Abstract
This study investigated the effects of chronic homocysteine administration on some parameters of inflammation, such as cytokines (TNF-α, IL-1β and IL-6), chemokine CCL2 (MCP-1), nitrite and prostaglandin E2 levels, as well as on immunocontent of NF-κB/p65 subunit in hippocampus and/or serum of rats. Since acetylcholinesterase has been associated with inflammation, we also evaluated the effect of homocysteine on this enzyme activity in hippocampus of rats. Wistar rats received daily subcutaneous injections of homocysteine (0.3–0.6 μmol/g body weight) or saline (control) from the 6th to the 28th days-of-age. One or 12 h after the last injection, rats were euthanized and hippocampus and serum were used. Results showed that chronic hyperhomocysteinemia significantly increased pro-inflammatory cytokines (TNF-α, IL-1β and IL-6), chemokine CCL2 (MCP-1) and prostaglandin E2 in hippocampus and serum of rats at 1 and 12 h after the last injection of homocysteine. Nitrite levels increased in hippocampus, but decreased in serum at 1 h after chronic hyperhomocysteinemia. Acetylcholinesterase activity and immunocontent of citoplasmic and nuclear NF-κB/p65 subunit were increased in hippocampus of rats subjected to hyperhomocysteinemia at 1 h, but did not alter at 12 h after the last injection of homocysteine. According to our results, chronic hyperhomocysteinemia increases inflammatory parameters, suggesting that this process might be associated, at least in part, with the cerebrovascular and vascular dysfunctions characteristic of some homocystinuric patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tissue levels of homocysteine (Hcy) are increased in homocystinuria, an inborn error of metabolism characterized biochemically by cystathionine β-synthase (CBS, EC 4.2.1.22) deficiency [1]. Hyperhomocysteinemia also occurs in neurodegenerative disorders, such as Alzheimer’s and Parkinson’s diseases [2–4], and has been identified as an independent risk factor for atherosclerosis, cerebrovascular and neuroinflammatory diseases [5–9].
Inflammation is fundamentally a protective response whose ultimate goal is to eliminate the injury-inducing agent, including a micro-organism, physical stimuli and chemical agent [10]. Some of the important mediators of inflammation are: histamine, serotonin, lysosomal enzymes, prostaglandins (PGs), leukotrienes (LTs), platelet activating factor, reactive oxygen species (ROS), nitric oxide (NO), cytokines, chemokines, acute-phase proteins, coagulation/fibrinolysis system, and complement system [10]. Exposure of the cells to diverse stimuli, such as inflammatory cytokines, oxidative stress, or bacterial endotoxins, results in activation of nuclear factor-kappaB (NF-κB) through the stimulation of phosphorylation and degradation of IκBα [11].
Activated NF-κB is then translocated to the nucleus, where it binds to the cis-acting κB enhancer element of target genes and activates the expression of pro-inflammatory mediators [12]. NF-κB plays an important role in inflammatory phenotypic changes in various pathophysiological conditions [13]. Like other members of the NF-κB family, NF-κB/p65 resides in the cytoplasm in an inactive form bound to inhibitory IκB proteins. Cellular activation results in the nuclear translocation of NF-κB/p65 for initiating gene transcription. The translocation of NF-κB/p65 from cytoplasm to nucleus is often taken as an indication of NF-κB activation and is related to the cellular response to oxidants or to the inflammatory and acute immune response [14].
Brain inflammation is characterized by activation of microglia, which releasing a number of factors that modulate pro- and anti-inflammatory mediators (cytokines, chemokines, NO, PGEs, growth factors and superoxide species [15], which in turn, up-regulate adhesion molecules, increase permeability of the blood–brain barrier, facilitate the invasion of peripheral immune cells, induce the release of potentially toxic molecules, that may compromise brain cells [16]. Thus, the central nervous system (CNS) can be affected not only by inflammatory mediators produced within the brain, but also through the actions of mediators originating from the periphery.
Among the diverse functions that are regulated by acetylcholinesterase (AChE), inflammation has recently emerged as one of the most interesting. Since acetylcholine (ACh) is a neurotransmitter and has regulatory effect on serotonin, dopamine and other neuropeptides, it is clear that a complex network of interaction exists between these molecule in the regulation of immune response and neurotransmission [17, 18].
