Abstract
In the present study, we investigate the effect of severe hyperhomocysteinemia on biochemical (creatine kinase activity), behavioral (memory tests), and histological assessments (hippocampal volume). A possible neuroprotective role of creatine on hyperhomocysteinemia effects was also evaluated. Severe hyperhomocysteinemia was induced in neonate rats (starting at 6 days of age) by treatment with homocysteine (0.3–0.6 μmol/g body weight) for 23 days. Creatine (50 mg/kg body weight) was administered concomitantly with homocysteine. Controls received saline in the same volumes. Twelve hours after the last injection, the rats were submitted to behavioral tests [(recognition task (NOR)] and inhibitory avoidance (IA)]. Following behavioral assessment, the animals were perfused and decapitated, the brain removed for subsequent morphological analysis of the hippocampus. Another group of animals was used to test creatine kinase activity in hippocampus. The results showed that rats treated with homocysteine decreased (44%) the exploration of the novel object in NOR. In the IA task, homocysteine-treated animals presented decreased latencies to step down the platform in short- (32%) and long-term (18%) testings (3 h and 7 days, respectively), evidencing aversive memory impairment. Hippocampal volume was not altered by homocysteine administration. Hyperhomocysteinemia decreased (45%) creatine kinase activity, and creatine was able to prevent such effect probably by creatine kinase/phosphocreatine/creatine homeostasis, which serves as energy circuit within of the cell. This finding may be associated, at least in part, with memory improvement, suggesting that creatine might represent an effective adjuvant to protect against the effects of high homocysteine plasma levels.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Homocysteine (Hcy) can be re-methylated to methionine via the re-methylation or 5-methyltetrahydrofolate pathway or it can undergo transsulfuration to form cysteine. Impaired metabolism due to genetic alteration in metabolic enzymes (methionine synthase, methyltetrahydrofolate reductase, cystathionine β-synthase (CβS), and cystathionine-γ-lyase) or deficiency in cofactors may lead to an acquired metabolic anomaly known as hyperhomocysteinemia (Bhatia and Singh 2015). Hyperhomocysteinemia is a common consequence of diet, behavioral, and pathological conditions and is epidemiologically related to different diseases, among which neurodegenerative ones are receiving progressively more attention in the last years. Patients with the rare homocystinuria have levels of >100 μmol/L and typically develop cognition deficits (Varga et al. 2005), being that in normal individuals, plasma Hcy levels range from 5 to 15 mmol/L.
Chronic Hcy administration in experimental animals has attracted the interest of scientific literature in recent decades because of the association between its concentration in the blood and the incidence of energy and memory impairment (Seshadri et al. 2008; Streck et al. 2004; Matté et al. 2009a, b; Ataie et al. 2010). Clinical investigations have shown elevated Hcy plasma levels in patients with neurodegenerative diseases (Bhatia and Singh 2015; Khan et al. 2015; Kunisawa et al. 2015) and cognitive disorders (Kunisawa et al. 2015; Zhao et al. 2015). There are clinical studies in humans (Imbard et al. 2015; McCully 2015; Vanzin et al. 2014, 2015; Sørensen et al. 2016) relating various changes and biochemical parameters found and used in experimental animal models of hyperhomocysteinemia. In this way, it is possible to clarify the consequences of high levels of Hcy on the physiology of human systems. It is the basic research to elucidate results as reliable sources in the literature capable of supporting promising clinical studies to better understand the pathophysiology of diseases in general.
Elevated levels of Hcy induce oxidative stress (Kolling et al. 2014; da Cunha et al. 2011). The reactive species generated by these abnormal levels of Hcy also increases the activation of matrix metalloproteinases (MMPs) (Gasche et al. 1999), particularly MMP-9 (Lee et al. 2012). These series of alterations may be responsible for the alterations in the plasticity and decrease in the speed of basic synaptic transmission in hippocampus of adult rats treated with Hcy (Christie et al. 2005). The alterations found in this region of the brain may be related to several studies that link elevated levels of Hcy with memory impairments and neurodegenerative diseases (Streck et al. 2004; Obeid et al. 2007). Furthermore, a recent study developed by Chung et al. (2016) demonstrated that Hcy enhances the interaction between fibrinogen and Aβ, promoting the formation of tighter fibrin clots and delaying clot fibrinolysis, suggesting that this may also be partially responsible for the significant impairments in learning and memory.
Creatine is well known for its protective properties, with studies showing that it can be beneficial in the treatment of neurodegenerative diseases (Bender et al. 2006; Chung et al. 2007; Tarnopolsky 2007; Beal 2011). Moreover, creatine has antioxidative properties per se (Lawler et al. 2002; Sestili et al. 2006). Therefore, creatine can prevent the oxidative stress that appears to play a role in the molecular changes that lead to memory deficits.
Energy metabolism is associated with important metabolic pathways. An important system that contributes in the maintenance of ATP levels is the creatine kinase (CK), which consists of a group of isoenzymes connected with a central role in energy metabolism and is responsible for providing and re-synthesizing ATP for tissues with high energy demands (Wallimann et al. 1992; Yang et al. 2010). There are few studies relating the involvement of CK with behavioral changes (Zhang et al. 2008; Hoyer and Lannert 2008).
