Abstract
The role of fatty acid-binding proteins (FABPs) in atherosclerosis has been investigated. The aim of this study was to verify the hypothesis that higher levels of serum fatty acid-binding protein 4 (FABP4) could be a prognostic factor in Chinese patients with type 2 diabetes (T2DM) and acute ischemic stroke (AIS). From September 2015 to August 2016, consecutive first-ever AIS patients combined with T2DM were included in this study. FABP4, NIH stroke scale (NIHSS), and conventional risk factors were evaluated to determine their value to predict functional outcomes within 3 months. Multivariate analyses were performed using logistic regression models. We measured FABP4 in 329 patients. The median age of patients included in this study was 63 (IQR, 56–72) years and 45.9% were women. FABP4 serum levels were obtained at a median of 8.5 h (IQR, 4.0–14.0 h) after the stroke onset with a median value of 21.4 ng/ml (IQR, 15.6–28.2 ng/ml). In multivariable models, FABP4 remained an independent stroke severity predictor with an adjusted OR of 1.05 (95% CI, 1.02–1.09). In multivariate models comparing the third (odd ratio (OR), 2.25; 95% confidence interval (CI), 1.59–3.54) and fourth quartiles (OR, 3.75; 95% CI, 2.48–5.03) against the first quartile of the FABP4, levels of FABP4 were associated with poor functional outcome. At 3 months, 38 patients (11.6%; 95%CI, 8.1–15.0%) had died. The mortality distribution across the FABP4 quartiles ranged between 3.7% (first quartile) and 20.7% (fourth quartile). Elevation of FABP4 is associated with an increased risk of death and poor functional outcome events in patients with type 2 diabetes and acute ischemic stroke and is independent of other established clinical risk predictors and biomarkers.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Stroke is a frequently occurring disease in old-aged people in China. The age-standardized incidence rate was 1114.8 per 100,000 people (Wang et al. 2017). Type 2 diabetes is a significant cause of premature mortality and morbidity especially those related to cardiovascular disease (i.e., stroke), while the severity of ischemic heart disease is markedly enhanced in type 2 diabetes (Song et al. 2015). Interestingly, a previous study suggested that diabetes was associated with a higher risk of death and disability after the onset of stroke (Kaarisalo et al. 2005).
Fatty acid-binding proteins (FABPs) are cytosolic fatty acid chaperones whose biological role and mechanisms of action are not well understood. Adipocyte fatty acid-binding protein (AFABP) (also known as aP2, FABP4, and adipocyte lipid-binding protein), a member of the lipid chaperone fatty acid-binding protein family, is produced in adipocytes and macrophages, with its gene expression being several orders of magnitude higher in adipocytes (Milner et al. 2009). Previous studies had shown that circulating AFABP levels are associated with the metabolic syndrome and obesity (Xu et al. 2006), type 2 diabetes (Tso et al. 2007), insulin resistance (Maeda et al. 2003), atherogenic dyslipidemia (Cabré et al. 2008), and fatty liver disease (Milner et al. 2009).
Results from previous studies demonstrated that FABP4 have a robust impact on multiple components of metabolic syndrome, integrating metabolic and inflammatory responses in mice and constituting a powerful target for the treatment of these diseases (Maeda et al. 2005). Yeung et al. (2007) reported that FABP4 was an independent determinant of carotid atherosclerosis in Chinese women, while another study suggested that FABP4 levels in atherosclerotic lesions were associated with an unstable plaque phenotype and an increased risk for cardiovascular events during follow-up (Peeters et al. 2011). Currently, no data were available on the role of FABP4 in the progression of stroke in patients with type 2 diabetes. Thus, we propose a hypothesis that the level of serum FABP4 could be associated with functional outcomes in type 2 diabetes with stroke. In this study, we therefore evaluated the short-term prognostic value of early measurement of serum FABP4 levels in Chinese type 2 diabetes with acute ischemic stroke (AIS).
Subjects and Methods
We conducted a prospective cohort study at the emergency department of our hospital. From September 2015 to August 2016, consecutive first-ever AIS patients combined with T2DM were identified. The study population was exclusively Chinese. All patients were admitted within 24 h of experiencing a stroke attack. Ischemic stroke was defined according to World Health Organization recommendations (defined stroke as a “neurological deficit of cerebrovascular cause that persists beyond 24 hours or is interrupted by death within 24 hours”) (Huang et al. 2016). The WHO diagnostic criteria for type 2 diabetes were used (glycated hemoglobin (HbA1c) level ≥ 6.4% or a fasting blood sugar (FBS) ≥ 6.11 mmol/l) (Guo et al. 2017).