The relative balance of pro and anti-inflammatory cytokine and chemokine expression is believed to play a significant role in the etiology of both thrombosis and atherogenesis [19]. Previous studies have indicated that Hcy may contribute to the development and progression of atherosclerosis by inducing endothelial dysfunction, increasing proliferation of vascular smooth muscle cell, promoting lipoprotein oxidation and platelet activation, and enhancing collagen synthesis [20]. In addition, a pro-inflammatory state, associated with hyperhomocysteinemia, has been demonstrated by several authors [7, 21, 22]. In vitro studies have shown that Hcy is able to induce mRNA and protein expression of the pro-inflammatory cytokines in cultured human aortic endothelial cells [23, 24]. Additionally, we have previously reported that acute Hcy administration increases pro-inflammatory cytokines (TNF-α, IL-1β and IL-6) and chemokine CCL2 (MCP-1) in brain and serum of rats [9].
In order to investigate whether chronic hyperhomocysteinemia could alter inflammatory markers, in the present study we evaluated the effect of chronic Hcy administration on some inflammatory parameters such as cytokines (TNF-α, IL-1β and IL-6), chemokine CCL2 (MCP-1), nitrite and prostaglandin E2 levels and the immunocontent of NF-κB/p65 subunit, as well as on AChE activity in hippocampus and/or serum of rats.
Materials and Methods
Animals and Reagents
Seventy-four Wistar rats were obtained from the Central Animal House of the Departamento de Bioquímica, Instituto de Ciências Básicas da Saúde, Universidade Federal do Rio Grande do Sul, Porto Alegre, RS, Brazil. Animals were maintained on a 12 h light/12 h dark cycle at a constant temperature (22 ± 1 °C), with free access to water and commercial protein chow. Animal care followed the NIH ‘‘Guide for the Care and Use of Laboratory Animals’’ (NIH publication no. 80-23, revised 1996) and was approved by the University Ethics Committee.
Acrylamide, bisacrylamide, SDS, and β-mercaptoethanol used in sodium dodecylsulfate polyacrylamide gel electrophoresis (SDS-PAGE) were purchased from Sigma Chemical Co. (St. Louis, MO, USA). Polyclonal antibodies were purchased from Cell Signaling Technology (Beverly, MA, USA). Anti-rabbit IgG peroxidase-conjugated and reagents to detect chemioluminescence (ECL) were purchased from Amersham Pharmacia Biotech (Piscataway, NJ, USA). Hybond-C nitrocellulose membranes were from Hybond-ECL-(Hybond- ECL- nitrocellulose membrane, Amersham Biosciences, Freiburg, Germany). X-ray films were purchased from Kodak (Kodak X-Omat, Rochester, NY, USA). All other reagents were purchased from Sigma Chemical Co. (St. Louis, MO, USA).
Chronic Homocysteine Treatment
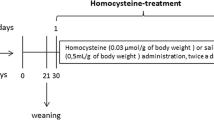
D,L-Hcy was dissolved in 0.9 % NaCl solution (saline) and buffered to pH 7.4. Hcy solution was administered subcutaneously twice a day from the 6th to 28th days-of-age. Hcy doses were calculated from pharmacokinetic parameters previously determined in our laboratory [25]. During the first week of treatment, animals received 0.3 μmol Hcy/g body weight. In the second week, 0.4 μmol Hcy/g body weight was administered to the animals, and in the last week rats received 0.6 μmol Hcy/g body weight. Plasma Hcy concentration in rats subjected to this treatment reached levels similar to those found in homocystinuric patients [1, 25]. Hcy reaches the brain maximum concentration between 15 and 30 min after injection and returns to baseline levels at 12 h [25]. Control animals received saline solution in the same volumes as those applied to Hcy-treated rats. The rats were euthanized by decapitation without anesthesia 1 or 12 h after the last injection; serum was separated and brain was quickly removed and hippocampus was dissected.
