonic acid monohydrate (MES) buffer 10−1 M at pH = 6.3 (Acros). The TRYCATs were then mixed with the BSA solution and supplemented with 15-mg N-hydroxysuccinimide (Sigma) and 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide (Acros) as coupling agents. The conjugates were synthesized by linking 3-OH-kynurenine (3HK, Sigma), kynurenic acid (KA, Acros), quinolinic acid (QA, Acros), anthranilic acid (AA, Acros), xanthurenic acid (XA, Akros), and picolinic acid (PA, Akros) to 20-mg BSA. The coupling reaction proceeded at 37 °C for 1 h in the dark. The coupling was stopped by adding 100-mg hydroxylamine (Sigma-Aldrich) per conjugate. Protein conjugates were dialyzed with 10−1-M NaCl solution for 72 h, and the bath solution was changed at least four times per day. The conjugated TRYCATs and BSA concentrations were evaluated by spectrophotometry. The coupling ratio of each conjugate was determined by measuring the concentration of TRYCATs and BSA at 310–330 nm and 280 nm, respectively. ELISA tests were used to determine plasma titers of immunoglobulins (Ig) A (IgA). Towards this end, polystyrene 96-well plates (NUNC) were coated with 200-μL solution containing 10–50-μg/mL TRYCAT conjugates in 0.05-M carbonate buffer (pH = 9.6). Well plates were incubated under agitation at 4 °C for 16 h. Then, 200-μL blocking buffer A (phosphate-buffered saline, PBS, 2.5 g/L BSA) was applied, and all samples were incubated at 37 °C for 1 h. Well plates were washed with PBS solution and filled up with 100-μL serum diluted 1:130 in blocking buffer and incubated at 37 °C for 1 h and 45 min. Well plates were washed three times with PBS, 0.05% Tween 20, incubated with peroxidase-labeled goat anti-human IgA (SouthernBiotech) antibodies at 37 °C for 1 h. The goat anti-human IgA antibody was diluted at 1:10.000 in blocking buffer (PBS, 2.5-g/L BSA). Plates were then washed three times with PBS, 0.05% Tween 20. Fifty microliters of TMB substrate (3,3′,5,5′-tetramethylbenzidine, SouthernBiotech) was added and incubated for 10 min in the dark. The reaction was stopped using 50 μL of TMB stop solution (SouthernBiotech). ODs were measured at 450 nm using Varioskan Flash (Thermo Scientific). All assays were carried out in one and the same run by the same operator who was blind to all clinical results. All assays were carried out in duplicate. The analytical intra-assay CV values were < 7%. We computed a noxious/protective TRYCAT ratio as z score of PA (zPA) plus zXA plus zOHK minus zAA minus zKA (NOX/PRO TRYCAT ratio) [50].
We used an enzyme-linked immunosorbent assay to measure IgM levels directed against conjugated MDA [51,52,53,54]. MDA was linked to fatty acid free-BSA, according to previously described methods [51,52,53,54]. Synthesis of the conjugates to delipidated BSA was performed as described before [53]. In order to mimic nitrosylation processes, NO-cysteinyl was synthesized by linking haptens to BSA (Sigma-Aldrich) using glutaraldehyde [52, 55, 56]. The synthesis of these conjugates has been described previously [57]. The hapten conjugate was nitrosylated using sodium nitrite (NaNO2) dissolved in 2 mL of each conjugate, in 0.5-M HCl at 37 °C for 2 h, while shaking in the dark. The conjugate was then dialyzed at 4 °C for 24 h against a phosphate-buffered saline (PBS: 10−2-M NaH2PO4, 12H2O; 0.15-M NaCl; pH 7.4) solution. S-Nitrosothiol bond formation was determined by spectrophotometry. The S-nitrosothiol compound possesses two absorbance maxima, at 336 and 550 nm, respectively: e336 nm = 900 M−1 cm−1 for the conjugates, e550nm = 4000 M−1 cm−1 for BSA. Absorbance was evaluated in order to determine NO concentrations linked to the compound. The detection of IgM autoantibodies to the conjugates was performed by indirect ELISA tests [54, 57]. Briefly, polystyrene 96-well plates (NUNC) were coated with 200-μL solution containing the conjugates or BSA in 0.05-M carbonate buffer at pH 9.6. Well plates were incubated at 4 °C for 16 h under agitation. Then, a 200 μL of blocking solution (PBS, 2.5 g/l BSA) was added for 1 h and placed at 37 °C. Following three washes with PBS, plates were filled up with 100 μL of sera diluted at 1:1000 in the blocking buffer A (PBS, 0.05% Tween 20, 10% glycerol, 2.5-g/l BSA, 1-g/l BSA-G) and incubated at 37 °C for 2 h. After three washes with PBS-0.05% Tween 20, plates were incubated at 37 °C for 1 h with peroxidase-labeled anti-human IgM secondary antibodies diluted respectively at 1: 15,000, in the blocking buffer (PBS, 0.05% Tween 20, 2.5 g/l BSA). They were then washed three times with PBS-0.05% Tween 20 and incubated with the detection solution for 10 min in the dark. Chromogen detection solution was used for the peroxidase assay at 8% in 0.1-M acetate and 0.01-M phosphate buffer (pH 5.0) containing 0.01% H2O2. The reaction was stopped with 25-μL 2-N HCl. ODs were measured at 492 nm using a multiscan spectrophotometer. All assays were carried out in duplicate. The intra-assay coefficients of variation (CV) were < 6%.
For the assays of cytokines/chemokines, 50 μL of serum (1:2 dilution in calibrator diluents) was mixed with 50 μL of microparticle cocktail containing CCL-11, sIL-1RA, IL-10, and MIP-1α (R&D Systems, Inc., Minneapolis, MN, USA) per well of a 96-well plate provided by manufacturer and incubated for 2 h at room temperature on a shaker at 800 rpm. The mixture was then washed three times with wash buffer, and 50-μL diluted biotin antibody cocktail was added and then incubated for 1 h. Wells were washed three times before another 50 μL of diluted streptavidin-PE was added and further incubated for 30 min. Finally, wells were washed three times, and 100 μL of wash buffer was added and left at room temperature for 2 min before being read with Bio-Plex® 200 System (Bio-Rad Laboratories, Inc.). The intra-assay CV values were < 7.0%. The least detectable dose was 1.82 pg/mL for eotaxin, 1.58 pg/mL for MIP-1, 5.98 pg/mL for IL-1RA, and 0.4 pg/mL for IL-10. We computed an immune activation index as z score of interleukin-10 (zIL-10) plus zMIP-1α plus zsIL-1RA [15, 16].
