Abstract
The neonatal exposure to general anesthetics has been associated with neuronal apoptosis and dendritic spines morphologic changes in the developing brain. Ketamine, a noncompetitive N-methyl-D-aspartate (NMDA) receptor antagonist, is widely used in pediatric patients to induce general anesthesia, analgesia, and perioperative sedation. In the present study, we investigated short- and long-term effects of a single ketamine (20 mg/kg, s.c.) neonatal exposure at postnatal day 7 in rats on the hippocampal and frontal cortical cellular viability. Additionally, putative neurochemical alterations and neurobehavioral impairments were evaluated in the adulthood. Ketamine neonatal administration selectively decreased cellular viability in the hippocampus, but not in the frontal cortex, 24 h after the treatment. Interestingly, a single ketamine neonatal exposure prevented the vulnerability to glutamate-induced neurotoxicity in the frontal cortex of adult rats. No short- or long-term damage to cellular membranes, as an indicative of cell death, was observed in hippocampal or cortical slices. However, ketamine induced a long-term increase in hippocampal glutamate uptake. Regarding behavioral analysis, neonatal ketamine exposure did not alter locomotor activity and anxiety-related parameters evaluated in the open-field test. However, ketamine administration disrupted the hippocampal-dependent object recognition ability of adult rats, while improved the motor coordination addressed on the rotarod. These findings indicate that a single neonatal ketamine exposure induces a short-term reduction in the hippocampal, but not in cortical, cellular viability, and long-term alterations in hippocampal glutamate transport, improvement on motor performance, and short-term recognition memory impairment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The acute and long-term consequences of children exposure to general anesthetics remain a debatable issue. On one side, there is the concern regarding the potential risks of anesthetic-induced brain damage, mainly in early stages of central nervous system (CNS) development (Davidson 2011). On the other hand, it is noted that the use of anesthetics in major surgery is obligatory and beneficial against pain and stress, which are also per se harmful to neurodevelopment (Yan and Jiang 2014). Preclinical studies have associated the exposure to these drugs with some degree of neuronal apoptosis and changes in morphology of dendritic spines in the developing brain (Briner et al. 2010; Yon et al. 2005). Additionally, clinical evidence shows that children up to 2 years old subjected to procedures requiring anesthesia could display some persistent neurobehavioral impairments such as attention-deficit//hyperactivity disorder and learning disabilities (Flick et al. 2011; Sprung et al. 2012). These effects have been reported for most of the volatile anesthetics as well as for ketamine and propofol (Davidson 2011).
Ketamine (6)-2-(2-chlorophenyl)-2-(methylamino)cyclohexanone, a noncompetitive glutamate N-methyl-D-aspartate (NMDA) receptor antagonist, is widely used in pediatric patients to induce general anesthesia, analgesia, and perioperative sedation (Yan and Jiang 2014). Ikonomidou et al. (1999) first described the link between NMDA receptor antagonism and neurotoxicity in the early stages of CNS development. NMDA receptors are involved in the regulation of dendritic growth, neuronal development, and circuit establishment by mediating synaptic activity (Gambrill and Barria 2011).
Current findings suggest that ketamine-induced neurotoxicity presents a dose- and time-dependent profile. For instance, apoptosis was found in the frontal cortex and hippocampus of postnatal day (PND) 7 rats that received 5 or 6 ketamine injections at a dose of 20 mg/kg (Soriano et al. 2010; Zou et al. 2009). Moreover, persistent cognitive deficits were observed both in PND7 rats subjected to repetitive administration of ketamine and in PNDs 5 and 6 Rhesus monkeys exposed to intravenous ketamine anesthesia for 24 h (Huang et al. 2012; Paule et al. 2011). However, the effects of a single dose of ketamine on the CNS in development and its possible long-term functional and neurobehavioral impairments remain unclear.
Therefore, this study aimed to investigate the cellular viability, functionality, and putative susceptibility to glutamate excitotoxicity of hippocampus and frontal cortex of neonatal rats (PND7) exposed to a single dose of ketamine (20 mg/kg) in a short- and long-term evaluation. In addition, neurobehavioral parameters related to anxiety, spatial short-term memory, and locomotor activity were evaluated in adulthood.
Material and Methods
Animals
All experimental procedures involving the animals were performed in accordance with National Institute of Health Guide for the Care and Use of Laboratory Animals (NIH Publications, 8th edition, 2011) and were designed to minimize suffering and limit the number of animals used. The experiments were performed after approval of the protocol by the local Institutional Ethics Committee for Animal Research (CEUA/UFSC PP955). Dams were monitored, and the day of birth of pups noted. Wistar rat pups (from the Federal University of Santa Catarina breeding colony) were randomly assigned to treatment groups and were maintained with their dams in sets of four females and four males at PND2. On PND7, rats were randomly assigned to saline or ketamine groups. For all experimental interventions, care was taken to minimize the duration of maternal separation and handling of pups, and this was the same for both control and treatment groups. The animals were maintained in an air-conditioned room at 22 ± 2 °C on a 12 h light/dark cycle (lights on at 7:00 a.m.). Rats were housed to a maximum of 6 in plastic cages with food and water ad libitum, with pups weaned into same-sex cages at PND21, and maintained until PND65. All manipulations were carried out between 9:00 and 16:00 h.