In contradiction to the study presented by Bender and Klopstock (2016), some studies show beneficial effects of creatine. We can infer that the amine should be used with caution and in appropriate doses; it can change important physiological functions, as well as compromise the energy homeostasis of different organs and tissues (Kolling et al. 2015). There are several well-documented studies showing its beneficial role and its protective properties, showing that it can help in the treatment of neurodegenerative diseases (Bender et al. 2006; Chung et al. 2007; Tarnopolsky 2007; Beal 2011). In addition, it has antioxidant properties per se that can be considered relevant for a number of clinical conditions in patients with brain disorders (Lawler et al. 2002; Sestili et al. 2006).
Natural dietary supplements with creatine was accompanied by favorable effects on neurobehavioral functioning, especially memory skills (Bender et al. 2008), and has emerged as a promising adjunct therapy in several pathological conditions (Gualano et al. 2011, 2012). There are studies showing that creatine contributes to the improvement of energy homeostasis in several tissues (Kolling and Wyse 2010; Kolling et al. 2012, 2014, 2015) and prevents behavioral alterations in open field tasks (Vasques et al. 2006). These findings pinpoint to a close correlation between the functional capacity of the creatine kinase/phosphocreatine/creatine system and the proper brain function (Wyss and Schulze 2002). On this, we can highlight that creatine biosynthesis contributes to Hcy fluctuating concentrations in the plasma. Based on these findings, it is possible that creatine could prevent the effects of Hcy, lowering the risk of developing energy and cognitive impairment.
In view of these considerations and the fact that, to our knowledge, there are no studies assessing the relationship between CK activity, behavioral alterations, and histological amendments after inducing severe hyperhomocysteinemia, in the present study we investigate the effects of chronic administration of Hcy on CK activity in the hippocampus of rats, cognitive tests such as NOR and IA, as well as on hippocampal volume. We also evaluate whether creatine administration could prevent the enzymatic alteration relating them to the commitment of animal memory on some behavioral tasks.
Methods
Animals
In our study, we used male and/or female to accomplish the biochemical and morphological tests, as in previous studies, there were no differences in these parameters in relation to sex. For the behavioral tests that used only males, it is known that females suffer from hormonal fluctuation, and the different stages of the estrous cycle of these can interfere with the analysis of these parameters.
Male or female Wistar rats (6-day-old) were obtained from the Central Animal House of the Department of Biochemistry, Institute of Basic Science of Health, Universidade Federal do Rio Grande do Sul (Porto Alegre, RS, Brazil). Animals were maintained on a 12/12-h light/dark cycle, in an air-conditioned (22 ± 1 °C) colony room. Rats had free access to a 20% (w/w) protein commercial feed, and to water. All animal experiments were approved by the Ethical Committee of the Universidade Federal do Rio Grande do Sul (RG, Brazil; document no. 21847), and performed in accordance with the Guide for the Care and Use of Laboratory Animals (NIH, revised 2011).
Sample Size
The sample size was calculated according to the aid of the Minitab® program. In this case, the sample size was informed by the program of nine animals per group for biochemical studies and 15 animals per group for behavioral studies.
-
For the enzymatic activity (creatine kinase) and morphological analysis:
-
Level: 4
-
Power: 0.8
-
Maximum difference: 25%
-
Standard deviation: 15%
-
Sample size: 9
-
Real power: 0.806077
-
Total animals: 9 × 4 = 36 (× 2) = 72
-
-
For the behavioral analysis:
-
Level: 4
-
Power: 0.8
-
Maximum difference: 25%
-
Standard deviation: 20%
-
Sample size: 15
-
Real power: 0.800969
-
Total animals: 15 × 4 = 60
-
Subjects and Reagents
In our study, we used anhydrous creatine (from Sigma-Aldrich C0780).
Chronic Treatments with Creatine and Homocysteine
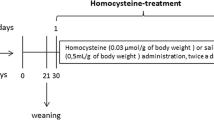
Creatine and Hcy were dissolved in 0.85% NaCl (pH 7.4), and administered to rats for 23 days, from the sixth day of age. Hcy was administered subcutaneously twice a day (morning and evening, with an 8-h gap between treatments), and at the following doses: 0.3 μmol Hcy/g in the first week of treatment, which was changed to 0.4 and 0.6 μmol Hcy/g in the second and third weeks of treatment, respectively. Plasma Hcy concentrations in rats subjected to this treatment regime achieve similar levels to those found in severe hyperhomocysteinemic patients (Streck et al. 2002, 2003a, b). Creatine (50 mg/kg) was injected intraperitoneally once a day, as described by Kolling and Wyse (2010). Control animals received saline solution (in the same volumes given to Hcy- and creatine-treated rats). Six animals were used for each group in all experiments. The behavioral tests began 12 h after the last injections of Hcy and creatine. Twenty-four hours after the behavioral tests, the animals were perfused and decapitated, the brain removed for subsequent morphological analysis of the hippocampus. Other animals were divided into the same experimental groups and were subjected to further treatment, following the same chronic model of severe hyperhomocysteinemia, and 12 h after the last injection, they were decapitated, the brain removed, and the hippocampus dissected for analysis of creatine kinase activity.