Statistical Analysis
Analysis of variance (ANOVA) was used to assess differences in scale variables among categories and analysis of contingency tables (Χ2 tests) was employed to assess associations between nominal variables. Correlation matrices were assessed to check associations between sets of scale variables using Pearson’s product moment and Spearman’s rank order correlation coefficients. Multiple regression analysis was employed to delineate the significant biomarkers predicting symptoms and cognitive scores. Binary and multinomial logistic regression analyses were used to delineate the significant predictors of diagnostic groups (either binary, e.g., deficit versus non-deficit SCZ or multiple groups, e.g., controls versus SCZ with or without deficit). Multivariate GLM analysis was used to examine the effects of explanatory variables (e.g., drug state, age, and sex) on the six PARA-TRANS proteins. Results of regression analyses were checked for multicollinearity. Moreover, all analyses were bootstrapped (1000 bootstraps), and we report the bootstrapped results if there are differences between analyses with and without bootstrapping. IgM to MDA and CCL-11 were processed in Ln transformations in order to normalize the data distribution of these variables. Tests were two-tailed, and a p value of 0.05 was used for statistical significance. All abovementioned statistical analyses were performed using IBM SPSS Windows version 25.
We used partial least squares (PLS) path modeling to analyze causal associations [58] between input variables, namely biomarkers including PARA/TRANS, E-cadherin, IgA to Gram-negative bacteria, the immune activation index, CCL-11, NOX/PRO TRYCAT ratio, IgM to MDA and NO-Cysteinyl, and symptom dimensions, including negative (the six symptoms of the SDS) and PHEM symptoms, which were used as output variables. Based on our new cognitive theory of schizophrenia [15, 16], we considered that memory dysfunctions may mediate the association between biomarkers and symptoms. We entered data in the PLS analysis as indicator variables, i.e., CCL-11, TRYCATs, PARA/TRANS, E-cadherin, IgM MDA, immune activation index, or as latent vectors (LV), namely an LV extracted from Gram-negative bacteria (“Gram-negative LV”), an LV extracted from negative (the 6 SDS symptoms) and PHEM indicators (“symptom LV”), and an LV extracted from memory indicators (VFT, WLM, and WL True Recall) and FTD values (“memory LV”). Based on our novel neurocognitive theory, we entered the memory LV as a predictor variable for the symptom LV, while the biomarkers predicted both the memory and symptom LVs. As such, we used a multistep path model with multiple mediators [59], including Gram-negative LV, CCL-11, NOX/PRO TRYCAT ratio, and memory LV. PLS path modeling was carried out only if the LVs and overall model quality data complied with specific criteria, namely (a) all indicators in the outer model have factor loadings > 0.400 (all p < 0.001), (b) model SRMR < 0.08; and (c) all LVs have adequate reliability as indicated by Cronbach’s alpha > 0.7, composite reliability > 0.8, and average variance extracted (AVE) > 0.5. We then applied complete, consistent bootstrapping (2000 bootstraps) to compute path coefficients with exact p values, total effects, and total indirect and specific indirect effects.
Results
Biomarkers and Schizophrenia Categories
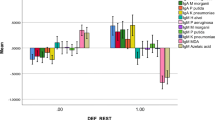
Table 1 shows the outcome of different multinomial logistic regression analyses with the diagnosis, namely controls, deficit schizophrenia (SCZ), and non-deficit SCZ, as dependent variables and the biomarkers as explanatory variables. Regression #1 shows a strong association between PARA/TRANS ratio and diagnosis with an effect size of 0.577 for the total model (including age, sex, and IgM MDA); 0.343 for PARA/TRANS alone; 0.268 for IgM MDA alone; 0.330 for age, sex, and IgM MDA combined; and 0.111 for age and sex. PARA/TRANS was significantly and positively associated with deficit SCZ as compared with non-deficit SCZ and controls and with non-deficit schizophrenia as compared with controls. Also, ANOVA showed that the PARA/TRANS ratio was significantly different (F = 24.41, df = 2/114, p < 0.001) between the three study groups and increased from controls (mean z score ± SE = − 0.698 ± 0.804) ➔ non-deficit SCZ (0.008 ± 0.817) ➔ deficit SCZ (0.654 ± 0.903). Figure 1 shows the dot plot of the PARA/TRANS ratio in the three study groups.
There were significant and positive correlations between IgM to MDA and IgM to zonulin (r = 0.624, p < 0.001), occludin (r = 0.670, p < 0.001), E-cadherin (r = 0.672, p < 0.001), talin (r = 0.660, p < 0.001), and actin (r = 0.712, p < 0.001), but not vinculin (r = 0.096, p = 0.302, all n = 117). Therefore, all regressions shown in Table 1 examine whether the IgM levels to the proteins contribute to the prediction of deficit SCZ after considering the significant association of IgM MDA and deficit SCZ [17], while controlling for the effects of age and sex (see below). Firstly, we have examined the effects of the LEAKY, PARA, PARA2, and PARA/TRANS indices and the effects of the separate IgM values.
Regression #2 and #3 show that the LEAKY and PARA indices had a significant impact and were significantly associated with deficit SCZ as compared with non-deficit SCZ and controls, while there were no significant differences between controls and non-deficit SCZ. Occludin (regression #4) yielded a higher effect size and showed that increased levels were significantly associated with deficit SCZ versus controls and non-deficit SCZ. Increased IgM to zonulin (regression #5) was significantly associated with both SCZ categories versus controls, without significant differences between the SCZ subgroups. Regression #6 shows that the TRANS proteins were associated with deficit versus non-deficit SCZ albeit with very low impact. There were no significant associations between vinculin and the diagnostic groups, while both talin (Wald = 8.59, df = 1, p = 0.003) and actin (F = 11.88, df = 1, p = 0.001) were associated with deficit versus non-deficit SCZ. PARA2 (regression #7) was significantly associated with deficit SCZ versus non-deficit SCZ and controls, while IgM to E-cadherin was significantly associated with deficit SCZ versus non-deficit SCZ (Wald = 18.20, df = 1, p < 0.001 with an odds ratio = 10.59 and 95% confidence intervals: 3.65–32.90). Regression #8 shows the combined effects of increased PARA/TRANS ratio and IgA against Gram-negative bacteria (z composite score of the five bacteria): both increased PARA/TRANS and Gram-negative bacteria were associated with deficit SCZ versus non-deficit SCZ and controls, while PARA/TRANS also differentiated non-deficit SCZ from controls.
Characteristics of Increased PARA/TRANS Ratio
Based on the high impact of the PARA/TRANS ratio, we examined the characteristics of SCZ subjects with an increased PARA/TRANS ratio versus patients with a lower ratio and controls. Table 2 shows the outcome of ANOVAs/Χ2 tests performed on socio-demographic, clinical, and biomarker data in three study groups, namely controls and SCZ patients with a lower PARA/TRANS (< 0.666 percentile) versus higher PARA/TRANS ratio (≥ 0.666 percentile). There were no significant differences in age, TUD, and BMI between the three study groups. There were somewhat more females in the lower PARA/TRANS group than in controls, while years of education were somewhat lower in SCZ patients with an increased PARA/TRANS ratio. The number of psychotic episodes was significantly lower in those with an increased PARA/TRANS ratio, while the latter showed increased SDS, SANS, PANSS negative, psychosis, excitements, and PMRI scores as compared with those with a lowered PARA/TRANS ratio. There were, however, no significant differences in PANSS positive symptoms, hostility, mannerism, FTD, and GIS symptoms between both SCZ subgroups. There was a significant association between the PARA/TRANS groups and deficit SCZ. IgA levels to Gram-negative bacteria and IgM to NO-cysteinyl were significantly greater in SCZ patients allocated to the high PARA/TRANS group. The other biomarkers and VFT, WLM, and WL True Recall did not differ significantly between both PARA/TRANS groups.