Experimental Design
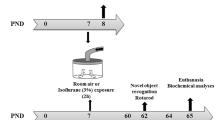
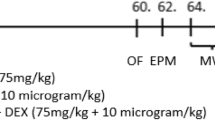
The experimental protocol conducted in the present study is summarized in Fig. 1. Rats were divided in two groups, saline and ketamine. Animals at PND7 received saline (0.9% NaCl, vehicle, 10 mL/kg) or ketamine (20 mg/kg, 10 mL/kg) by subcutaneous route using a 0.3-mL syringe with a 30-gauge needle. Animals were returned to their dam after injection. Two protocols were carried out independently: short- and long-term protocols. In the short-term protocol (n = 3–4 animals per group), the rats were killed by decapitation 24 h after saline or ketamine injection (PND8) (Fig. 1a). Moreover, rats from long-term protocol (10 animals per group) were maintained with their dam until weaning and subjected from PND60 to behavioral tests. At PND65, the animals were killed by decapitation (Fig. 1b). Hippocampi and frontal cortices were removed in both protocols and the biochemical analyses were performed.
Schematic representation of experimental procedure protocols. Rats at PND7 received saline or ketamine at a dose of 20 mg/kg by subcutaneous route. a Short-term protocol: biochemical analyses in hippocampal and frontal cortical slices were performed 24 h after the ketamine treatment (PND8). b Long-term protocol: behavioral tests began at PND60. At PND65, biochemical assays were carried out in the hippocampal and frontal cortical slices
Chemicals
(RS)-2-(2-chlorophenyl)-2-methylamino-cyclohexan-1-one (ketamine hydrochloride) and 3-(4,5-dimethylthiazol-2-yl)-2,5 diphenyltetrazolium bromide (MTT) were obtained from Sigma-Aldrich Chemical Co (MO, USA). L-[3H]glutamate (31.0 Ci/mmol) was obtained from GE-Healthcare (UK). All other reagents were of analytical grade.
Biochemical Analyses
Preparation and Incubation of Hippocampal and Frontal Cortical Slices
Hippocampi and frontal cortices were placed in an ice-cold Krebs–Ringer bicarbonate buffer (KRB) containing the following, in mM: 122 NaCl, 3 KCl, 1.2 MgSO4, 1.3 CaCl2, 0.4 KH2PO4, 25 NaHCO3, and 10 D-glucose. The buffer was bubbled with 95% O2–5% CO2 up to pH 7.4 (Dal-Cim et al. 2013). Slices (0.4 mm) were prepared using a McIlwain Tissue Chopper, separated in KRB at 4 °C, using a soft brush. Immediately after sectioning, slices were transferred to a 24-well plate and were maintained in KRB at 37 °C for 30 min to recover from slicing trauma (pre-incubation period).
Glutamate Toxicity
A previously established protocol of evaluation of glutamate toxicity in vitro was used (Ludka et al. 2016; Molz et al. 2011; Molz et al. 2009). After pre-incubation period, slices were exposed to 10 mM glutamate (in KRB) for 1 h. The medium was then removed, and slices were maintained during 4 h in nutritive incubation medium (NIM) composed of 50% KRB, 50% Dulbecco’s modified Eagle’s medium (DMEM, Gibco), 20 mM HEPES, at 37 °C in a CO2 atmosphere. The slices corresponding to the control groups were incubated only in KRB solution for 1 h and then maintained in the NIM, to preserve slice viability in a medium similar to the physiological conditions.
Cellular Viability
The ability of cells to reduce MTT was used to determine the cellular viability as previously described (Mosmann 1983). Slices from hippocampus and frontal cortex were incubated with MTT (0.5 mg/mL) in KRB for 30 min at 37 °C. The tetrazolium ring of MTT can be cleaved by active dehydrogenases to produce a precipitated formazan which reduction can be used as an index of cellular viability. The medium was withdrawn and precipitated formazan was solubilized with dimethyl sulfoxide (DMSO) and quantified spectrophotometrically at a wavelength of 540 nm.
Propidium Iodide Incorporation
Propidium iodide (PI) incorporation is a parameter to evaluate cellular membrane damage as an indicative of cell death. Cell damage was assessed by evaluating the uptake of the fluorescent exclusion dye, PI, which is a polar compound that enters only cells with damaged membranes. Once inside the cells, PI complexes with DNA and emits an intense red fluorescence (630 nm) when excited by green light (495 nm). Hippocampal and frontal cortical slices were prepared and incubated with PI (7 μg/mL) for 30 min at 37 °C, and then washed with KRB for analysis on fluorescence microplate reader (TECAN) (Piermartiri et al. 2009).
L-[3H]Glutamate Uptake
L-[3H]glutamate uptake into hippocampal and frontal cortical slices was evaluated as previously described (Molz et al. 2009). Slices were prepared and incubated in KRB for 30 min at 37 °C in order to normalize to physiological conditions. Hippocampal and frontal cortical slices were then washed for 15 min at 37 °C in a Hank’s balanced salt solution (HBSS), composition in mM: 1.29 CaCl2, 136.9 NaCl, 5.36 KCl, 0.65 MgSO4, 0.27 Na2HPO4, 1.1 KH2PO4, and 5 HEPES. Uptake was assessed by adding 0.33 μCi/mL L-[3H]glutamate in the presence of 100 μM unlabeled glutamate in a final volume of 300 μL. Incubation was stopped immediately after 7 min by discarding the incubation medium, and slices were submitted to two ice-cold washes with 1 mL HBSS. Slices were solubilized by adding a solution with 0.1% NaOH/0.01% SDS and incubated overnight. Aliquots of slice lysates were taken for determination of the intracellular content of L-[3H]glutamate by scintillation counting. Sodium-independent uptake was determined by using choline chloride, instead of sodium chloride in the HBSS buffer. Unspecific sodium-independent uptake was subtracted from total uptake to obtain the specific sodium-dependent glutamate uptake. Results were obtained and presented as nmol of L-[3H]glutamate taken up per milligram of protein per minute.