Creatine Kinase Activity
CK activity was measured in total homogenate preparation from the hippocampus of rats. The reaction mixture consisted of the following medium: 65 mM Tris-HCl buffer pH 7.5, containing 7 mM phosphocreatine, 9 mM MgSO4, and approximately 0.4–1.2 μg protein in a final volume of 0.1 mL. EMA (1 and 5 mM) was supplemented to the medium, whereas controls did not contain this acid. After 5 min of preincubation at 37 °C, the reaction was started by the addition of 3.2 mM ADP plus 0.8 mM reduced glutathione. The reaction was stopped after 10 min by the addition of 1 μmol p-hydroxymercuribenzoic acid. The creatine formed was estimated according to the colorimetric method of Hughes (1962). The color was developed by the addition of 0.1 mL 2% α-naphthol and 0.1 mL 0.05% diacetyl in a final volume of 1 mL and read after 20 min at 540 nm. None of the substances added to the assay medium interfered with the color development or spectrophotometric readings. Results are expressed as micromoles of creatine per minute per milligram of protein.
Novel Object Recognition (Non-aversive Task)
The novel object recognition task assesses declarative memory (Clark and Martin 2005). In the first phase of the test, each animal was confronted with two different objects, placed in an open-field box, and the time of object exploration is registered in 5 min. Following this phase, the rodent was removed from the open-field box to another separate box for a period of 5 min. In the second phase, each animal was exposed to two objects placed in the same open-field box: one familiar object, used in the first phase, and one novel object. The time spent exploring the novel object and the familiar object was described by Benice and Raber (2009) and Rodrigues et al. (2016). A discrimination index was calculated in the test session (second), as follows: the difference in exploration time divided by the total time spent exploring the two objects (B – A / B + A, where B is the new object and A is the familiar object) (Okuda et al. 2004).
Inhibitory Avoidance (Aversive Task)
An acrylic box was used (50 × 25 × 25 cm), with the left-most 7 cm of the box floor was occupied by a 3-cm-high platform. The box floor was a grid of parallel stainless steel bars (1.5 mm diameter) spaced 1 cm apart. Animals were gently placed on the platform, and their latencies to step down placing their four paws on the grid were measured with an automatic device. On stepping down, they received a 0.5-mA, 60-Hz scrambled foot shock for 2 s, and were withdrawn from the box. Animals were tested for retention 3 h and 7 days later. The test session was procedurally similar to the training one except that foot shock was omitted; step-down latency in test was used as an index of retention (Arteni et al. 2003 and Netto et al. 1985).
Morphological Analysis
Wistar rats were anesthetized with ketamine and xylazine (100 and 10 mg/kg body weight, respectively, i.p.) and perfused through the left cardiac ventricle with 0.9% saline followed by 4% paraformaldehyde with phosphate buffer. The brains were quickly removed and placed in 4% paraformaldehyde buffered solution. After 4 h, the samples were immersed in a solution of 30% sucrose for cryoprotection for 2 days. Following this, the samples were sectioned using a cryostat (CM1850, Leica, São Paulo, SP, Brazil) in coronal slices of 40 μm thickness. Coronal sections containing the entire hippocampus and striatum area were placed on gelatinized glass slides and stained with hematoxylin and eosin. Images were then captured and digitalized using the Adobe Photoshop software, and the areas of each hemisphere were measured using NIH ImageJ software.
Hippocampal Volume
The entire hippocampal and dentate gyrus area, in accordance with Paxinos and Watson (1986), was delineated in all sections. The hippocampus volume was calculated by the sum of the areas multiplied by the section interval, according to the Cavalieri method (Pereira et al. 2007; Arteni et al. 2010; Sanches et al. 2013). The anterior limit was set to find the first slice containing the hippocampus and the posterior one in which the ventral hippocampus first appeared (i.e., between coordinates—1.60 and 6.84 mm of the Paxinos Atlas). Ammon’s horn volume was calculated as the difference between the entire hippocampus and dentate gyrus volumes (Rodrigues et al. 2004).
Statistical Analysis
The parametric data for four groups were analyzed by one-way analysis of variance (ANOVA) followed by post hoc Tukey test when F test was significant. Non-parametric data for four groups were analyzed by Kruskal-Wallis test followed post hoc Dunn test when F test was significant. The creatine kinase activity and morphological analysis of the hippocampus were analyzed by parametric statistical test. The behavioral parameters were analyzed by non-parametric statistical test.
Values of p < 0.05 were considered statistically significant. All analyzes and graphics were performed using GraphPad Prism 5.1 software program in a compatible computer.
Results
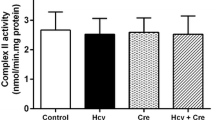
Hcy Alters CK(total) Activity
Hcy decreased the activity of CK(total) in hippocampus of rats (p < 0.01). Creatine was able to prevent this reduction (Fig. 1).
Hcy Impairs Memory Formation
Novel Object Recognition
Firstly, the novel object recognition test was applied to access the effect of Hcy on recognition memory. Control rats spent less time on the familiar object in the test session when compared to the training; thus, the time exploring the novel object increased in the control group (p < 0.05), and decreased in treated animals (p < 0.05). Creatine per se did not exert any effect on this parameter and when associated with Hcy was not able to prevent such effects (Fig. 2a). The group treated with Hcy also exhibited a reduced discrimination index (p < 0.05) (Fig. 2b).