Multivariate Biomarker Prediction of SCZ and Deficit SCZ
Table 3 examines the best prediction of deficit versus non-deficit SCZ and SCZ versus healthy controls using logistic regression analyses with PARA/TRANS, IgA to Gram-negative bacteria, IgM to NO-cysteinyl, and MDA, CCL-11, and NOX/PRO TRYCAT ratio as explanatory variables. Logistic regression #1 shows that deficit SCZ (versus non-deficit SCZ) was highly predicted (effect size = 0.773) by IgA Gram-negative bacteria, PARA/TRANS ratio, and IgM to NO-cysteinyl (all positively) and IgM to MDA (inversely); 88.6% of all cases were correctly classified with a sensitivity of 87.2% and a specificity of 90.0%. We found that zonulin, occludin, IgA to Gram-negative bacteria, IgM to NO-cysteinyl, and CCL-11 (all positively) and IgM to MDA (inversely) significantly discriminated deficit from non-deficit SCZ (Χ2 = 109.50, df = 6, p < 0.001, Nagelkerke = 1) with a sensitivity and specificity of 100%. Regression #2 shows that SCZ (versus controls) was highly predicted by CCL-11, the immune activation index, the NOX/PRO TRYCAT ratio, PARA/TRANS (all positively), and age (inversely); 92.3% of all cases were correctly classified with a sensitivity of 94.9% and a specificity of 86.8%. A second logistic regression analysis showed that PARA/TRANS could be replaced by zonulin levels (Wald = 6.73, df = 1, p = 0.009) and that zonulin together with the variables shown in Table 3, regression #2, yielded an effect size of 0.829; 93.2% of the cases were correctly classified with a sensitivity of 94.9% and a specificity of 89.5%.
Effects of Extraneous Variables
We have also examined possible effects of the drug state of the patients on the IgM responses to paracellular and transcellular proteins. Multivariate GLM analysis did not show a significant effect of use of risperidone (F = 1.59, df = 6/96, p = 0.158; n = 34), clozapine (F = 1.16, df = 6/96, p = 0.335; n = 9), haloperidol (F = 0.69, df = 6/96, p = 0.661; n = 11), perphenazine (F = 1.25, df = 6/96, p = 0.290; n = 21), antidepressants (F = 0.40, df = 6/96, p = 0.880; n = 26), mood stabilizers (F = 0.37, df = 6/96, p = 0.899; n = 13), and anxiolytics/hyponotics (F = 1.04, df = 6/96, p = 0.406; n = 29) on the six proteins. Previously, we have shown that the same drugs have no effect on CCL-11, TRYCATs, IgM to MDA and NO-cysteinyl, IgA/IgM to Gram-negative bacteria, and the clinical assessments [14,15,16,17, 25, 45]. There was however a significant effect of age (F = 4.58, df = 6/106, p < 0.001), but not sex (F = 2.05, df = 6/106, p = 0.065) on the proteins. Parameter estimates showed significant inverse associations between age and IgM to E-cadherin (F = 15.28, df = 1/111, p < 0.001) and vinculin (F = 10.62, df = 1/111, p = 0.001). In any case, all regression analyses were controlled for possible effects of age and sex. There were no significant effects of TUD (F = 0.26, df = 6/105, p = 0.953) and BMI (F = 1.05, df = 6/100, p = 0.400) on the IgM levels to PARA-TRANS proteins.
Prediction of SCZ Symptomatology Using Biomarkers
In SCZ patients, we have examined the effects of PARA/TRANS, E-cadherin, IgM to Gram-negative bacteria, IgA to K. pneumonia or M. Morganii on negative, and PHEM symptoms, while also allowing for the effects of age, sex, and education. Table 4, regression #1, shows that 58.6% of the variance in the total SDS score was explained by the regression on PARA/TRANS, IgA to K. pneumonia and E-cadherin (all positively), and IgM to MDA and education (inversely). Regressions #2–7 show that a large part of the variance (35.3–53.3%) in the items of the SDS was predicted by PARA/TRANS and IgM to MDA with or without Gram-negative bacteria, E-cadherin, and education. There were no associations between the explanatory variables used here and PANSS positive symptoms (regression #8), while a large part of the variance in PANSS negative symptoms (regression #9) was explained by the regression on PARA/TRANS, IgA to K. pneumonia and E-Cadherin (all positively), and IgM to MDA (inversely). The same four variables together with education also predicted the SANS total score with an explained variance of 50.8% (regression #10). Regression #11 shows that 46.8% of the variance in psychomotor retardation was explained by PARA/TRANS, IgA to K. pneumoniae, E-cadherin (all positively), and IgM MDA (inversely). We found that 23.3% of the variance in psychotic symptoms (regression #12) was explained by two explanatory variables, namely PARA/TRANS and IgA to K. pneumoniae, while 33.2% of the variance in excitation (regression #13) was explained by PARA/TRANS, IgA to K. pneumonia (both positively) and education, and IgM to MDA (both inversely).
Prediction of SCZ Symptomatology Using all Biomarkers
Table 5 shows the prediction of key symptoms using the variables shown in Table 4 combined with CCL-11, NOX/PRO TRYCAT ratio, IgM NO-cysteinyl, and immune activation index, while allowing for possible effects of age, sex, and education. We used multiple regression analyses performed in the total study group to examine these associations. Regression #1 shows that 18.4% of the variance in GIS symptoms was explained by the immune activation index and zonulin levels. We found that (regression #2) 37.9% of the variance in WLM was explained by immune activation and PARA/TRANS (both inversely) and education and IgM MDA (both positively). 50.1% of the variance in PMRI (regression #3) was explained by the multiple regression on PARA/TRANS, IgA to K. pneumoniae, CCL-11, E-cadherin, and the immune activation index (all positively) and IgM MDA (inversely). FTD (regression #4) was best predicted by IgA NOX/PRO, PARA/TRANS, and male sex (26.9% of the variance). A large part of the variance in SDS score (57.1%, regression #5) was explained by PARA/TRANS, IgA to K. pneumoniae, CCL-11, IgA NOX/PRO, E-cadherin, and the immune activation index (all positively) and IgM MDA (inversely). A large part of the variance in psychosis (regression #6) was explained by PARA/TRANS, IgA to K. pneumoniae, IgA to NOX/PRO, and male sex. A relatively smaller part of the variances in hostility and mannerism (regressions # 7 and 9) were explained by PARA/TRANS and male sex and immune activation index. We found that PARA/TRANS, IgA to K. pneumoniae, IgA to NOX/PRO and CCL-11 (all positively), and IgM MDA (inversely) were significantly associated with excitation (regression #8).