Protein Measurement
Protein content was evaluated by the method of Lowry et al. (1951), using bovine serum albumin (Sigma-Aldrich Chemical Co, MO, USA) as standard.
Behavioral Tests
Behavioral tests were carried out from PND60 to PND64 according to the sequence shown in Fig. 1b. All tests were performed between 9:00 and 14:00 h, and they were scored by the same rater in an observation sound-attenuated room under low-intensity light (12 lx), where the rats had been habituated for at least 1 h before the beginning of the tests. Behavior was monitored through a video camera positioned above the apparatuses and the videos were later analyzed with the ANY Maze® video tracking system (Stoelting Co., Wood Dale, IL, USA). The apparatuses were cleaned with 10% ethanol between animals to avoid odor cues.
Open Field
Spontaneous locomotor activity was assessed in the open field apparatus. Rats were placed in the center of a wooden arena (100 × 100 cm, gray walls and gray floor) and allowed to freely explore it during 10 min. The total distance traveled and the average speed as indicators of spontaneous locomotor activity. The time spent in the center of the open field was used as a measure of anxiety-like behavior (Walsh and Cummins 1976).
Rotarod
The balance and motor coordination of rats were addressed in the accelerating rotarod apparatus (Insight Scientific Equipments, Ribeirão Preto, SP, Brazil). Rotarod apparatus consists of a grooved metal roller (6 cm in diameter) separated by 9-cm-wide compartments, elevated 16 cm. First, a habituation session was performed, in which each rat remained on the apparatus (absent rotation) for 30 s. Rats were permitted as many trials as necessary to reach this criterion. Next, a training session was performed, in which the animals had three trials to stay for 90 s on the rotating apparatus (5 rpm). Those animals that were able to perform for 90 s during the training session were chosen for the experiment. During the test session (performed 30 min after training), the starting speed was 5 rpm and it was increased by 0.1 rev/s over a maximal period of 300 s, and the latency to fall (in seconds) from the accelerating rotarod was determined (Jiang et al. 2004).
Novel Object Recognition Task
The short-term recognition memory was addressed in novel object recognition task, performed as previously described by Abe et al. (2004). The task consists of three phases: habituation, training, and test phases. The habituation phase consisted of 2 days before testing, which all rats were allowed to explore the open field arena for 15 min once a day. The habituation phase aims to reduce stress, anxiety, and environmental exploration of animals on test day. The training and testing phases occurred 24 h after the last habituation day, during 3 min each, separated by an interval of 30 min to evaluate the short-term memory. The time spent by animals investigating each object in both phases was recorded. In the training phase, rats were exposed to two identical objects (A1 and A2) for 3 min. These objects were fixed in opposite corners 20 cm away from walls and 60 cm apart from each other. In the test phase, rats were exposed for 3 min to one of the familiar objects and the other was replaced by a new object (B), which had a similar shape and size with different color.
Statistical Analysis
The results are presented as the mean ± SEM. Comparisons between saline and ketamine groups were performed by the Student’s t test. Data from cell viability evaluation were analyzed by two-way ANOVA followed by post hoc of Newman-Keuls. Additionally, we used a t test to compare the percentage of recognition against a hypothetical value of 50% in the novel object recognition task. Probability values less than 0.05 (P < 0.05) were considered as statistically significant. Statistical analysis was performed using the GraphPad Prism 6.0 (GraphPad, San Diego, CA, USA) and Statistica 13.0 software’s (StatSoft Inc., La Jolla, CA, USA).
Results
Effects of a Single Neonatal Ketamine Exposure on Cellular Viability Following In Vitro Glutamate Challenge in the Hippocampus and Frontal Cortex of Neonatal and Adult Rats
Neonatal rats (PND7) were subjected to ketamine (20 mg/kg, s.c.), and at PND8, their hippocampi and frontal cortices were obtained, sliced, and assessed for cellular viability. Moreover, the response of the brain slices to glutamate excitotoxicity also was evaluated. As expected, the hippocampal and frontal cortical slices of neonatal rats (PND8) showed tolerance to glutamate-induced excitotoxicity (Fig. 2a, b). However, two-way ANOVA revealed a per se effect of a single ketamine neonatal exposure on the cellular viability in the hippocampus of these animals [F(1,12) = 28.021; P = 0.0002]. Twenty-four hours after ketamine treatment (PND8), a reduced hippocampal cellular viability as compared to slices from saline-treated group was observed (Fig. 2a). On the other hand, cellular viability of the frontal cortical slices was altered by neither ketamine neonatal treatment nor in vitro glutamate challenge in the short-term protocol (Fig. 2b).
Cellular viability evaluation in hippocampal and frontal cortical slices from neonatal and adult rats subjected to a single ketamine neonatal exposure. Cellular viability at PND8 of a hippocampal and b frontal cortical slices obtained from saline- or ketamine-treated rats. c Hippocampal and d frontal cortical slices viability of rats at PND65. Data are presented as means ± SEM of 4–5 animals per group. *P < 0.05 as compared to control group (two-way ANOVA followed by post hoc of Newman-Keuls)
In contrast, hippocampal and frontal cortical slices obtained from adult rats (PND65) exhibited marked susceptibility to glutamatergic excitotoxicity (Fig. 2c, d). Thus, in vitro glutamate challenge decreased hippocampal viability in both control and ketamine-treated groups [F(1,12) = 30.375; P = 0.00005] (Fig. 2c). Two-way ANOVA revealed that a single ketamine neonatal exposure did not modify the cellular toxicity evoked by glutamate on hippocampal slices of adult rats. Furthermore, glutamate also decreased the cellular viability in the frontal cortices slices from control (saline-treated) adult rats [F(1,12) = 9.279; P = 0.0082]. The neonatal ketamine treatment did not alter the cellular viability in comparison to slices from saline-treated group in PND65. However, a single ketamine neonatal exposure (PND7) attenuated the glutamate-induced decrease on cellular viability in frontal cortical slices obtained from PND65 rats (Fig. 2d).