Effect of Hcy, creatine, and Hcy plus creatine on novel object recognition. Time spent in each object (a) and discrimination index (b) in the test session. Note A is the old object and B is the novel object, and the discrimination index was calculated by the formula [(B − A) / (A + B)]. Data are expressed as mean ± S.E.M. for 13–16 animals in each group. Statistical difference as p < 0.05. *Different from control. Hcy homocysteine
Inhibitory Avoidance Task
Short and long aversive memory was evaluated in the inhibitory avoidance task (3 h and 7 days after training sessions). Kruskal-Wallis showed difference between groups at 3 h (p < 0.05) and 7 days (p < 0.05). Control groups increase their latencies to step down the platform for 3 h and 7 days, respectively (p < 0.05). The Hcy group showed long-term memory impairment since the latencies between training and test session were not statistically different (p > 0.05), demonstrating that animals did not remember the shock, that way down the platform in similar times in training and testing. We note that creatine when associated with Hcy increases the latency in the test section, preventing a possible damage in the memory (p < 0.05) (Fig. 3).
Effect of Hcy, creatine, and Hcy plus creatine on step-down inhibitory avoidance task. Latency to step down the platform in the training, short-term (3 h), and long-term (7 days) test sessions in the inhibitory avoidance task. Results are expressed as medians and 25–75% percentiles. Non-parametric data were analyzed by Kruskal-Wallis test. Statistical difference as p < 0.05. *Difference from control. #Difference between testing and training session. Hcy homocysteine
Histological Assessment
The Cavalieri method was used for volumetric brain analysis: statistical analysis revealed that all groups present a similar mean volume between right and left hemispheres (p > 0.05) (Fig. 4).
Effect of Hcy, creatine, and Hcy plus creatine on hippocampal volumes. Representative micrographs of hematoxylin-eosin-stained coronal sections at −2.80 mm hippocampal level from control and Hcy animals. Data are displayed as mean ± SEM. There was no statistical difference (p > 0.05). Hcy homocysteine
Discussion
It has been reported that Hcy alters phosphatase, kinase, and methylation activities (Ho et al. 2002; Chan et al. 2008); perturbs the methionine cycle (Miller 2003); and induces oxidative damage in brain tissue (Obeid and Herrmann 2006) which may be the cause of cognitive decline. Initially in our study, we observed that the chronic administration of Hcy causes a decrease in CK activity. A decrease in the CK activity in the brain may be associated with the disruption of neuronal functions, loss of hippocampal mossy fiber bodies, and changes in mitochondrial structure that are typical for pathological conditions, such as encephalomyopathies (Watanabe et al. 2002), which can be neurological signs found in the severe hyperhomocysteinemia. We observed that creatine administration prevents the decrease in CK activity. The enzyme CK and phosphocreatine form an extremely efficient energy buffering system, serving as a spatial “energy shuttle” or energy circuit within the cell (Adhihetty and Beal 2008). We suggest that the beneficial impact of creatine administration may have occurred as a result of its well-defined functional improvements on creatine kinase activity.
We assessed the locomotor and/or exploratory function of the animals by open field task. We did not observe locomotor and/or exploratory changes in rats subjected by chronic hyperhomocysteinemia in this test, indicating that they were able to carry out the aversive task. The influence of Hcy administration on memory was initially investigated in the novel object recognition task, which consists a non-aversive task. As a normal behavior, control rats spent less time on the familiar object in the test session when compared to the training. When we compare the training and testing sessions of animals treated with Hcy, the exploration of the novel object in NOR decreased. The Hcy group showed a significant reduction in exploration earlier than the control group in both phases. In relation to differential exploration between objects in the test phase, an early decrease in the exploration of the novel object was observed in the Hcy group, also suggesting that low performance in this parameter should be anticipated.
The inhibitory avoidance is one of the most widely used behavioral tasks to assess aversive memory and learning. Short and long aversive memories were evaluated in the inhibitory avoidance task (3 h and 7 days after training sessions). We observe in this test that rats submitted to Hcy administration exhibit similar behavior to control animals (3 h and 7 days after training session). When we compare training and testing sessions of animals submitted to chronic hyperhomocysteinemia, we did not observe significant differences. On the other hand, when comparing the training and testing sessions of animals treated with creatine, we observe that they learned in 3 h and 7 days after the training.
Correlating our results, some aspects could contribute to the memory consolidation impairment elicited by Hcy. Some researchers observed that a reduction of CK activity may potentially impair energy homeostasis, contributing to brain damage that may lead to cognitive deficits (Burbaeva et al. 1999; Sackeim 2000), such as that observed in this study.
Regarding morphology, we note that the hippocampus may suffer the insults caused by Hcy, since there are studies showing relevant changes in this structure (Machado et al. 2011; da Cunha et al. 2012a, b; Kolling et al. 2015). Hippocampal atrophy is observed in some studies related to hyperhomocysteinemia, and it can be quantified with a computer-assisted analysis using a magnetic resonance imaging device (Firbank et al. 2010; Shimomura et al. 2011). Thus, we decided to evaluate the hippocampal impairment, analyzing whether there is any morphological change in the volume of this structure after the induction of the severe hyperhomocysteinemia model. In our study, we observed that control and Hcy groups present similar mean volumes between right and left hemispheres, demonstrating that there was no change in hippocampal volume.