Results of SmartPLS Analysis
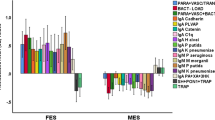
Figure 2 shows the results of a PLS analysis with as final outcome variable the symptom LV and as direct explanatory variable the memory LV. The biomarkers were entered as input variables, including the Gram-negative LV (two Gram-negative bacteria did not load significantly and therefore were deleted from the model). Figure 2 shows the multistep path model with multiple mediators, including the Gram-negative LV, CCL-11, TRYCATs, and memory LV. We found that the overall model fit was excellent, namely SRMR = 0.047, the construct reliability, and discriminant validity of all LVs were good to excellent, namely all Cronbach’s alpha > 0.787, composite reliability > 0.865, and AVE > 0.618, and all outer loadings of the 3 LVs were > 0.701.
Results of partial least squares (PLS) path modeling. This figure shows the results of PLS analysis with as final outcome variable a LV extracted from 6 negative SDS (schedule of the deficit syndrome) and 4 PHEM (psychosis, hostility, excitation, mannerism) symptoms (named: “symptoms LV”) with as direct explanatory variable a LV extracted from memory indicators, namely Verbal Fluency Test (VFT), Word List Memory (WLM), WL True Recall, and formal thought disorders (FTD) (named “memory LV”). Biomarkers are entered as input variables predicting both the symptom and memory LV: a the ratio between IgM responses to zonulin and occludin versus talin, actin, and vinculin (PARA/TRANS) and a LV extracted from IgA to H. alvei, K. pneumoniae, and M. morganii (named “Gram-negative LV”); b IgM to malondialdehyde (MDA); and c an immune activation indicator (composite score of soluble interleukin-1 receptor antagonist, interleukin-10 and macrophage inflammatory protein-1α), CCL-11 or eotaxin, and the noxious/protective tryptophan catabolite (TRYCAT) ratio. Shown are path coefficients with exact p values for the inner model and t values for the indicators in the outer model
A large part of the variance in the symptoms LV (90.8%) was explained by the regression on memory LV, the Gram-negative LV, PARA/TRANS, E-cadherin (all positively), and IgM MDA (inversely); 53.3% of the variance in the memory LV was explained by Gram-negative LV, PARA/TRANS, CCL-11, TRYCATs, and the immune activation index. The Gram-negative LV was predicted by PARA/TRANS, while CCL-11 and TRYCATs were both predicted by the immune activation index (both positively), which in turn was predicted by IgM MDA (inversely). We found significant total effects of E-cadherin (t = 3.59, p < 0.001), CCL-11 (t = 4.68, p < 0.001), Gram-negative bacteria LV (t = 2.07, p < 0.0001), IgM MDA (t = − 4.90, p < 0.001), immune activation index (t = 3.85, p < 0.001), PARA/TRANS (t = 4.75, p < 0.001), and NOX/PRO TRYCAT ratio (t = 4.75, p < 0.001) on the symptom LV. There were significant total effects of CCL-11 (t = 5.33, p < 0.001), Gram-negative bacteria LV (t = 2.25, p = 0.024), immune activation index (t = 4.39, p < 0.001), PARA/TRANS (t = 2.77, p = 0.006), and the NOX/PRO TRYCAT ratio (t = 2.52, p = 0.012) on the memory LV. There were significant specific indirect effects of PARA/TRANS (t = 2.94, p = 0.003), Gram-negative bacteria LV (t = 2.27, p = 0.023), CCL-11 (t = 4.68, p < 0.001), TRYCATs (t = 2.50, p = 0.013), and immune activation index (t = 3.85, p < 0.001) on the symptoms LV, which were all mediated by the memory LV.
Discussion
The first major finding of this study is that the PARA/TRANS ratio (i.e., increased IgM responses to zonulin plus occludin versus talin plus actin plus viculin) and IgM responses to occludin are positively associated with deficit schizophrenia versus non-deficit schizophrenia and controls. Table 6 summarizes the major findings of this study with regard to the schizophrenia diagnoses. The distance in the PARA/TRANS ratio between deficit schizophrenia and controls was 1.35 standard deviations and between deficit and non-deficit schizophrenia 0.65 standard deviations. The appearance of IgM antibodies to occludin in the peripheral blood of patients with deficit schizophrenia may indicate damage to the TJ system and breakdown of occludin TJs leading to an upregulated intestinal paracellular pathway [34]. Moreover, we found that the increased PARA/TRANS ratio was significantly associated with increased IgA responses to Gram-negative bacteria, suggesting that an upregulated paracellular leak pathway allowed ingress of these pathogenic bacteria leading to breakdown of oral tolerance. Occludin is one of the key transmembrane proteins of the TJs that contribute to TJ stability, maintenance of selective paracellular permeability, and TJ integrity [60,61,62,63,64,65]. TJ strands from adjacent epithelial cells associate with strands from the opposing membrane to form a TJ fence, and occludin is together with claudin a main functional component of this TJ fence [66]. Increased expression of occludin in cultured Madin-Darby canine kidney (MDCK) cells elevates transepithelial resistance of these cells, whereas introduction of truncated occludin into MDCK cells increases paracellular leakage of small molecules [67, 68]. Moreover, mice lacking occludin show a complex phenotype characterized by chronic inflammation of the gastric epithelium, growth retardation, male sterility, testicular atrophy, and calcifications in the brain [66]. A leaky paracellular pathway is involved in autoimmune and inflammatory disorders, including celiac disease, diabetes mellitus type 1, and inflammatory bowel disease [6, 7, 69].
Another finding is that IgM antibodies to E-cadherin are significantly and positively associated with deficit versus non-deficit schizophrenia and explain part of the variance in negative symptoms and psychomotor retardation. Cadherin molecules are core components of AJs (or intermediate junctions) that give stability, mediate cell-cell adhesion, and regulate the turnover of the actin cytoskeleton through binding of catenins [70,71,72,73]. AJs are important for the integrity of the epithelium and tissue morphogenesis, and they mediate transcriptional regulation and intracellular signaling [73]. Moreover, we also detected that there was a weak albeit significant association of IgM antibodies to the cytoskeletal proteins talin and actin and deficit schizophrenia, although the increased PARA/TRANS ratio indicates that the paracellular pathway is significantly more upregulated than the transcellular pathway. Actin is a key component in cellular functions including regulation of transcription and maintenance of cell structure, polarity, and shape [74], while talin is another cytoskeletal molecule than links integrins to the actin cytoskeleton and functions in the attachment of microfilaments to the plasma membrane [75]. TJs are closely associated with the actin cytoskeleton, whilst occludin may regulate the structural maintenance of the actin cytoskeleton in endothelial cells [76, 77]. Therefore, it is plausible that the minor changes in the cytoskeleton observed in this study are secondary to degradation of the TJs (and AJs), which in part regulate the cytoskeleton.