Effects of a Single Neonatal Ketamine Exposure on PI Incorporation in the Hippocampus and Frontal Cortex of Neonatal and Adult Rats
The PI incorporation, which is a marker of damaged cellular membranes, was evaluated in hippocampal and frontal cortical slices obtained from neonatal (PND8) and adult rats (PND65), following ketamine exposure. Student’s t test indicated that a single neonatal ketamine exposure did not increase PI incorporation in both hippocampus and frontal cortex slices obtained from PND8 or PND65 rats (Fig. 3). This is suggestive that a single neonatal ketamine exposure does not come with short- or long-term gross damage in brain slices.
Evaluation of cellular membrane damage due to propidium iodide (PI) incorporation in hippocampal and frontal cortical slices from neonatal and adult rats subjected to a single ketamine neonatal exposure. PI incorporation in a hippocampal and b frontal cortical slices of rats at PND8. Cellular membrane damage evaluated in c hippocampal and d frontal cortical slices from adult rats subjected to a single ketamine neonatal exposure. Data are presented as means ± SEM of 3–5 animals per group (Student’s t test)
Effects of a Single Neonatal Ketamine Exposure on L-[3H]Glutamate Uptake in the Hippocampus and Frontal Cortex of Neonatal and Adult Rats
Figure 4 shows the effects of a single neonatal ketamine exposure on L-[3H]glutamate uptake into hippocampal and frontal cortices slices from PND8 and PND65 rats. Student’s t test indicated that ketamine neonatal exposure did not alter the Na+-dependent L-[3H]glutamate uptake in PND8 rats. Regarding adult rats, Student’s t test revealed that ketamine administration increased hippocampal Na+-dependent L-[3H]glutamate uptake [t(6) = 3.365; P = 0.015] (Fig. 4c). Ketamine also seems to alter frontal cortical L-[3H]glutamate uptake, although it did not reach statistical significance (Fig. 4d).
Evaluation of glutamate uptake into hippocampal and frontal cortical slices from adult rats subjected to a single ketamine neonatal exposure. Na+-dependent L-[3H] glutamate uptake in the a hippocampus and b frontal cortex slices of rats at PND8. c Hippocampal and d frontal cortical Na+-dependent L-[3H] glutamate uptake in slices from rats at PND65 subjected to a single ketamine neonatal exposure. Data are presented as means ± SEM of 3–5 animals per group. *P < 0.05 as compared to saline group (Student’s t test)
Long-Term Neurobehavioral Effects of a Single Neonatal Ketamine Exposure
Adult rats subjected to ketamine neonatal treatment displayed behavioral alterations on the motor performance and cognitive function. Open field test was carried out in order to verify putative changes on spontaneously locomotor activity and anxiety-like behaviors of the animals (PND60). No significant alterations were observed in the parameters analyzed: total distance traveled, mean speed, and central time (Table 1). Conversely, Student’s t test indicated significant difference in the rotarod test. A single ketamine neonatal exposure induced an increase of the latency to fall of rotarod as compared to those of the saline group (PND62) [t(18) = 2.164; P = 0.044], indicating an improvement in the balance and motor coordination (Fig. 5).
Motor performance evaluation in adult rats subjected to a single ketamine neonatal exposure. Latency to fall of rod in the rotarod test of adult rats (PND62) subjected to ketamine neonatal exposure. Data are presented as means ± SEM of 10 animals per group. *P < 0.05 as compared to saline group (Student’s t test)
Regarding to the cognitive function, short-term recognition memory was evaluated by the novel object recognition task, at PND62. As illustrated in Fig. 6a, the two identical objects (A1 and A2) were equally explored in the training phase. Remarkably, a significant difference was found between the percentage of recognition of saline group and hypothetical value of 50% in the test phase [t(6) = 3.664; P = 0.0105], indicating that these animals spent more time exploring the novel object. This difference was not observed in the ketamine group, demonstrating a short-term memory impairment induced by a single ketamine neonatal exposure (Fig. 6b).
Short-term memory evaluation in the novel object recognition task in adult rats (at PND62) subjected to a single ketamine neonatal exposure. a Exploration time of the identical objects (a1 and a2) in the training phase. b Percentage of the novel object recognition in the test phase. Data are demonstrated as means ± SEM of 6–7 animals per group. *P < 0.05 as compared to hypothetical value of 50% (Student’s t test)
Discussion
Our data revealed that a single ketamine administration (20 mg/kg, s.c.) at PND7 decreased the hippocampal cellular viability at 24 h after its administration as well as increased L-[3H]glutamate uptake in this brain structure in the adulthood (PND65). On the other hand, a single ketamine neonatal administration prevented glutamate-induced excitotoxicity in the frontal cortex of adult rats. These neural consequences of ketamine exposure were accompanied by an improvement in the balance and motor coordination and by short-term recognition memory impairment.