Patients with malignant tumor, renal insufficiency (creatinine > 1.5 mg/dl), history of brain trauma, and disturbance of consciousness were excluded. In addition, patients refused to be included and lost blood samples were also excluded. The present study has been approved by the ethics committee of the Hongqi Hospital of Mudanjiang Medical University. All participants or their relatives were informed of the study protocol, and their written informed consents were obtained.
Clinical information was collected. Demographic data (age and sex), body mass index (BMI), and history of risk factors (hypertension, diabetes mellitus, atrial fibrillation, hyperlipidemia, and smoking habit) were obtained at admission. Pre-stroke therapy (anticoagulants, antiplatelet agents, and intensive glucose treatment) and acute treatment (IV thrombolysis and/or mechanical thrombectomy) were also recorded. Routine blood and biochemical tests, electrocardiogram, and a baseline brain computer tomography (CT) or magnetic resonance imaging (MRI) scan were performed in all patients at admission. All patients received treatment according to current guidelines. Stroke severity was assessed on admission using the National Institutes of Health Stroke Scale (NIHSS) by a neurologist (Brott et al. 1989). Strokes were classified according to the criteria of the TOAST (Trial of Org 10172 in Acute Stroke Treatment) classification and clinical stroke syndrome was determined applying the criteria of the Oxfordshire Community Stroke Project (OCSP). MRI with diffusion-weighted imaging (DWI) was available. The infarct volume was calculated by using the formula 0.5 × a × b × c (where a is the maximal longitudinal diameter, b is the maximal transverse diameter perpendicular to a, and c is the number of 10-mm slices containing infarct) (Sims et al. 2009).
Functional outcome was obtained on month 3 according to the modified Rankin Scale (mRS) (Bonita 1988) blinded to FABP4 levels. The primary end point of this study was good functional outcome of stroke patients after 3 months from baseline, defined as a mRS score of 0 to 2 points. Secondary end point in stroke patients was death from all-cause within 3 months follow-up. Outcome assessment was performed by one trained medical staff blinded to FABP4 levels with a structured follow-up telephone interview with the patient or, if not possible, with the relative.
All blood samples were collected on the first morning after admission at 6:00 under fasting state and within 48 h of symptom onset (within 0–6 h (n = 102), 6–12 h (n = 112), 12–24 h (n = 65), and 24–48 h (n = 53) from symptom onset). Fasting venous blood was collected by vacutainer tubes and quickly centrifuged to avoid glycolysis. All serum samples were kept at − 80 °C until assay. Routine serum biomarkers, for instance, triglyceride, cholesterol, high-density lipoprotein (HDL), low-density lipoprotein (LDL), homocysteine (HCY), fasting blood glucose(FBG), and high-sensitivity C-reactive protein (Hs-CRP), were tested using standard detection methods. Serum levels of FABP4 were batch analyzed, blind to stroke outcome status, using a commercially available ELISA assay from R & D Systems (Minneapolis, MN). In our study, the lower detection limit for FABP4 was 2.0 ng/ml, and the detection range was 2.0–120 ng/ml. Inter-assay and intra-assay coefficients of variation were 4.5–8.0 and 3.2–4.8%, respectively.
Statistical Analysis
Results are expressed as percentages for categorical variables and as medians (interquartile ranges, IQRs) for the continuous variables. Univariate data on demographic and clinical features were compared by Mann–Whitney U test or chi-square test as appropriate. Correlations among continuous variables were assessed by the Spearman rank-correlation coefficient. In addition, associations between FABP4 and NIHSS score were also assessed using ordered logistic regression models in multivariate adjustment with possible confounders.