The third major finding of this study is that IgM responses to zonulin were significantly and positively associated with schizophrenia (as compared with controls) and with GIS, indicating that zonulin may play a role in schizophrenia and GIS symptoms in that disorder. The expression of zonulin in plasma is elevated in several autoimmune disorders and neurodegenerative disorders, including autoimmune disorders, diabetes mellitus type 1, and celiac disease [6]. In fact, the latter author concluded that plasma levels of zonulin may be employed as a biomarker of impaired TJ functions for these disorders. Nevertheless, the assay of plasma zonulin levels is not very reproducible, whereas measurements of plasma antibodies directed to zonulin may be more reliable and sensitive to measure zonulin status [34]. Zonulin may displace zona occludens proteins from the TJ complex thereby modulating the permeability of TJs [6], and therefore, this molecule was called the doorway to inflammation, autoimmunity, and cancer [6]. We reported that schizophrenia is accompanied by increased Hp-2 gene frequency, Hp 2-2 phenotype, and increased Hp levels [1]. Also, Wan et al. [78] reported increased Hp levels in schizophrenia and a significant association between the Hp gene and schizophrenia. Because pre-Hp-2 is similar to zonulin [6], it is safe to hypothesize that Hp-2 carriers are more prone to develop schizophrenia [1] through effects on zonulin with consequent breakdown of the TJs.
Nevertheless, it should be underscored that zonulin levels are affected not only by the Hp-2 gene but also by gliadin and other bacterial toxins, which may induce the release of zonulin and consequently displace the zonula occludens, thereby opening the paracellular leak pathway, which allows pathogens, bacterial antigens, and proteins to be translocated into the plasma [6]. In this respect, it is interesting to note that gluten sensitivity, antigliadin antibodies, and celiac disease may play a role in schizophrenia [79,80,81] and that gut dysbiosis may occur in some patients with schizophrenia [82]. However, increased levels of pro-inflammatory cytokines, including IL-6, IFN-γ, and TNF-α, cause gut barrier dysfunctions and disassemble TJs and AJs, thereby upregulating the paracellular leak pathway [83,84,85,86]. Thus, the primary IRS during acute episodes of schizophrenia may affect the apical junction complex (TJs and AJs) especially in Hp-2 carriers and in people with lowered natural IgM defenses against immune-inflammatory injuries. The consequent upregulation of the paracellular leak pathway with increased translocation of Gram-negative bacteria (and possibly undigested food macromolecules and other microbiota) may, in turn, contribute to the ongoing immune-inflammatory response.
The fourth major finding of this study is that the upregulated paracellular leak pathway and increased bacterial translocation coupled with lower IgM to MDA, immune activation, and increased CCL-11 and noxious TRYCAT levels are, together, strong predictors of deficit schizophrenia (versus non-deficit schizophrenia and controls), negative symptoms, psychosis, excitation, psychomotor retardation, FTD, and memory impairments. A large part of the variance (90.8%) in negative and PHEM (psychosis, hostility, excitation, and mannerism) symptoms was explained by Gram-negative bacteria, PARA/TRANS ratio, E-cadherin, IgM to MDA, and memory dysfunctions, which in turn were predicted (53.3% of the variance) by an increased PARA/TRANS ratio, Gram-negative bacteria, CCL-11, TRYCATs, and immune activation. One mechanistic explanation is that breakdown of TJs and AJs with increased bacterial translocation may induce different neuro-immune pathways that ultimately cause neuroprogression. We have reviewed elsewhere the mechanism by which gut microbiota, CCL-11, some pro-inflammatory cytokines, and noxious TRYCATs may cause cognitive decline and impairments in long-term memory performance [14,15,16,17, 25]. Interestingly, based on our new cognitive model of schizophrenia [15,16,17], we found that the effects of an upregulated paracellular pathway with increased bacterial translocation are at least in part mediated by memory deficits. As such, the upregulated paracellular leak pathway in deficit schizophrenia may aggravate the cognitive decline in semantic and episodic memory and, as a consequence, schizophrenia symptomatology. Nevertheless, our PLS analysis showed that different pathways may lead to schizophrenia phenomenology: (a) the paracellular path with bacterial translocation; (b) immune activation with increased TRYCAT and eotaxin production; and (c) lowered natural IgM, which aggravates the effects of the first two paths. These findings also suggest that the paracellular leak path may affect other (unknown) pathways that were not assayed in our study comprising other cytokines and microbiota and food hypersensitivities (including gliadin and gluten).
The results of the current study should be interpreted with regard to its limitations. Firstly, this is a cross-sectional study, which precludes the establishment of firm causal inferences. Secondly, while the associations of the PARA/TRANS ratio with deficit schizophrenia and its phenomenology are highly significant, the IgM antibody data to PARA/TRANS proteins appear to be useful as disease markers when combined with other biomarkers of schizophrenia. Furthermore, the strong associations between IgM to MDA (an established natural IgM to an oxidation-associated neo-determinant) and IgM to PARA/TRANS proteins suggest that the latter reflects two phenomena, namely natural IgM and induced IgM responses secondary to possible damage to the paracellular proteins. It is well established that IgM antibodies to MDA have housekeeping properties protecting against uncontrolled inflammation [87], while also IgM to various proteins may have protective properties [88], e.g., IgM responses to tubulin (another cytoskeleton protein) in neuroinflammatory disorders [89].
In conclusion, we found that the paracellular leak pathway was upregulated in deficit schizophrenia and that mainly TJs (but also AJs) contribute to this phenomenon. An increased paracellular pathway and associated translocation of Gram-negative bacteria and lower IgM to MDA coupled with immune activation and increased CCL-11 and TRYCATs predict memory dysfunctions (including FTD), while the latter coupled with an upregulated paracellular leak pathway, Gram-negative bacteria, and lowered natural IgM predict schizophrenia symptomatology comprising negative and PHEM symptoms. The results suggest that breakdown of the TJs and dysfunctions in the intestinal paracellular route contribute to the phenomenology of deficit schizophrenia.