Slices obtained from control neonatal rats (PND8) are less susceptible to in vitro glutamate challenge than slices from adult brains, as evidenced by lack of cellular viability reduction. Glutamatergic receptors and transporter expression are developmentally regulated, and their immature expression and distribution in neonatal brain avoid excitotoxic events (Dumas 2005; Hestrin 1992; Yuan and Bellone 2013). In contrast, developing brain is particularly sensitive to agents reducing the glutamatergic neurotransmission (e.g., NMDA receptor antagonists) during synaptogenesis, also known as brain’s growth spurt (BGS) period. This period begins at mid-gestation and remains for 2 to 3 years of age in humans and happens during the first 2 to 3 weeks after birth in rodents (Liu et al. 2011; Rice and Barone 2000; Sun 2010). During the BGS period, NMDA receptors are overexpressed and pyramidal neurons of CA1 area show enhanced sensibility (Hamon and Heinemann 1988). NMDA receptor subunit composition also suffers changes during the development, leading to modifications in their functional proprieties (Lecointre et al. 2015). Additionally, the stage of brain and the dose and/or duration of exposure are critical factors for neurotoxic action of general anesthetics (Wang 2012). Moreover, development of CNS happens heterogeneously in different brain regions, changing their vulnerability to neurotoxic agents (Zhao et al. 2016).
Corroborating early findings, the current data indicates that hippocampus and frontal cortex were differentially affected by a single ketamine exposure at PND7. The hippocampi from neonatal rats were susceptible to neurotoxicity induced by ketamine administration while the frontal cortex was not affected. It is believed that this regional discrepancy may be linked to ketamine-induced neurotoxic effects on neuroplasticity and synaptogenesis (Jevtovic-Todorovic et al. 2013; Zhao et al. 2016).
However, this early exposure of frontal cortex to ketamine resulted in long-lasting changes in this brain region. In fact, it is interesting to see that a single ketamine neonatal exposure promoted a preconditioning-like effect against glutamate-induced toxicity in the frontal cortex. Preconditioning was firstly described by Janoff (1964) to explain the tolerance response of an organism to lethal stress induced by prior exposure to low doses of toxic agents or stimuli. Therefore, a sublethal insult can activate endogenous protective mechanisms, which confer a state of cellular protection against subsequent damaging insults (Constantino et al. 2014). Herein, it is feasible that ketamine neonatal exposure acted as a sublethal insult that induced subsequent in vitro glutamate challenge tolerance in the frontal cortex. Although the increased glutamate uptake seen in frontal cortical slices exposed to ketamine was not statistically significant, we cannot exclude its contribution to the glutamate tolerance observed herein. Moreover, ketamine exposure could also be triggering changes in NMDA subunit density as previously shown. For example, Lecointre et al. (2015) described an age-dependent vulnerability of cerebral cortex to ketamine injection. These authors reported a decrease in the GluN2A-NMDA receptor subunit expression in cortical layers I to IV, and an increase in all NMDA receptor subunit expression in deeper layers (V-VI) 24 h after mice were exposed to a single ketamine injection (40 mg/kg, s.c.) at PNDs 5 and 10, respectively. In this way, the decrease in cortical GluN2A-NMDA receptor subunit levels could impair the developmental switch, in which GluN2B is progressively replaced by GluN2A-NMDA subunit around PND5/6 (Paoletti et al. 2013). Of note, GluN2A-NMDA receptor subunit may mediate the neuronal survival (Liu et al. 2007).
Even though ketamine induced hippocampal susceptibility during neonatal period, no indicative of cellular membrane damage at PND8 and PND65 were evidenced. This is suggestive that ketamine induced a decrease in hippocampal cellular metabolic activity which was reversible and did not translate into cell death.
However, changes in the hippocampal glutamate uptake in the adulthood were observed after the single neonatal ketamine exposure. Glutamate uptake was evaluated as an indicative of short- and long-term glutamatergic neurotransmission functionality or changes following neonatal ketamine exposure. Hippocampal slices from ketamine-treated rats demonstrated increased Na+-dependent glutamate uptake. Astrocytic glutamate transporters, GLAST and GLT-1, represent the main glutamate transporters, being their activities essential for maintenance of low extracellular glutamate concentrations and also favors glutamate turnover to neurons (Robinson and Jackson 2016). Herein, a single neonatal ketamine administration was effective in increasing glutamate uptake in adult hippocampal slices, but not in hippocampal slices from young rats (PND8). However, whether this ketamine-induced change in the hippocampal glutamate uptake is linked to avoiding excitotoxic events, such as chronic receptor activation and their consequent desensitization, remains to be identified. Nonetheless, this increase in hippocampal glutamate uptake was not sufficient to prevent exogenous glutamate-induced (in vitro glutamate challenge) loss of cellular viability. Interestingly, no significant alterations were observed in cortical glutamate uptake. A recent study showed that repeated ketamine administration (30 mg/kg for 5 days) promoted an increased synaptic glutamate release in the cortex and a decreased expression of GLT-1 (Lisek et al. 2017), suggesting that glutamate transport alterations may represent a novel mechanism of ketamine actions. Similarly, we also found alterations in glutamate transport, despite of the differences in ketamine administration protocols and brain regions affected.
Regarding behavioral analyses, adult rats subjected to a single neonatal ketamine exposure displayed improvement on motor performance and short-term recognition memory impairment. As mentioned previously, the BGS is a specifically vulnerable period during neonatal brain development in which novel sensory and motor faculties are acquired and result in a peak in spontaneous behavior (Bolles and Woods 1964). In this way, changes to the course of the BGS can affect the structural and functional integrity of different areas at different times with putative consequences for neuronal circuitry in development and, eventually, translating into behavioral changes in adulthood (Chen et al. 1999; Olney et al. 2000).