Creatine has been shown as a safe approach in the prevention and treatment of central nervous system disorders, such as neurodegenerative diseases, cognitive impairments, and even hyperhomocysteinemia (Andres et al. 2008; Allen 2012; Suresh et al. 2015). Considering that more studies are necessary to establish the effect of creatine on experimental models, we also evaluated the effect of this compound on impairments elicited by Hcy administration. Results showed that creatine efficiently prevented the deficits in CK activity and the consolidation of memory in hyperhomocysteinemic rats, evaluated by inhibitory avoidance task. To our knowledge, there are a limited number of studies about the mechanisms involved on creatine cognition-related effects; thus, our findings may be identified as a promising study.
We believe that homocysteine levels should be measured in this study in order to determine whether there is a real decrease due to the creatine administration. In relation to bioenergetics, more parameters should be evaluated, as well as CK: how to check the activity of the enzymes of the respiratory chain, measure the levels of ATP, and evaluate the activity of Na+, K+-ATPase, an important enzyme for neuronal excitability. Furthermore, other morphological evaluations can be carried out to complement the study in relation to the state that the hippocampus is after induction of the experimental model.
In summary, in this work we demonstrated for the first time that Hcy alters important parameters of the phosphoryl transfer network in rat brain, as CK. The enzymatic and behavioral alterations are probably consequences of cell and tissue damages. Therefore, it is possible to hypothesize that the inhibition in CK activity associated with changes of other parameters may contribute to brain damage causing impairment in energy homeostasis and behavioral and cognitive deficits. If this also occurs in severe hyperhomocysteinemia, it is possible that creatine administration might be beneficial to homocystinuric patients.
References
Adhihetty P, Beal M (2008) Creatine and its potential therapeutic value for targeting cellular energy impairment in neurodegenerative diseases. NeuroMolecular Med 10(4):275–290. doi:10.1007/s12017-008-8053-y
Allen P (2012) Creatine metabolism and psychiatric disorders: does creatine supplementation have therapeutic value? Neurosci Biobehav Rev 36(5):1442–1462. doi:10.1016/j.neubiorev.2012.03.005
Andres R, Ducray A, Schlattner U, Wallimann T, Widmer H (2008) Functions and effects of creatine in the central nervous system. Brain Res Bull 76(4):329–343. doi:10.1016/j.brainresbull.2008.02.035
Arteni N, Salgueiro J, Torres I, Achaval M, Netto C (2003) Neonatal cerebral hypoxia–ischemia causes lateralized memory impairments in the adult rat. Brain Res 973(2):171–178. doi:10.1016/s0006-8993(03)02436-3
Arteni N, Pereira L, Rodrigues A, Lavinsky D, Achaval M, Netto C (2010) Lateralized and sex-dependent behavioral and morphological effects of unilateral neonatal cerebral hypoxia-ischemia in the rat. Behav Brain Res 210(1):92–98. doi:10.1016/j.bbr.2010.02.015
Ataie A, Sabetkasaei M, Haghparast A, Moghaddam A, Ataee R, Moghaddam S (2010) Curcumin exerts neuroprotective effects against homocysteine intracerebroventricular injection-induced cognitive impairment and oxidative stress in rat brain. J Med Food 13(4):821–826. doi:10.1089/jmf.2009.1278
Beal MF (2011) Neuroprotective effects of creatine. Amino Acids 40(5):1305–1313
Bender A, Klopstock T (2016) Creatine for neuroprotection in neurodegenerative disease: end of story? Amino Acids 48(8):1929–1940
Bender A, Samtleben W, Elstner M, Klopstock T (2008) Long-term creatine supplementation is safe in aged patients with Parkinson disease. Nutr Res 28(3):172–178. doi:10.1016/j.nutres.2008.01.001
Bender A, Koch W, Elstner M, Schombacher Y, Bender J, Moeschl M, Gekeler F, Muller-Myhsok B, Gasser T, Tatsch K, Klopstock T (2006) Creatine supplementation in Parkinson disease: a placebo-controlled randomized pilot trial. Neurology 67(7):1262–1264
Benice T, Raber J (2009) Dihydrotestosterone modulates spatial working-memory performance in male mice. J Neurochem 110(3):902–911. doi:10.1111/j.1471-4159.2009.06183.x
Bhatia P, Singh N (2015) Homocysteine excess: delineating the possible mechanism of neurotoxicity and depression. Fundam Clin Pharmacol 29(6):522–528. doi:10.1111/fcp.12145
Breckenridge D, Germain M, Mathai J, Nguyen M, Shore G (2003) Regulation of apoptosis by endoplasmic reticulum pathways. Oncogene 22(53):8608–8618. doi:10.1038/sj.onc.1207108
Burbaeva GSh, Savushkina OK, Dmitriev AD (1999) Brain isoforms of creatine kinase in health and mental diseases: Alzheimer's disease and schizophrenia. Vestn Ross Akad Med Nauk (1):20–4