References
Maes M, Delanghe J, Bocchio Chiavetto L, Bignotti S, Tura GB, Pioli R, Zanardini R, Altamura CA (2001) Haptoglobin polymorphism and schizophrenia: Genetic variation on chromosome 16. Psychiatry Res 104(1):1–9
Ritzmann SE, Daniels JC (1976) Haptoglobin, in serum protein abnormalities: Diagnostic and clinical aspects. Little, Brown, Boston
Dalan R, Liew H, Goh LL, Gao X, Chew DE, Boehm BO, Leow MK (2016) The haptoglobin 2-2 genotype is associated with inflammation and carotid artery intima-media thickness. Diab Vasc Dis Res 13(5):373–376
Lazalde B, Huerta-Guerrero HM, Simental-Mendía LE, Rodríguez-Morán M, Guerrero-Romero F (2014) Haptoglobin 2-2 genotype is associated with TNF-α and IL-6 levels in subjects with obesity. Dis Markers 2014:912756
Marvasti TB, Moody AR, Singh N, Maraj T, Tyrrell P, Afshin M (2017) Haptoglobin 2-2 genotype is associated with presence and progression of MRI depicted atherosclerotic intraplaque hemorrhage. Int J Cardiol Heart Vasc 18:96–100
Fasano A (2012) Zonulin, regulation of tight junctions, and autoimmune diseases. Ann N Y Acad Sci 1258:25–33
Fasano A (2012) Leaky gut and autoimmune diseases. Clin Rev Allergy Immunol 42(1):71–78
Smith RS, Maes M (1995) The macrophage-T-lymphocyte theory of schizophrenia: Additional evidence. Med Hypotheses 45:135–141
Noto MN, Maes M, Nunes SO, Ota VK, Rossaneisf AC, Verri Jr WA, Cordeiro Q, Belangero SI et al (2018) Activation of the immune-inflammatory response system and the compensatory immune-regulatory reflex system in antipsychotic naive first episode psychosis. Preprints Preprints 201809.0314.v2.
Roomruangwong C, Noto C, Kanchanatawan B, Anderson G, Kubera M, Carvalho AF, Maes M (2018) The role of aberrations in the immune-inflammatory response system (IRS) and the compensatory immune-regulatory reflex system (CIRS) in different phenotypes of schizophrenia: The IRS-CIRS theory of schizophrenia. Preprint, September 2018. https://doi.org/10.20944/preprints201809.0289.v1
Maes M, Carvalho AF (2018) The compensatory immune-regulatory reflex system (CIRS) in depression and bipolar disorder. Mol Neurobiol 55(12):8885–8903
Davis J, Moylan S, Harvey BH, Maes M, Berk M (2014) Neuroprogression in schizophrenia: Pathways underpinning clinical staging and therapeutic corollaries. Aust N Z J Psychiatry 48:512–529
Davis J, Eyre H, Jacka FN, Dodd S, Dean O, McEwen S, Debnath M, McGrath J et al (2016) A review of vulnerability and risks for schizophrenia: Beyond the two hit hypothesis. Neurosci Biobehav Rev 65:185–194
Kanchanatawan B, Hemrungrojn S, Thika S, Sirivichayakul S, Ruxrungtham K, Carvalho AF, Geffard M, Anderson G et al (2018) Changes in tryptophan catabolite (TRYCAT) pathway patterning are associated with mild impairments in declarative memory in schizophrenia and deficits in semantic and episodic memory coupled with increased false-memory creation in deficit schizophrenia. Mol Neurobiol 55(6):5184–5201
Sirivichayakul S, Kanchanatawan B, Thika S, Carvalho AF, Maes M (2018) A new schizophrenia model: Immune activation is associated with induction of different neurotoxic products which together determine memory impairments and schizophrenia symptom dimensions. CNS Neurol Disord Drug Targets. https://doi.org/10.2174/1871527317666181119115532
Sirivichayakul S, Kanchanatawan B, Thika S, Carvalho AF, Maes M (2019) Eotaxin, an endogenous cognitive deteriorating chemokine (ECDC), is a major contributor to cognitive decline in Normal people and to executive, memory, and sustained attention deficits, formal thought disorders, and psychopathology in schizophrenia patients. Neurotox Res 35(1):122–138
Maes M, Kanchanatawan B, Sirivichayakul S, Carvalho AF (2018) In schizophrenia, deficits in natural IgM isotype antibodies including those directed to malondialdehyde and azelaic acid strongly predict negative symptoms, neurocognitive impairments, and the deficit syndrome. Mol Neurobiol. https://doi.org/10.1007/s12035-018-1437-6
Binder CJ (2012) Naturally occurring IgM antibodies to oxidation-specific epitopes. Adv Exp Med Biol 750:2–13
Weismann D, Binder CJ (2012) The innate immune response to products of phospholipid peroxidation. Biochim Biophys Acta 1818:2465–2475
Díaz-Zaragoza M, Hernández-Ávila R, Viedma-Rodríguez R, Arenas-Aranda D, Ostoa-Saloma P (2015) Natural and adaptive IgM antibodies in the recognition of tumor-associated antigens of breast cancer (review). Oncol Rep 34:1106–1114
Thiagarajan D, Frostegård AG, Singh S, Rahman M, Liu A, Vikström M, Leander K, Gigante B, Hellenius ML, Zhang B, Zubarev RA, de Faire U, Lundström SL, Frostegård J (2016) Human IgM antibodies to malondialdehyde conjugated with albumin are negatively associated with cardiovascular disease among 60-year-olds. J Am Heart Assoc 5(12). https://doi.org/10.1161/JAHA.116.004415
McMahon M, Skaggs B (2016) Autoimmunity: Do IgM antibodies protect against atherosclerosis in SLE? Nat Rev Rheumatol 12:442–444
Aziz M, Holodick NE, Rothstein TL, Wang P (2015) The role of B-1 cells in inflammation. Immunol Res 63:153–166
Roomruangwong C, Barbosa DS, de Farias CC, Matsumoto AK, Baltus THL, Morelli NR, Kanchanatawan B, Duleu S et al (2018) Natural regulatory IgM-mediated autoimmune responses directed against malondialdehyde regulate oxidative and nitrosative pathways and coupled with IgM responses to nitroso adducts attenuate depressive and physiosomatic symptoms at the end of term pregnancy. Psychiatry Clin Neurosci 72:116–130
Maes M, Kanchanatawan B, Sirivichayakul S, Carvalho AF (2018) In schizophrenia, increased plasma IgM/IgA responses to gut commensal Bacteria are associated with negative symptoms, neurocognitive impairments, and the deficit phenotype. Neurotox Res 35:684–698. https://doi.org/10.1007/s12640-018-9987-y
Rothstein TL, Griffin DO, Holodick NE, Quach TD, Kaku H (2013) Human B-1 cells take the stage. Ann N Y Acad Sci 1285:97–114
Lucas K, Maes M (2013) Role of the toll like receptor (TLR) radical cycle in chronic inflammation: Possible treatments targeting the TLR4 pathway. Mol Neurobiol 48:190–204
Braniste V, Al-Asmakh M, Kowal C, Anuar F, Abbaspour A, Tóth M, Korecka A, Bakocevic N et al (2014) The gut microbiota influences blood-brain barrier permeability in mice. Sci Transl Med 6(263):263ra158