Long-term effects manifested as cognitive deficits and induction of hyperactive phenotype have also been found in adult animals after ketamine neonatal exposure (Fredriksson and Archer 2003; Fredriksson and Archer 2004; Lecointre et al. 2015; Paule et al. 2011). In this sense, it has been proposed that the repetitive exposure to anesthetics during the neurodevelopment may increase the risk of developing attention-deficit/hyperactivity disorder (ADHD) (Sprung et al. 2012).
In the present study, we demonstrated that the single ketamine exposure (20 mg/kg, s.c.) at PND7 induces hyperactivity assessed by locomotor performance on the rotarod apparatus. According to our data, hyperlocomotion was also found in ketamine-treated mice at PND 2 or 10 (Fredriksson and Archer 2003; Fredriksson and Archer 2004; Lecointre et al. 2015). In addition, Rhodes et al. (2003) have reported that frontal and sensory cortices are implicated in sensorimotor integration and coordination during motor control of daily functional activities and that hippocampus modulates the running intensity. Thus, the motor alterations here reported could be, at least in part, related to hippocampal and frontal cortical susceptibility to a single ketamine neonatal injection observed at PND8. Nonetheless, other brain areas involved in the regulation of locomotor activity, such as substantia nigra, putamen, caudate, thalamus, and cerebellum, may be also affected by ketamine neonatal treatment (Ikonomidou et al. 1999; Young et al. 2005). Moreover, these motor alterations came without alterations in spontaneous locomotor activity as assessed in the open field apparatus.
Moreover, acute hippocampal susceptibility promoted by a single ketamine neonatal administration may be also associated to short-term memory deficit exhibited by the ketamine group in the novel object recognition test. Of note, to the best of our knowledge, this is the first evidence that mnemonic process in adulthood rat can be impaired by a single ketamine neonatal exposure at PND7. Additionally, mice subjected to postnatal ketamine exposure (50 mg/kg, s.c.) at PND10 also showed cognitive deficits in the radial arm maze and circular swim maze (Fredriksson and Archer 2004). Persistent cognitive decline following to repetitive neonatal exposure of ketamine both in rats and primates already were been described (Huang et al. 2012; Paule et al. 2011). Lastly, clinical data indicate the association between the general anesthetics use in the early life and the appearance of late-onset learning disabilities (Ing et al. 2012; Kalkman et al. 2009).
In conclusion, the current data indicate that a single neonatal ketamine exposure at PND7 promotes an acute reduction in hippocampal, but not in cortical slice viability. It also reveals a hippocampal susceptibility to glutamate challenge and increased glutamate uptake in adulthood. Moreover, it is suggested that hippocampal and cortical alterations may be linked, at least in part, with the enhancement of motor performance and short-term memory impairment in adulthood. Due to these long-term consequences, a careful evaluation of the benefit/risk ratio should be realized before the ketamine use in neonates.
References
Abe H, Ishida Y, Iwasaki T (2004) Perirhinal N-methyl-D-aspartate and muscarinic systems participate in object recognition in rats. Neurosci Lett 356:191–194. https://doi.org/10.1016/j.neulet.2003.11.049
Bolles RC, Woods PJ (1964) The ontogeny of behaviour in the albino rat. Anim Behav 12:427–441. https://doi.org/10.1016/0003-3472(64)90062-4
Briner A, De Roo M, Dayer A, Muller D, Habre W et al (2010) Volatile anesthetics rapidly increase dendritic spine density in the rat medial prefrontal cortex during synaptogenesis. Anesthesiology 112:546–556. https://doi.org/10.1097/ALN.0b013e3181cd7942
Chen WA, Parnell SE, West JR (1999) Early postnatal alcohol exposure produced long-term deficits in brain weight, but not the number of neurons in the locus coeruleus. Dev Brain Res 118:33–38. https://doi.org/10.1016/S0165-3806(99)00128-5
Constantino LC, Tasca CI, Boeck CR (2014) The role of NMDA receptors in the development of brain resistance through pre- and postconditioning. Aging Dis 5:430–441. https://doi.org/10.14336/AD.2014.0500430
Dal-Cim T, Ludka FK, Martins WC, Reginato C, Parada E, Egea J, López MG, Tasca CI (2013) Guanosine controls inflammatory pathways to afford neuroprotection of hippocampal slices under oxygen and glucose deprivation conditions. J Neurochem 126:437–450. https://doi.org/10.1111/jnc.12324
Davidson AJ (2011) Anesthesia and neurotoxicity to the developing brain: the clinical relevance. Paediatr Anaesth 21:716–721. https://doi.org/10.1111/j.1460-9592.2010.03506.x
Dumas TC (2005) Developmental regulation of cognitive abilities: modified composition of a molecular switch turns on associative learning. Prog Neurobiol 76:189–211. https://doi.org/10.1016/j.pneurobio.2005.08.002
Flick RP, Katusic SK, Colligan RC, Wilder RT, Voigt RG, Olson MD, Sprung J, Weaver AL, Schroeder DR, Warner DO (2011) Cognitive and behavioral outcomes after early exposure to anesthesia and surgery. Pediatrics 128:e1053–e1061. https://doi.org/10.1542/peds.2011-0351