Chan A, Alsaraby A, Shea T (2008) Folate deprivation increases tau phosphorylation by homocysteine-induced calcium influx and by inhibition of phosphatase activity: alleviation by S-adenosyl methionine. Brain Res 1199:133–137. doi:10.1016/j.brainres.2008.01.008
Christie L, Riedel G, Algaidi S, Whalley L, Platt B (2005) Enhanced hippocampal long-term potentiation in rats after chronic exposure to homocysteine. Neurosci Lett 373(2):119–124
Chung YC, Kruyer A, Yao Y, Feierman E, Richards A, Strickland S, Norris EH (2016) Hyperhomocysteinemia exacerbates Alzheimer's disease pathology by way of the Î2-amyloid fibrinogen interaction. J Thromb Haemost 14(7):1442–1452
Chung Y-l, Alexanderson H, Pipitone N, Morrison C, Dastmalchi M, Ståhl-Hallengren C, Selwyn R, Louise Thomas E, Hamilton G, Bell JD, Lundberg IE, Scott DL (2007) Creatine supplements in patients with idiopathic inflammatory myopathies who are clinically weak after conventional pharmacologic treatment: six-month, double-blind, randomized, placebo-controlled trial. Arthritis Rheum 57(4):694–702
Clark R, Martin S (2005) Interrogating rodents regarding their object and spatial memory. Curr Opin Neurobiol 15(5):593–598. doi:10.1016/j.conb.2005.08.014
da Cunha AA, Ferreira AG, da Cunha MJ, Pederzolli CD, Becker DL, Coelho JG, Dutra-Filho CS, Wyse AT (2011) Chronic hyperhomocysteinemia induces oxidative damage in the rat lung. Mol Cell Biochem 358(1–2):153–160. doi:10.1007/s11010-011-0930-2
da Cunha A, Ferreira A, Loureiro S, da Cunha M, Schmitz F, Netto C, Wyse A (2012a) Chronic hyperhomocysteinemia increases inflammatory markers in hippocampus and serum of rats. Neurochem Res 37(8):1660–1669. doi:10.1007/s11064-012-0769-2
da Cunha AA, Scherer E, da Cunha MJ, Schmitz F, Machado FR, Lima DD, Delwing D, Wyse AT (2012b) Acute hyperhomocysteinemia alters the coagulation system and oxidative status in the blood of rats. Mol Cell Biochem 360(1–2):205–214. doi:10.1007/s11010-011-1058-0
Firbank MJ, Narayan SK, Saxby BK, Ford GA, O'Brien JT (2010) Homocysteine is associated with hippocampal and white matter atrophy in older subjects with mild hypertension. Int Psychogeriatr 22(05):804–811
Gasche Y, Fujimura M, Morita-Fujimura Y, Copin J, Kawase M, Massengale J, Chan P (1999) Early appearance of activated matrix metalloproteinase-9 after focal cerebral ischemia in mice: a possible role in blood-brain barrier dysfunction. Journal Of Cerebral Blood Flow & Metabolism 1020–1028
Gualano B, Lugaresi R, Painelli de Salles V, Queiroz A, Artioli G, Roschel H et al (2011) Creatine supplementation does not augment muscle carnosine content in type 2 diabetic patients. Appl Physiol Nutr Metab 36(5):764–767. doi:10.1139/h11-083
Gualano B, Roschel H, Lancha AH, Brightbill CE, Rawson ES (2012) In sickness and in health: the widespread application of creatine supplementation. Amino Acids 43(2):519–529. doi:10.1007/s00726-011-1132-7
Ho P, Ortiz D, Rogers E, Shea T (2002) Multiple aspects of homocysteine neurotoxicity: glutamate excitotoxicity, kinase hyperactivation and DNA damage. J Neurosci Res 70(5):694–702. doi:10.1002/jnr.10416
Hoyer S, Lannert H (2008) Long-term effects of corticosterone on behavior, oxidative and energy metabolism of parietotemporal cerebral cortex and hippocampus of rats: comparison to intracerebroventricular streptozotocin. J Neural Transm 115(9):1241–1249. doi:10.1007/s00702-008-0079-7
Hughes B (1962) A method for the estimation of serum creatine kinase and its use in comparing creatine kinase and aldolase activity in normal and pathological sera. Clin Chim Acta 7(5):597–603. doi:10.1016/0009-8981(62)90137-7
Imbard A, Benoist JF, Esse R, Gupta S, Lebon S, de Vriese AS, de Baulny HO, Kruger W, Schiff M, Blom HJ (2015) High homocysteine induces betaine depletion. Biosci Rep 35(4). doi:10.1042/BSR20150094
Khan R, Hossain M, Nai Q, Yousif A, Sen S (2015) Hyperhomocysteinemia association with transient global amnesia: a rare case report. N Am J Med Sci 7(8):374. doi:10.4103/1947-2714.163647
Kolling J, Wyse A (2010) Creatine prevents the inhibition of energy metabolism and lipid peroxidation in rats subjected to GAA administration. Metab Brain Dis 25(3):331–338. doi:10.1007/s11011-010-9215-9
Kolling J, Scherer E, Siebert C, Hansen F, Torres F, Scaini G et al (2012) Homocysteine induces energy imbalance in rat skeletal muscle: is creatine a protector? Cell Biochem Funct. doi:10.1002/cbf.2938
Kolling J, Scherer EB, Siebert C, Marques EP, Dos Santos TM, Wyse AT (2014) Creatine prevents the imbalance of redox homeostasis caused by homocysteine in skeletal muscle of rats. Gene 545(1):72–9. doi: 10.1016/j.gene.2014.05.005