Zakaria R, Wan Yaacob WM, Othman Z, Long I, Ahmad AH, Al-Rahbi B (2017) Lipopolysaccharide-induced memory impairment in rats: A model of Alzheimer’s disease. Physiol Res 66:553–565
Muraca M, Putignani L, Fierabracci A, Teti A, Perilongo G (2015) Gut microbiota-derived outer membrane vesicles: Under-recognized major players in health and disease? Discov Med 19:343–348
Anand D, Chaudhuri A (2016) Bacterial outer membrane vesicles: New insights and applications. Mol Membr Biol 33:125–137
Ellis TN, Kuehn MJ (2010) Virulence and immunomodulatory roles of bacterial outer membrane vesicles. Microbiol Mol Biol Rev 74:81–94
Ellis TN, Leiman SA, Kuehn MJ (2010) Naturally produced outer membrane vesicles from Pseudomonas aeruginosa elicit a potent innate immune response via combined sensing of both lipopolysaccharide and protein components. Infect Immun 78:3822–3831
Vojdani A, Vojdani E (2019) Food-associated autoimmunities: When food turns your immune system against you. In press
Maes M, Mihaylova I, Leunis JC (2007) Increased serum IgA and IgM against LPS of enterobacteria in chronic fatigue syndrome (CFS): Indication for the involvement of gram-negative enterobacteria in the etiology of CFS and for the presence of an increased gut-intestinal permeability. J Affect Disord 99(1–3):237–240
Maes M, Kubera M, Leunis JC (2008) The gut-brain barrier in major depression: Intestinal mucosal dysfunction with an increased translocation of LPS from gram negative enterobacteria (leaky gut) plays a role in the inflammatory pathophysiology of depression. Neuro Endocrinol Lett 29(1):117–124
Kirkpatrick B, Buchanan RW, McKenney PD, Alphs LD, Carpenter WT Jr (1989) The Schedule for the Deficit Syndrome: An instrument for research in schizophrenia. Psychiatry Res 30:119–123
Kittirathanapaiboon P, Khamwongpin M (2005) The validity of the Mini international neuropsychiatric interview (M.I.N.I.) Thai version. J Ment Health Thailand 13(3):125–135
Kay SR, Fiszbein A, Opler LA (1987) The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr Bull 13:261–276
Andreasen NC (1989) The Scale for the Assessment of Negative Symptoms (SANS): Conceptual and theoretical foundations. Br J Psychiatry Suppl 7:49–58
Overall JE, Gorham DR (1962) The Brief Psychiatric Rating Scale. Psychol Rep 10:799–812
Hamilton M (1960) A rating scale for depression. J Neurol Neurosurg Psychiatry 23:56–62
Hamilton M (1959) The assessment of anxiety states by rating. Br J Med Psychol 32(1):50–55
Zachrisson O, Regland B, Jahreskog M, Kron M, Gottfries CG (2002) A rating scale for fibromyalgia and chronic fatigue syndrome (the FibroFatigue scale). J Psychosom Res 52(6):501–509
Kanchanatawan B, Thika S, Sirivichayakul S, Carvalho AF, Geffard M, Maes M (2018) In schizophrenia, depression, anxiety, and Physiosomatic symptoms are strongly related to psychotic symptoms and excitation, impairments in episodic memory, and increased production of neurotoxic tryptophan catabolites: A multivariate and machine learning study. Neurotox Res 33(3):641–655
CERAD (1986) CERAD—An overview: The consortium to establish a registry for Alzheimer’s disease; http://cerad.mc.duke.edu/. Accessed 4 Apr 2019
Duleu S, Mangas A, Sevin F, Veyret B, Bessede A, Geffard M (2010) Circulating antibodies to IDO/THO pathway metabolites in Alzheimer’s disease. Int J Alzheimers Dis 15:2010
Roomruangwong C, Kanchanatawan B, Sirivichayakul S, Anderson G, Carvalho AF, Duleu S, Geffard M, Maes M (2017) IgA/IgM responses to tryptophan and tryptophan catabolites (TRYCATs) are differently associated with prenatal depression, physio-somatic symptoms at the end of term and premenstrual syndrome. Mol Neurobiol 54(4):3038–3049
Roomruangwong C, Kanchanatawan B, Carvalho AF, Sirivichayakul S, Duleu S, Geffard M, Maes M (2018) Body image dissatisfaction in pregnant and non-pregnant females is strongly predicted by immune activation and mucosa-derived activation of the tryptophan catabolite (TRYCAT) pathway. World J Biol Psychiatry 19:200–209
Kanchanatawan B, Sirivichayakul S, Ruxrungtham K, Carvalho AF, Geffard M, Ormstad H, Anderson G, Maes M (2018) Deficit, but not nondeficit, schizophrenia is characterized by mucosa-associated activation of the tryptophan catabolite (TRYCAT) pathway with highly specific increases in IgA responses directed to picolinic, xanthurenic, and quinolinic acid. Mol Neurobiol 55(2):1524–1536
Daverat P, Geffard M, Orgogozo JM (1989) Identification and characterization of anti-conjugated azelaic acid antibodies in multiple sclerosis. J Neuroimmunol 22(2):129–134
Boullerne A, Petry KG, Geffard M (1996) Circulating antibodies directed against conjugated fatty acids in sera of patients with multiple sclerosis. J Neuroimmunol 65(1):75–81
Amara A, Constans J, Chaugier C, Sebban A, Dubourg L, Peuchant E, Pellegrin JL, Leng B et al (1995) Autoantibodies to malondialdehyde-modified epitope in connective tissue diseases and vasculitides. Clin Exp Immunol 101(2):233–238
Faiderbe S, Chagnaud JL, Geffard M (1992) Anti-phosphoinositide auto-antibodies in sera of cancer patients: Isotypic and immunochemical characterization. Cancer Lett 66(1):35–41
Geffard M, Bodet D, Dabadie MP, Arnould L (2003) Identification of antibodies in sera of breast cancer patients. Immuno-Analyse & Biologie Special 18:248–253
Boullerne AI, Petry KG, Meynard M, Geffard M (1995) Indirect evidence for nitricoxide involvement in multiple sclerosis by characterization of circulating antibodies directed against conjugated S-nitrosocysteine. J Neuroimmunol 60(1–2):117–124
Boullerne AI, Rodriguez JJ, Touil T, Brochet B, Schmidt S, Abrous ND, Le Moal M, Pua JR et al (2002) Anti-S-nitrosocysteine antibodies are a predictive marker for demyelination in experimental autoimmune encephalomyelitis: Implications for multiple sclerosis. J Neurosci 22(1):123–132
Ringle CM, da Silva D, Bido D (2014) Structural equation modeling with the SmartPLS. Brazilian Journal of Marketing—BJM Revista Brasileira de Marketing—ReMark Edição Especial 13(2):56–73
Cepeda-Carrion G, Cegarra-Navarro J-G, Cillo V (2018) Tips to use partial least squares structural equation modelling (PLS-SEM) in knowledge management. J Knowl Manag 23:67–89. https://doi.org/10.1108/JKM-05-2018-0322