Fredriksson A, Archer T (2003) Hyperactivity following postnatal NMDA antagonist treatment: reversal by D-amphetamine. Neurotox Res 5:549–564
Fredriksson A, Archer T (2004) Neurobehavioural deficits associated with apoptotic neurodegeneration and vulnerability for ADHD. Neurotox Res 6:435–456
Gambrill AC, Barria A (2011) NMDA receptor subunit composition controls synaptogenesis and synapse stabilization. Proc Natl Acad Sci U S A 108:5855–5860. https://doi.org/10.1073/pnas.1012676108
Hamon B, Heinemann U (1988) Developmental changes in neuronal sensitivity to excitatory amino acids in area CA1 of the rat hippocampus. Brain Res 466:286–290
Hestrin S (1992) Developmental regulation of NMDA receptor-mediated synaptic currents at a central synapse. Nature 357:686–689. https://doi.org/10.1038/357686a0
Huang L, Liu Y, Jin W, Ji X, Dong Z (2012) Ketamine potentiates hippocampal neurodegeneration and persistent learning and memory impairment through the PKCgamma-ERK signaling pathway in the developing brain. Brain Res 1476:164–171. https://doi.org/10.1016/j.brainres.2012.07.059
Ikonomidou C, Bosch F, Miksa M, Bittigau P, Vockler J et al (1999) Blockade of NMDA receptors and apoptotic neurodegeneration in the developing brain. Science 283:70–74. https://doi.org/10.1126/science.283.5398.70
Ing C, DiMaggio C, Whitehouse A, Hegarty MK, Brady J, von Ungern-Sternberg BS, Davidson A, Wood AJJ, Li G, Sun LS (2012) Long-term differences in language and cognitive function after childhood exposure to anesthesia. Pediatrics 130:e476–e485. https://doi.org/10.1542/peds.2011-3822
Janoff A (1964) Alterations in lysosomes (intracellular enzymes) during shock; effects of preconditioning (tolerance) and protective drugs. Int Anesthesiol Clin 2:251–269
Jevtovic-Todorovic V, Absalom AR, Blomgren K, Brambrink A, Crosby G, Culley DJ, Fiskum G, Giffard RG, Herold KF, Loepke AW, Ma D, Orser BA, Planel E, Slikker W Jr, Soriano SG, Stratmann G, Vutskits L, Xie Z, Hemmings HC Jr (2013) Anaesthetic neurotoxicity and neuroplasticity: an expert group report and statement based on the BJA Salzburg seminar. Br J Anaesth 111:143–151. https://doi.org/10.1093/bja/aet177
Jiang C, Wan X, Jankovic J, Christian ST, Pristupa ZB, Niznik HB, Sundsmo JS, le W (2004) Dopaminergic properties and experimental anti-parkinsonian effects of IPX750 in rodent models of Parkinson disease. Clin Neuropharmacol 27:63–73
Kalkman CJ, Peelen L, Moons KG, Veenhuizen M, Bruens M, Sinnema G, de Jong TP (2009) Behavior and development in children and age at the time of first anesthetic exposure. Anesthesiology 110:805–812. https://doi.org/10.1097/ALN.0b013e31819c7124
Lecointre M, Vezier C, Benard M, Ramdani Y, Dupre N et al (2015) Age-dependent alterations of the NMDA receptor developmental profile and adult behavior in postnatally ketamine-treated mice. Dev Neurobiol 75:315–333. https://doi.org/10.1002/dneu.22232
Lisek M, Ferenc B, Studzian M, Pulaski L, Guo F, Zylinska L, Boczek T (2017) Glutamate deregulation in ketamine-induced psychosis-a potential role of PSD95, NMDA receptor and PMCA interaction. Front Cell Neurosci 11:181. https://doi.org/10.3389/fncel.2017.00181
Liu Y, Wong TP, Aarts M, Rooyakkers A, Liu L, Lai TW, Wu DC, Lu J, Tymianski M, Craig AM, Wang YT (2007) NMDA receptor subunits have differential roles in mediating excitotoxic neuronal death both in vitro and in vivo. J Neurosci 27:2846–2857. https://doi.org/10.1523/JNEUROSCI.0116-07.2007
Liu F, Paule MG, Ali S, Wang C (2011) Ketamine-induced neurotoxicity and changes in gene expression in the developing rat brain. Curr Neuropharmacol 9:256–261. https://doi.org/10.2174/157015911795017155
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Ludka FK, Dal-Cim T, Binder LB, Constantino LC, Massari C, Tasca CI (2016) Atorvastatin and fluoxetine prevent oxidative stress and mitochondrial dysfunction evoked by glutamate toxicity in hippocampal slices. Mol Neurobiol 54:3149–3161. https://doi.org/10.1007/s12035-016-9882-6
Molz S, Dal-Cim T, Tasca CI (2009) Guanosine-5′-monophosphate induces cell death in rat hippocampal slices via ionotropic glutamate receptors activation and glutamate uptake inhibition. Neurochem Int 55:703–709. https://doi.org/10.1016/j.neuint.2009.06.015
Molz S, Dal-Cim T, Budni J, Martin-de-Saavedra MD, Egea J et al (2011) Neuroprotective effect of guanosine against glutamate-induced cell death in rat hippocampal slices is mediated by the phosphatidylinositol-3 kinase/Akt/ glycogen synthase kinase 3beta pathway activation and inducible nitric oxide synthase inhibition. J Neurosci Res 89:1400–1408. https://doi.org/10.1002/jnr.22681
Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65:55–63. https://doi.org/10.1016/0022-1759(83)90303-4
Olney JW, Farber NB, Wozniak DF, Jevtovic-Todorovic V, Ikonomidou C (2000) Environmental agents that have the potential to trigger massive apoptotic neurodegeneration in the developing brain. Environ Health Perspect 108(Suppl 3):383–388