Kolling J, Scherer EB, Siebert C, Longoni A, Loureiro S, Weis S, Petenuzzo L, Wyse AT (2015) Severe hyperhomocysteinemia decreases respiratory enzyme and Na+−K+ ATPase activities, and leads to mitochondrial alterations in rat amygdala. Neurotox Res 29(3):408–418. doi:10.1007/s12640-015-9587-z
Kunisawa K, Nakashima N, Nagao M, Nomura T, Kinoshita S, Hiramatsu M (2015) Betaine prevents homocysteine-induced memory impairment via matrix metalloproteinase-9 in the frontal cortex. Behav Brain Res 292:36–43. doi:10.1016/j.bbr.2015.06.004
Lawler JM, Barnes WS, Wu G, Song W, Demaree S (2002) Direct antioxidant properties of Creatine. Biochem Biophys Res Commun 290(1):47–52
Lee S, Lee Y, Seo K, Bae J, Kim G, Park S, Kim C (2012) Homocysteine enhances MMP-9 production in murine macrophages via ERK and Akt signaling pathways. Toxicol Appl Pharmacol 260(1):89–94. doi:10.1016/j.taap.2012.01.026
Machado F, Ferreira A, da Cunha A, Tagliari B, Mussulini B, Wofchuk S, Wyse A (2011) Homocysteine alters glutamate uptake and Na+,K+−ATPase activity and oxidative status in rats hippocampus: protection by vitamin C. Metab Brain Dis 26(1):61–67. doi:10.1007/s11011-011-9232-3
Matté C, Pereira L, Dos Santos T, Mackedanz V, Cunha A, Netto C, Wyse A (2009a) Acute homocysteine administration impairs memory consolidation on inhibitory avoidance task and decreases hippocampal brain-derived neurotrophic factor immunocontent: prevention by folic acid treatment. Neuroscience 163(4):1039–1045. doi:10.1016/j.neuroscience.2009.07.023
Matté C, Stefanello FM, Mackedanz V, Pederzolli CD, Lamers ML, Dutra-Filho CS, Dos Santos MF, Wyse AT (2009b) Homocysteine induces oxidative stress, inflammatory infiltration, fibrosis and reduces glycogen/glycoprotein content in liver of rats. Int J Dev Neurosci 27(4):337–344. doi:10.1016/j.ijdevneu.2009.03.005
McCully KS (2015) Homocysteine and the pathogenesis of atherosclerosis. Expert Rev Clin Pharmacol 8(2):211–219. doi:10.1586/17512433.2015.1010516 Review
Miller AL (2003) The methionine-homocysteine cycle and its effects on cognitive diseases. Altern Med Rev 8(1):7–19
Netto C, Dias R, Izquierdo I (1985) Interaction between consecutive learnings: inhibitory avoidance and habituation. Behav Neural Biol 44(3):515–520. doi:10.1016/s0163-1047(85)91048-9
Obeid R, Herrmann W (2006) Mechanisms of homocysteine neurotoxicity in neurodegenerative diseases with special reference to dementia. FEBS Lett 580(13):2994–3005. doi:10.1016/j.febslet.2006.04.088
Obeid R, McCaddon A, Herrmann W (2007) The role of hyperhomocysteinemia and B-vitamin deficiency in neurological and psychiatric diseases. Clin Chem Lab Med 45(12). doi:10.1515/cclm.2007.356
Okuda S, Roozendaal B, McGaugh J (2004) Glucocorticoid effects on object recognition memory require training-associated emotional arousal. Proc Natl Acad Sci 101(3):853–858. doi:10.1073/pnas.0307803100
Paxinos G, Watson C (1986) The rat brain in stereotaxic coordinates 2ed. Academic Press, Sydney
Pereira L, Arteni N, Petersen R, da Rocha A, Achaval M, Netto C (2007) Effects of daily environmental enrichment on memory deficits and brain injury following neonatal hypoxia-ischemia in the rat. Neurobiol Learn Mem 87(1):101–108. doi:10.1016/j.nlm.2006.07.003
Rodrigues A, Arteni N, Abel C, Zylbersztejn D, Chazan R, Viola G et al (2004) Tactile stimulation and maternal separation prevent hippocampal damage in rats submitted to neonatal hypoxia–ischemia. Brain Res 1002(1–2):94–99. doi:10.1016/j.brainres.2003.12.020
Rodrigues AF, Biasibetti H, Zanotto BS, Sanches EF, Pierozan P, Schmitz F, Parisi MM, Barbé-Tuana F, Netto CA, Wyse ATS (2016) Intracerebroventricular d-galactose administration impairs memory and alters activity and expression of acetylcholinesterase in the rat. Int J Dev Neurosci 50:1–6
Sanches E, Arteni N, Scherer E, Kolling J, Nicola F, Willborn S et al (2013) Are the consequences of neonatal hypoxia–ischemia dependent on animals’ sex and brain lateralization? Brain Res 1507:105–114. doi:10.1016/j.brainres.2013.02.040
Seshadri S, Wolf P, Beiser A, Selhub J, Au R, Jacques P et al (2008) Association of plasma total homocysteine levels with subclinical brain injury. Arch Neurol 65(5). doi:10.1001/archneur.65.5.642
Sestili P, Chiara M, Bravi G, Piccoli G, Curci R, Battistelli M, Falcieri E, Agostini D, Gioacchini AM, Stocchi V (2006) Creatine supplementation affords cytoprotection in oxidatively injured cultured mammalian cells via direct antioxidant activity. Free Radic Biol Med 40(5):837–849
Shimomura T, Futoshi A, Takayuki M, Umeno Y, Eshima N, Saikawa T, Yoshimatsu H, Fujiki M, Kobayashi H (2011) Homocysteine levels are associated with hippocampus volume in type 2 diabetic patients. Eur J Clin Investig 41(7):751–758