The relationship between FABP4 and stroke severity (minor stoke was defined as NIHSS score ≤ 5) (Daubail et al. 2013) were performed by binary logistic regression analysis, which allows adjustment for confounding factors (for age, sex, time from onset to blood collection, stroke syndrome, stroke etiology, pre-stroke treatment, acute treatment, vascular risk factors and serum levels of Hs-CRP, FBG, and HCY). Results were expressed as adjusted odds ratios (OR) with the corresponding 95% confidence interval (CI). To investigate whether FABP4 allows predicting of functional outcome, different statistical methods were used. First, the relation of FABP4 with the end point was investigated with the use of logistic regression models. For multivariate analysis, we included confounders, known risk factors, and other outcome predictors as assessed in univariate analysis. For a more detailed exploration of the FABP4 and outcome relationship, we also used multivariate analysis models to estimate adjusted OR and 95% CIs of outcome for FABP4 quartiles (with first FABP4 quartile as reference). Second, we compared different prognostic risk scores from different predictive models by calculating receiver operating characteristic curves (ROC) analysis. ROC was used to test the overall prognostic accuracy of MBL, the NIHSS, and other serum biomarkers and results were reported as area under the curve (AUC). Integrated discrimination improvement (IDI) and net reclassification improvement (NRI) indices were calculated to determine the clinical utility of the addition of FABP4 to established risk factors and the ability of FABP4 to improve functional outcome prediction (Pencina et al. 2008). All statistical analysis was performed with SPSS for Windows, version 22.0 (SPSS Inc., Chicago, IL, USA). Statistical significance was defined as P < 0.05.
Results
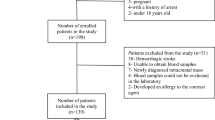
From 465 screened patients (stroke admission), 389 ischemic strokes with T2DM were included in the analysis. By the time of follow-up, at 3 months post-stroke, 28 declined the invitation to participate and 32 lost follow-up, leaving 329 individuals. However, these 329 patients were similar in terms of baseline characteristics (age (P = 0.76), sex (P = 0.55), BMI (P = 0.26), and NIHSS score (P = 0.18)) compared to the overall cohort. The median age of patients included in this study was 63 (IQR, 56–72) years and 45.9% were women. FABP4 serum levels were obtained at a median of 8.5 h (IQR, 4.0–14.0 h) after the stroke onset with a median value of 21.4 ng/ml (IQR, 15.6–28.2 ng/ml). Basal characteristics of those patients were provided in Table 1.
Serum FABP4 levels increased with increasing severity of stroke as defined by the NIHSS score. There was a modest correlation between levels of FABP4 and NIHSS score (r = 0.192, P < 0.001). There was still a significant positive correction between FABP4 serum levels and NIHSS score, using ordered logistic regression after multivariate adjustment for possible confounders (P = 0.015). At admission, 152 patients (46.2%) had a minor stroke (NIHSS ≤ 5). In these patients, the median FABP4 level was lower than that observed in patients with moderate-to-high clinical severity (18.7 (14.1–25.6) ng/ml vs. 23.5 (18.5–29.9) ng/ml; Z = 4.411, P < 0.001; Fig. 1). In multivariable models adjusted for age, gender, and other significant risk factors, FABP4 remained an independent stroke severity predictor with an adjusted OR of 1.05 (95% CI, 1.02–1.09). In addition, serum FABP4 levels were also positive correlated with triglyceride, cholesterol, and LDL (P < 0.05, all). In patients for whom MRI data were available (n = 210), there was a positive correlation between levels of FABP4 and the infarct volume (r = 0.210, P < 0.001).
In the 103 patients with poor functional outcomes, serum FABP4 levels were higher compared with those in patients with good outcomes (26.9 (IQR, 20.1–32.1) ng/ml vs. 18.9 (IQR, 14.2–25.5) ng/ml; Z = 6.322; P < 0.001). With an unadjusted OR of 1.15 (95% CI, 1.09–1.20), FABP4 had a strong association with poor functional outcome. After adjusting for all other significant outcome predictors, FABP4 remained an independent outcome predictor with an adjusted OR of 1.10 (95% CI, 1.04–1.15). For a more detailed exploration of the FABP4 concentration-functional outcome relationship, we also used multivariate analysis models to estimate adjusted OR and 95% CIs of unfavorable functional outcome for FABP4 quartiles (with first quartile as reference). In multivariate models comparing the third and fourth quartiles against the first quartile of the FABP4 (Table 2), levels of FABP4 were associated with poor functional outcome. In the subgroup of patients (n = 210) in whom MRI evaluations were performed, FABP4 was an independent poor outcome predictor with an OR of 1.12 (95% CI, 1.06–1.17; P < 0.001) after adjustment for both lesion size and the NIHSS score.