Paoletti P, Bellone C, Zhou Q (2013) NMDA receptor subunit diversity: impact on receptor properties, synaptic plasticity and disease. Nat Rev Neurosci 14:383–400. https://doi.org/10.1038/nrn3504
Paule MG, Li M, Allen RR, Liu F, Zou X, Hotchkiss C, Hanig JP, Patterson TA, Slikker W Jr, Wang C (2011) Ketamine anesthesia during the first week of life can cause long-lasting cognitive deficits in rhesus monkeys. Neurotoxicol Teratol 33:220–230. https://doi.org/10.1016/j.ntt.2011.01.001
Piermartiri TC, Vandresen-Filho S, de Araujo Herculano B, Martins WC, Dal'agnolo D et al (2009) Atorvastatin prevents hippocampal cell death due to quinolinic acid-induced seizures in mice by increasing Akt phosphorylation and glutamate uptake. Neurotox Res 16:106–115. https://doi.org/10.1007/s12640-009-9057-6
Rhodes JS, Garland T Jr, Gammie SC (2003) Patterns of brain activity associated with variation in voluntary wheel-running behavior. Behav Neurosci 117:1243–1256. https://doi.org/10.1037/0735-7044.117.6.1243
Rice D, Barone S Jr (2000) Critical periods of vulnerability for the developing nervous system: evidence from humans and animal models. Environ Health Perspect 108(Suppl 3):511–533
Robinson MB, Jackson JG (2016) Astroglial glutamate transporters coordinate excitatory signaling and brain energetics. Neurochem Int 98:56–71. https://doi.org/10.1016/j.neuint.2016.03.014
Soriano SG, Liu Q, Li J, Liu JR, Han XH, Kanter JL, Bajic D, Ibla JC (2010) Ketamine activates cell cycle signaling and apoptosis in the neonatal rat brain. Anesthesiology 112:1155–1163. https://doi.org/10.1097/ALN.0b013e3181d3e0c2
Sprung J, Flick RP, Katusic SK, Colligan RC, Barbaresi WJ, Bojanić K, Welch TL, Olson MD, Hanson AC, Schroeder DR, Wilder RT, Warner DO (2012) Attention-deficit/hyperactivity disorder after early exposure to procedures requiring general anesthesia. Mayo Clin Proc 87:120–129. https://doi.org/10.1016/j.mayocp.2011.11.008
Sun L (2010) Early childhood general anaesthesia exposure and neurocognitive development. Br J Anaesth 105(Suppl 1):i61–i68. https://doi.org/10.1093/bja/aeq302
Walsh RN, Cummins RA (1976) The open-field test: a critical review. Psychol Bull 83:482–504. https://doi.org/10.1037//0033-2909.83.3.482
Wang C (2012) Advanced pre-clinical research approaches and models to studying pediatric anesthetic neurotoxicity. Front Neurol 3:142. https://doi.org/10.3389/fneur.2012.00142
Yan J, Jiang H (2014) Dual effects of ketamine: neurotoxicity versus neuroprotection in anesthesia for the developing brain. J Neurosurg Anesthesiol 26:155–160. https://doi.org/10.1097/ANA.0000000000000027
Yon JH, Daniel-Johnson J, Carter LB, Jevtovic-Todorovic V (2005) Anesthesia induces neuronal cell death in the developing rat brain via the intrinsic and extrinsic apoptotic pathways. Neuroscience 135:815–827. https://doi.org/10.1016/j.neuroscience.2005.03.064
Young C, Jevtovic-Todorovic V, Qin YQ, Tenkova T, Wang H, Labruyere J, Olney JW (2005) Potential of ketamine and midazolam, individually or in combination, to induce apoptotic neurodegeneration in the infant mouse brain. Br J Pharmacol 146:189–197. https://doi.org/10.1038/sj.bjp.0706301
Yuan T, Bellone C (2013) Glutamatergic receptors at developing synapses: the role of GluN3A-containing NMDA receptors and GluA2-lacking AMPA receptors. Eur J Pharmacol 719:107–111. https://doi.org/10.1016/j.ejphar.2013.04.056
Zhao T, Li C, Wei W, Zhang H, Ma D, Song X, Zhou L (2016) Prenatal ketamine exposure causes abnormal development of prefrontal cortex in rat. Sci Rep 6:26865. https://doi.org/10.1038/srep26865
Zou X, Patterson TA, Sadovova N, Twaddle NC, Doerge DR, Zhang X, Fu X, Hanig JP, Paule MG, Slikker W, Wang C (2009) Potential neurotoxicity of ketamine in the developing rat brain. Toxicol Sci 108:149–158. https://doi.org/10.1093/toxsci/kfn270
Funding
This research was supported by grants from Brazilian funding agencies Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq #307319/2012-1), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES/PVE 052/2012; CAPES-FCT 2014), Programa de Apoio aos Núcleos de Excelência (PRONEX-Project NENASC), Fundação de Apoio à Pesquisa do Estado de Santa Catarina (FAPESC), Instituto Nacional de Ciência e Tecnologia (INCT for Excitotoxicity and Neuroprotection); and by Portuguese funding agencies Fundação para a Ciência e Tecnologia (FCT, Portugal) (Strategic Project 2015-UID/NEU/04539/2013), COMPETE-FEDER (POCI-01-0145-FEDER-007400), Centro 2020 Regional Operational Programmes (CENTRO-01-0145-FEDER-000012: HealthyAging 2020 and CENTRO-01-0145-FEDER-000008: BrainHealth 2020). C.I.T. and R.D.P. are recipient of research fellowship from CNPq.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Sampaio, T.B., de Oliveira, L.F., Constantino, L.C. et al. Long-Term Neurobehavioral Consequences of a Single Ketamine Neonatal Exposure in Rats: Effects on Cellular Viability and Glutamate Transport in Frontal Cortex and Hippocampus. Neurotox Res 34, 649–659 (2018). https://doi.org/10.1007/s12640-018-9927-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12640-018-9927-x