Yu AS, McCarthy KM, Francis SA, McCormack JM, Lai J, Rogers RA, Lynch RD, Schneeberger EE (2005) Knockdown of occludin expression leads to diverse phenotypic alterations in epithelial cells. Am J Physiol Cell Physiol 288(6):C1231–C1241
Suzuki T, Elias BC, Seth A, Shen L, Turner JR, Giorgianni F, Desiderio D, Guntaka R et al (2009) PKC eta regulates occludin phosphorylation and epithelial tight junction integrity. Proc Natl Acad Sci U S A 106(1):61–66
Elias BC, Suzuki T, Seth A, Giorgianni F, Kale G, Shen L, Turner JR, Naren A et al (2009) Phosphorylation of Tyr-398 and Tyr-402 in occludin prevents its interaction with ZO-1 and destabilizes its assembly at the tight junctions. J Biol Chem 284(3):1559–1569
Furuse M, Hirase T, Itoh M, Nagafuchi A, Yonemura S, Tsukita S, Tsukita S (1993) Occludin: A novel integral membrane protein localizing at tight junctions. J Cell Biol 123(6 Pt 2):1777–1788
Balda MS, Matter K (2000) Transmembrane proteins of tight junctions. Semin Cell Dev Biol 11(4):281–289
Cummins PM (2012) Occludin: One protein, many forms. Mol Cell Biol 32(2):242–250
Saitou M, Furuse M, Sasaki H, Schulzke JD, Fromm M, Takano H, Noda T, Tsukita S (2000) Complex phenotype of mice lacking occludin, a component of tight junction strands. Mol Biol Cell 11(12):4131–4142
McCarthy KM, Skare IB, Stankewich MC, Furuse M, Tsukita S, Rogers RA, Lynch RD, Schneeberger EE (1996) Occludin is a functional component of the tight junction. J Cell Sci 109(Pt 9):2287–2298
Balda MS, Whitney JA, Flores C, González S, Cereijido M, Matter K (1996) Functional dissociation of paracellular permeability and transepithelial electrical resistance and disruption of the apical-basolateral intramembrane diffusion barrier by expression of a mutant tight junction membrane protein. J Cell Biol 134(4):1031–1049
Edelblum KL, Turner JR (2009) The tight junction in inflammatory disease: Communication breakdown. Curr Opin Pharmacol 9(6):715–720
van Roy F, Berx G (2008) The cell-cell adhesion molecule E-cadherin. Cell Mol Life Sci 65(23):3756–3788
Gomez GA, McLachlan RW, Wu SK, Caldwell BJ, Moussa E, Verma S, Bastiani M, Priya R et al (2015) An RPTPα/Src family kinase/Rap1 signaling module recruits myosin IIB to support contractile tension at apical E-cadherin junctions. Mol Biol Cell 26(7):1249–1262
Brüser L, Bogdan S (2017) Adherens junctions on the move-membrane trafficking of E-cadherin. Cold Spring Harb Perspect Biol 1:9(3)
Hartsock A, Nelson WJ (2008) Adherens and tight junctions: Structure, function and connections to the actin cytoskeleton. Biochim Biophys Acta 1778(3):660–669
Dominguez R, Holmes KC (2011) Actin structure and function. Annu Rev Biophys 40:169–186
Burridge K, Connell L (1983) Talin: A cytoskeletal component concentrated in adhesion plaques and other sites of actin-membrane interaction. Cell Motil 3(5–6):405–417
Balda MS, Matter K (1989) Tight junctions. J Cell Sci 111(Pt 5):541–547
Kuwabara H, Kokai Y, Kojima T, Takakuwa R, Mori M, Sawada N (2001) Occludin regulates actin cytoskeleton in endothelial cells. Cell Struct Funct 26(2):109–116
Wan C, La Y, Zhu H, Yang Y, Jiang L, Chen Y, Feng G, Li H et al (2007) Abnormal changes of plasma acute phase proteins in schizophrenia and the relation between schizophrenia and haptoglobin (Hp) gene. Amino Acids 32(1):101–108
Dohan FC, Grasberger JC (1973) Relapsed schizophrenics: Earlier discharge from the hospital after cereal-free, milk-free diet. Am J Psychiatry 130(6):685–688
Ergün C, Urhan M, Ayer A (2018) A review on the relationship between gluten and schizophrenia: Is gluten the cause? Nutr Neurosci 21(7):455–466
Rowland LM, Demyanovich HK, Wijtenburg SA, Eaton WW, Rodriguez K, Gaston F, Cihakova D, Talor MV et al (2017) Antigliadin antibodies (AGA IgG) are related to neurochemistry in schizophrenia. Front Psych 8:104
Nguyen TT, Kosciolek T, Maldonado Y, Daly RE, Martin AS, McDonald D, Knight R, Jeste DV (2019) Differences in gut microbiome composition between persons with chronic schizophrenia and healthy comparison subjects. Schizophr Res 204:23–29. https://doi.org/10.1016/j.schres.2018.09.014
Al-Sadi R, Ye D, Boivin M, Guo S, Hashimi M, Ereifej L, Ma TY (2014) Interleukin-6 modulation of intestinal epithelial tight junction permeability is mediated by JNK pathway activation of claudin-2 gene. PLoS One 9(3):e85345
Al-Sadi R, Boivin M, Ma T (2009) Mechanism of cytokine modulation of epithelial tight junction barrier. Front Biosci (Landmark Ed) 14:2765–2778
Utech M, Mennigen R, Bruewer M (2010) Endocytosis and recycling of tight junction proteins in inflammation. J Biomed Biotechnol 2010:484987
Bruewer M, Samarin S, Nusrat A (2006) Inflammatory bowel disease and the apical junctional complex. Ann N Y Acad Sci 1072:242–252
Grönwall C, Vas J, Silverman GJ (2012) Protective roles of natural IgM antibodies. Front Immunol 3:66
Sokoloff AV, Bock I, Zhang G, Hoffman S, Dama J, Ludtke JJ, Cooke AM, Wolff JA (2001) Specific recognition of protein carboxy-terminal sequences by natural IgM antibodies in normal serum. Mol Ther 3(6):821–830
Manfredini E, Nobile-Orazio E, Allaria S, Scarlato G (1995) Anti-alpha- and beta-tubulin IgM antibodies in dysimmune neuropathies. J Neurol Sci 133(1–2):79–84
Acknowledgements
The study was supported by the Asahi Glass Foundation, Chulalongkorn University Centenary Academic Development Project and Ratchadapiseksompotch Funds, Faculty of Medicine, Chulalongkorn University, grant numbers RA60/042 (to BK) and RA61/050 (to MM).
Author information
Authors and Affiliations
Contributions
All the contributing authors have participated in the manuscript. MM and BK designed the study. BK recruited patients and completed diagnostic interviews and rating scale measurements. MM carried out the statistical analyses. All authors (BK, MM, SS, and AV) contributed to interpretation of the data and writing of the manuscript. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors have no conflict of interest with any commercial or other association in connection with the submitted article.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Maes, M., Sirivichayakul, S., Kanchanatawan, B. et al. Upregulation of the Intestinal Paracellular Pathway with Breakdown of Tight and Adherens Junctions in Deficit Schizophrenia. Mol Neurobiol 56, 7056–7073 (2019). https://doi.org/10.1007/s12035-019-1578-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-019-1578-2