Sørensen JT, Gaustadnes M, Stabler SP, Allen RH, Mudd SH, Hvas AM (2016) Molecular and biochemical investigations of patients with intermediate or severe hyperhomocysteinemia. Mol Genet Metab 117(3):344–350. doi:10.1016/j.ymgme.2015.12.010
Streck E, Zugno A, Tagliari B, Sarkis J, Wajner M, Wannmacher C, Wyse A (2002) On the mechanism of the inhibition of Na+, K+−ATPase activity caused by homocysteine. Int J Dev Neurosci 20(2):77–81. doi:10.1016/s0736-5748(02)00043-6
Streck EL, Delwing D, Tagliari B, Matté C, Wannmacher CM, Wajner M, Wyse AT (2003a) Brain energy metabolism is compromised by the metabolites accumulating in homocystinuria. Neurochem Int 43(6):597–602
Streck EL, Matté C, Vieira PS, Calcagnotto T, Wannmacher CM, Wajner M, Wyse AT (2003b) Impairment of energy metabolism in hippocampus of rats subjected to chemically-induced hyperhomocysteinemia. Biochim Biophys Acta 1637(3):187–192
Streck E, Bavaresco C, Netto C, Wyse A (2004) Chronic hyperhomocysteinemia provokes a memory deficit in rats in the Morris water maze task. Behav Brain Res 153(2):377–381. doi:10.1016/j.bbr.2003.12.013
Suresh P, Ayyappan A, Nandini J, Ismail T (2015). Cognitive deficits and behavioral disorders in children: a comprehensive multidisciplinary approach to management. Annals of Behavioural Science
Tarnopolsky MA (2007) Clinical use of creatine in neuromuscular and neurometabolic disorders. Subcell Biochem 46:183–204
Vanzin CS, Manfredini V, Marinho AE, Biancini GB, Ribas GS, Deon M, Wyse AT, Wajner M, Vargas CR (2014) Homocysteine contribution to DNA damage in cystathionine β-synthase-deficient patients. Gene 539(2):270–274. doi:10.1016/j.gene.2014.02.015
Vanzin CS, Mescka CP, Donida B, Hammerschimidt TG, Ribas GS, Kolling J, Scherer EB, Vilarinho L, Nogueira C, Coitinho AS, Wajner M, Wyse AT, Vargas CR (2015) Lipid, oxidative and inflammatory profile and alterations in the enzymes paraoxonase and butyrylcholinesterase in plasma of patients with homocystinuria due CBS deficiency: the vitamin B12 and folic acid importance. Cell Mol Neurobiol 35(6):899–911. doi:10.1007/s10571-015-0185-7
Varga EA, Sturm AC, Misita CP, Moll S (2005) Homocysteine and MTHFR mutations: relation to thrombosis and coronary artery disease. Circulation 111(19):e289–e293. doi:10.1161/01.cir.0000165142.37711.e7
Vasques V, Brinco F, Viegas C, Wajner M (2006) Creatine prevents behavioral alterations caused by methylmalonic acid administration into the hippocampus of rats in the open field task. J Neurol Sci 244(1–2):23–29. doi:10.1016/j.jns.2005.12.005
Wallimann T, Wyss M, Brdiczka D, Nicolay K, Eppenberger H (1992) Intracellular compartmentation, structure and function of creatine kinase isoenzymes in tissues with high and fluctuating energy demands: the ‘phosphocreatine circuit’ for cellular energy homeostasis. Biochem J 281(1):21–40. doi:10.1042/bj2810021
Watanabe A, Kato N, Kato T (2002) Effects of creatine on mental fatigue and cerebral hemoglobin oxygenation. Neurosci Res 42(4):279–285
Wyss M, Schulze A (2002) Health implications of creatine: can oral creatine supplementation protect against neurological and atherosclerotic disease? Neuroscience 112(2):243–260. doi:10.1016/s0306-4522(02)00088-x
Yang Y, Fann M, Chang W, Tai L, Jiang J, Kao L (2010) Regulation of sodium-calcium exchanger activity by creatine kinase under energy-compromised conditions. J Biol Chem 285(36):28275–28285. doi:10.1074/jbc.m110.141424
Zhao H, Ji Z, Liu C, Yu X (2015) Neuroprotection and mechanisms of atractylenolide III in preventing learning and memory impairment induced by chronic high-dose homocysteine administration in rats. Neuroscience 290:485–491. doi:10.1016/j.neuroscience.2015.01.060
Zhang X-l, An L-j, Bao Y-m, Wang J-y, Jiang B (2008) D-galactose administration induces memory loss and energy metabolism disturbance in mice: protective effects of catalpol. Food Chem Toxicol 46(8):2888–2894
Acknowledgments
This work was partly supported by grants from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure
The following work has not been published previously and is not under consideration for publication elsewhere, and its publication is approved by all authors. If accepted, it will not be published elsewhere including electronically, in English or in any other language, without the written consent of the copyright holder.
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Kolling, J., Longoni, A., Siebert, C. et al. Severe Hyperhomocysteinemia Decreases Creatine Kinase Activity and Causes Memory Impairment: Neuroprotective Role of Creatine. Neurotox Res 32, 585–593 (2017). https://doi.org/10.1007/s12640-017-9767-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12640-017-9767-0