With an AUC of 0.79 (95% CI, 0.72–0.86), FABP4 showed a significantly greater discriminatory ability as compared with Hs-CRP (AUC, 0.68; 95% CI, 0.60–0.74; P < 0.001), FBG (AUC, 0.60; 95% CI, 0.52–0.66; P < 0.0001), and NIHSS score (AUC, 0.73; 95% CI, 0.66–0.80; P = 0.003). Interestingly, FABP4 improved the NIHSS score (AUC of the combined model, 0.80; 95% CI, 0.76–0.85; P < 0.001). The AUC was significantly increased by adding FABP4 to established risk factors (difference, 0.04 (95% CI, 0.02–0.06); P = 0.01) (Table 3). The NRI statistic showed that the addition of FABP4 to established risk factors significantly increased the correct reclassification of unfavorable outcome (P = 0.002). In addition, the IDI statistic found that the FABP4 level significantly increased discrimination between patients with unfavorable functional outcome and with favorable outcome (P = 0.03).
At 3 months, 38 patients (11.6%; 95% CI, 8.1–15.0%) had died. Non-survivors had significantly higher FABP4 levels than survivors (31.3 (IQR, 28.3–35.3) ng/ml vs. 20.1 (IQR, 15.0–25.1) ng/ml; Z = 7.132, P < 0.0001). The mortality distribution across the FABP4 quartiles ranged between 3.7% (first quartile) and 20.7% (fourth quartile), P for trend < 0.001 (Fig. 2).
Relation between baseline FABP4 quartiles and stroke mortality. Bars show the probability of achieving stroke mortality across FABP4 quartiles. Serum FABP4 levels in quartile 1 (< 15.6 ng/ml), quartile 2 (15.6–21.4 ng/ml), quartile 3 (21.5–28.2 ng/ml), and quartile 4 (> 28.2 ng/ml). FABP4 fatty acid-binding protein 4
Discussion
In this study, we have demonstrated that serum FABP4 was independently associated with functional outcomes, and the effects were independent of conventional cardiovascular risk factors. Furthermore, high FABP4 levels in blood samples taken at admission were associated with early mortality (within 3 months). It is imperative to emphasize targeted lifestyle intervention and more frequent medical interventions for stroke patients with type 2 diabetes, especially for these patients with the concentration of FABP4 in the range of fourth quartile (> 28.2 ng/ml).
Consistent with our finding, another study confirmed that serum FABP4 was significantly associated with ischemic stroke in a case-control study, and may serve as a useful prognostic indicator for early mortality (Tso et al. 2011). Tu et al. (2017) suggested that FABP4 was a novel independent prognostic marker improving the currently used risk stratification of stroke patients. Two studies have shown enhanced FABP4 expression within human carotid atherosclerotic lesions in association with poor prognosis (Agardh et al. 2011; Peeters et al. 2011), and other data suggest that higher levels of FABP4 are associated with elevated CVD mortality among men with type 2 diabetes mellitus (Liu et al. 2016). Interestingly, Holm et al. (2011) reported that FABP4 was linked to atherogenesis, plaque instability, and adverse outcome in patients with carotid atherosclerosis and acute ischemic stroke. Serum FABP4 level was also reported to be associated with coronary artery disease, but the effects were ameliorated after adjustment for age, sex, and body mass index (BMI) (Rhee et al. 2009). However, in this study, FABP4 still could be seen as one independent short-term prognostic marker of functional outcome even after correcting for above possible confounding factors.
Although our study design prevents firm conclusions in humans about a cause-and-effect relationship, increased FABP4 production could contribute to macrophage activity, possibly by enhancing fatty acid availability to hepatocytes and/or macrophages, with activation of inflammatory pathways, resulting in inflammation (Milner et al. 2009). An alternative hypothesis more in line with published evidence is that the elevated circulating FABP4 reflects increased cellular production in both adipocytes and macrophages in response to increased lipid availability, with increased Kupffer cell production of FABP4 mediating an increased inflammatory response (Furuhashi et al. 2007).
The mechanism of increased serum concentrations of FABP4 with poor outcome and mortality following an acute stroke in patients with type 2 diabetes is not yet established. First, FABP4 has been shown to play a crucial role in mediating the endoplasmic reticulum stress observed in macrophages upon lipotoxic signal exposure, which contributes to atherosclerosis, inflammation, and perhaps plaque vulnerability (Erbay et al. 2009). Second, it is known that acute stroke is associated with increased monocyte infiltration in the cerebral tissue, and Toll-like receptor-4 expression in monocytes had been found to be associated with poor outcome in stroke (Urra et al. 2009). Third, elevated levels of pro-inflammatory cytokines can be detected in the CSF after stroke, and interleukin-6 level, for example, is associated with early clinical deterioration after stroke (Vila et al. 2000). High levels of FABP4 may contribute to adverse prognosis via inducing endoplasmic reticulum stress in macrophages and upregulating pro-inflammatory cytokine production. Indeed, recent work by our group has shown that FABP4 modulates inflammatory responses in macrophages through a positive feedback loop involving c-Jun NH2-terminal kinases and activator protein-1 (Hui et al. 2010). Lastly, the increase of circulating endothelial progenitor cells (EPC) after acute ischemic stroke is suggested to be associated with good functional outcome and reduced infarct growth. A direct inversely effect of plasma FABP4 on the vascular endothelium in those with type 2 diabetes had been suggested (Aragonès et al. 2010).
Several limitations of this study should be considered. First, the relatively small sample size (N = 329) may limit the generalization of the results of this study. Before broad implementation, additional studies (multicenter, large sample) are needed for external validation. Second, this study measured FABP4 in serum, not in cerebral spinal fluid (CSF). It is still uncertain whether peripheral MBL levels reflect similar changes in the central nervous system (CNS). Further study is needed to confirm the correction between FABP4 serum levels and CSF. Third, the FABP4 levels were measured only at admission. We did not have convalescent serum of the acute stroke survivors at 3 months, and had to confirm our findings using another cohort. Finally our cohort study, being cross-sectional in nature, did not allow for conclusions to be drawn regarding causality and we cannot exclude the possibility of reverse causality.
Conclusions
Our study suggested that FABP4 levels at admission may reliably predict short-term stroke prognosis in Chinese patients with type 2 diabetes and acute ischemic stroke. We recommend that further studies should be carried out with respect to the role of FABP4 in the pathology of the stroke outcomes.
References
Agardh HE, Folkersen L, Ekstrand J, Marcus D, Swedenborg J, Hedin U, Gabrielsen A, Paulsson-Berne G (2011) Expression of fatty acid-binding protein 4/aP2 is correlated with plaque instability in carotid atherosclerosis. J Intern Med 269:200–210
Aragonès G, Ferré R, Lázaro I, Cabré A, Plana N, Merino J, Heras M, Girona J, Masana L (2010) Fatty acid-binding protein 4 is associated with endothelial dysfunction in patients with type 2 diabetes. Atherosclerosis 213:329–331
Bonita RBR (1988) Modification of Rankin scale: recovery of motor function after stroke. Stroke 19:1497–1500
Brott T, Adams HP Jr, Olinger CP, Marler JR, Barsan WG, Biller J, Spilker J, Holleran R, Eberle R, Hertzberg V (1989) Measurements of acute cerebral infarction: a clinical examination scale. Stroke 20:864–870
Cabré A, Lázaro I, Girona J, Manzanares JM, Marimón F, Plana N, Heras M, Masana L (2008) Plasma fatty acid binding protein 4 is associated with atherogenic dyslipidemia in diabetes. J Lipid Res 49:1746–1751
Daubail B, Jacquin A, Guilland JC, Hervieu M, Osseby GV, Rouaud O, Giroud M, Béjot Y (2013) Serum 25-hydroxyvitamin D predicts severity and prognosis in stroke patients. Eur J Neurol 20:57–61
Erbay E, Babaev VR, Mayers JR, Makowski L, Charles KN, Snitow ME, Fazio S, Wiest MM, Watkins SM, Linton MF, Hotamisligil GS (2009) Reducing endoplasmic reticulum stress through a macrophage lipid chaperone alleviates atherosclerosis. Nat Med 15:1383–1392
Furuhashi M, Tuncman G, Görgün CZ, Makowski L, Atsumi G, Vaillancourt E, Kono K, Babaev VR, Fazio S, Linton MF, Sulsky R, Robl JA, Parker RA, Hotamisligil GS (2007) Treatment of diabetes and atherosclerosis by inhibiting fatty-acid-binding protein aP2. Nature 447:959–965
Guo M, Liu H, Li SS, Jiang FL, Xu JM, Tang YY (2017) Low serum brain-derived neurotrophic factor but not brain-derived neurotrophic factor gene Val66Met polymorphism is associated with diabetic retinopathy in Chinese type 2 diabetic patients. Retina 37:350–358
Holm S, Ueland T, Dahl TB, Michelsen AE, Skjelland M, Russell D, Nymo SH, Krohg-Sørensen K, Clausen OP, Atar D, Januzzi JL (2011) Fatty acid binding protein 4 is associated with carotid atherosclerosis and outcome in patients with acute ischemic stroke. PLoS One 6:e28785
Huang H, Zheng T, Wang S, Wei L, Wang Q, Sun Z (2016) Serum 25-hydroxyvitamin D predicts early recurrent stroke in ischemic stroke patients. Nutr Metab Cardiovasc Dis 26:908–914
Hui X, Li H, Zhou Z, Lam KS, Xiao Y, Wu D, Ding K, Wang Y, Vanhoutte PM, Xu A (2010) Adipocyte fatty acid–binding protein modulates inflammatory responses in macrophages through a positive feedback loop involving c-Jun NH2- terminal kinases and activator protein-1. J Biol Chem 285:10273–10280
Kaarisalo MM, Räihä I, Sivenius J, Immonen-Räihä P, Lehtonen A, Sarti C, Mähönen M, Torppa J, Tuomilehto J, Salomaa V (2005) Diabetes worsens the outcome of acute ischemic stroke. Diabetes Res Clin Pract 69:293–298
Liu G, Ding M, Chiuve SE, Rimm EB, Franks PW, Meigs JB, Hu FB, Sun Q (2016) Plasma levels of fatty acid–binding protein 4, retinol-binding protein 4, high-molecular-weight adiponectin, and cardiovascular mortality among men with type 2 diabetes. Arterioscler Thromb Vasc Biol 36:2259–2267
Maeda K, Uysal KT, Makowski L, Görgün CZ, Atsumi G, Parker RA, Brüning J, Hertzel AV, Bernlohr DA, Hotamisligil GS (2003) Role of the fatty acid binding protein mal1 in obesity and insulin resistance. Diabetes 52:300–307
Maeda K, Cao H, Kono K, Gorgun CZ, Furuhashi M, Uysal KT, Cao Q, Atsumi G, Malone H, Krishnan B, Minokoshi Y, Kahn BB, Parker RA, Hotamisligil GS (2005) Adipocyte/macrophage fatty acid binding proteins control integrated metabolic responses in obesity and diabetes. Cell Metab 1:107–119
Milner KL, van der Poorten D, Xu A, Bugianesi E, Kench JG, Lam KS, Chisholm DJ, George J (2009) Adipocyte fatty acid binding protein levels relate to inflammation and fibrosis in nonalcoholic fatty liver disease. Hepatology 49:1926–1934
Peeters W, de Kleijn DP, Vink A, van de Weg S, Schoneveld AH, Sze SK, van der Spek PJ, de Vries JP, Moll FL, Pasterkamp G (2011) Adipocyte fatty acid binding protein in atherosclerotic plaques is associated with local vulnerability and is predictive for the occurrence of adverse cardiovascular events. Eur Heart J 32:1758–1768
Pencina MJ, D’Agostino RB Sr, D’Agostino RB Jr, Vasan RS (2008) Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med 27:157–172
Rhee EJ, Lee WY, Park CY, Oh KW, Kim BJ, Sung KC, Kim BS (2009) The association of serum adipocyte fatty acid–binding protein with coronary artery disease in Korean adults. Eur J Endocrinol 160:165–172
Sims JR, Gharai LR, Schaefer PW, Vangel M, Rosenthal ES, Lev MH, Schwamm LH (2009) ABC/2 for rapid clinical estimate of infarct, perfusion, and mismatch volumes. Neurology 72:2104–2110
Song FY, Wu MH, Zhu LH, Zhang ZQ, Qi QD, Lou CL (2015) Elevated serum mannose-binding lectin levels are associated with poor outcome after acute ischemic stroke in patients with type 2 diabetes. Mol Neurobiol 52:1330–1340
Tso AW, Xu A, Sham PC, Wat NM, Wang Y, Fong CH, Cheung BM, Janus ED, Lam KS (2007) Serum adipocyte fatty acid–binding protein as a new biomarker predicting the development of type 2 diabetes. Diabetes Care 30:2667–2672
Tso AW, Lam TK, Xu A, Yiu KH, Tse HF, Li LS, Law LS, Cheung BM, Cheung RT, Lam KS (2011) Serum adipocyte fatty acid–binding protein associated with ischemic stroke and early death. Neurology 76:1968–1975
Tu WJ, Zeng XW, Deng A, Zhao SJ, Luo DZ, Ma GZ, Wang H, Liu Q (2017) Circulating FABP4 (fatty acid-binding protein 4) is a novel prognostic biomarker in patients with acute ischemic stroke. Stroke 48:1531–1538
Urra X, Cervera A, Obach V, Climent N, Planas AM, Chamorro A (2009) Monocytes are major players in the prognosis and risk of infection after acute stroke. Stroke 40:1262–1268
Vila N, Castillo J, Davalos A, Chanmorro A (2000) Proinflammatory cytokines and early neurological worsening in ischemic stroke. Stroke 31:2325–2329
Wang W, Jiang B, Sun H, Ru X, Sun D, Wang L, Wang L, Jiang Y, Li Y, Wang Y, Chen Z, Wu S, Zhang Y, Wang D, Wang Y, Feigin VL, NESS-China Investigators (2017) Prevalence, incidence and mortality of stroke in China: results from a nationwide population-based survey of 480,687 adults. Circulation 135:759–771
Xu A, Wang Y, Xu JY, Stejskal D, Tam S, Zhang J, Wat NM, Wong WK, Lam KS (2006) Adipocyte fatty acid–binding protein is a plasma biomarker closely associated with obesity and metabolic syndrome. Clin Chem 52:405–413
Yeung DC, Xu A, Cheung CW, Wat NM, Yau MH, Fong CH, Chau MT, Lam KS (2007) Serum adipocyte fatty acid-binding protein levels were independently associated with carotid atherosclerosis. Arterioscler Thromb Vasc Biol 27:1796–1802
Acknowledgments
We are grateful to the Department of Neurology and Emergency; the nurses, physicians, and patients who participated in our study; and the staff of the central laboratory of the Hospital. Authors also acknowledge the contribution of the editors and reviewers who have helped us to improve the manuscript.
Conflict of Interest
The authors declare that they have no conflict of interest.
Funding
This study was supported by grants from the Scientific research project of health and Family Planning Commission of Heilongjiang province (No. 2017–337), Graduate Innovative Project of Heilongjiang province (No. 2016YJSCX-20MY), and Scientific research project of Mudanjiang science and Technology Bureau (No. Z2016s0066).
Role of the Sponsor
The funding organizations had no role in the design and concept of the study; the collection, management, analysis, and interpretation of the data; or the preparation, review, or approval of the manuscript.
Author information
Authors and Affiliations
Contributions
Yin had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Li, Bi, Zhao, Lian, Zhu, Xu, Ding, Wang, Yin.
Acquisition of data: Li, Bi, Zhao, Lian, Yin.
Analysis and interpretation of data: Li, Zhu, Xu, Ding, Wang, Yin.
Drafting of the manuscript: Li, Bi, Zhao, Lian, Yin.
Critical revision of the manuscript for important intellectual content: Zhu, Xu, Ding, Wang.
Administrative, technical, or material support: Li, Bi, Zhao, Lian, Zhu, Xu, Ding, Wang, Yin.
Obtain funding: Yin.
Study supervision: Yin.
Corresponding author
Rights and permissions
About this article
Cite this article
Li, S., Bi, P., Zhao, W. et al. Prognostic Utility of Fatty Acid-Binding Protein 4 in Patients with Type 2 Diabetes and Acute Ischemic Stroke. Neurotox Res 33, 309–315 (2018). https://doi.org/10.1007/s12640-017-9792-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12640-017-9792-z