Western Blotting for Cytosolic and Nuclear NF-κB/p65 Subunit
Tissue hippocampus were homogenized in 300 μL hypotonic lysis buffer (10 mM HEPES (pH 7.9), 1.5 mM MgCl2, 10 mM KCl, 0.5 mM phenylmethylsulfonyl fluoride, 1 mM dithiothreitol, 5 mM NaF, 1 mM sodium orthovanadate plus protease inhibitor cocktail). Hippocampus homogenate were than lysed with 18 μL 10 % IGEPAL. The homogenate was centrifuged (14,000xg, 30 s, 4 °C), and supernatants containing the cytosolic fraction were stored at −80 °C. The nuclear pellet was resuspended in 200 μL ice-cold hypertonic extraction buffer (10 mM HEPES (pH 7.9), 0.40 M NaCl, 1.5 mM MgCl2, 10 mM KCl, 0.5 mM phenylmethylsulfonyl fluoride, 1 mM dithiothreitol, 5 mM NaF, 1 mM sodium orthovanadate, 0.25 mM EDTA, 25 % glycerol plus protease inhibitor cocktail). After 40 min of intermittent mixing, extracts were centrifuged (14,000xg, 10 min, 4 °C), and supernatants containing nuclear protein were secured [26]. Cytosolic and nuclear fractions were used for NF-κβ/p65 subunit Western blotting. Aliquots were taken for protein determination and, for electrophoresis analysis, were dissolved in 25 % (v/v) of a solution containing 40 % glycerol, 5 % mercaptoethanol, 50 mM Tris–HCl, pH 6.8. Equal protein concentrations were loaded onto 10 % polyacrylamide gels and analyzed by SDS-PAGE. Protein samples were separated by 10 % SDS-PAGE (50 μg/lane of total protein) and transferred (Trans-blot SD semidry transfer cell, BioRad) to nitrocellulose membranes for 1 h at 15 V in transfer buffer (48 mM Trizma, 39 mM glycine, 20 % methanol, and 0.25 % SDS). The blot was then washed for 10 min in Tris-buffered saline (TBS) (0.5 M NaCl, 20 mM Trizma, pH 7.5), followed by 2 h incubation in blocking solution [TBS plus 5 % bovine serum albumin (BSA)]. After incubation, the blot was washed twice for 5 min with blocking solution plus 0.05 % Tween-20 (T-TBS) and then incubated overnight at 4 °C in blocking solution containing one of the following antibodies: anti-NF-kB/p65 (1:1,000; Santa Cruz Biotechnology) and anti-β-actin (1:1,000, Sigma Chemical Co.). The blot was then washed twice for 5 min with T-TBS and incubated for 2 h in antibody solution containing peroxidase-conjugated anti-mouse IgG or peroxidase-conjugated anti-rabbit IgG diluted 1:1,000. The blot was again washed twice for 5 min with T-TBS and twice for 5 min with TBS. The blot was developed using a chemiluminescence ECL kit (Amersham, Oakville, Ontario). The chemioluminescence was detected using X-ray films that were scanned and analyzed using the Optiquant Software (Packard Instruments).
Tissue Preparation
For acquisition of serum, whole blood was centrifuged at 1,000xg for 5 min and the serum was immediately removed. Hippocampus was homogenized 1:5 (w/v) in saline solution (0.9 % NaCl). The homogenate was centrifuged at 800xg for 10 min at 4 °C and the supernatant was used in assays.
Cytokine (TNF-α, IL-1β and IL-6) and Chemokine CCL2 (MCP-1) Assay
TNF-α, IL-1β, IL-6 and MCP-1 levels in hippocampus and serum were quantified by Multiplexed Immunoassay with a commercially available kit, and analyzed on a Luminex 200®TM.
Nitrite Assay
Nitrite levels were measured using the Griess reaction; 100 μL of rat hippocampus supernatant or serum were mixed with 100 μL Griess reagent (1:1 mixture of 1 % sulfanilamide in 5 % phosphoric acid and 0.1 % naphthylethylenediamine dihydrochloride in water) and incubated in 96-well plates for 10 min at room temperature. The absorbance was measured on a microplate reader (SpectraMax M5/M5 Microplate Reader, Molecular Devices, MDS Analytical Technologies, Sunnyvale, California, USA) at a wavelength of 543 nm. Nitrite concentration was calculated using sodium nitrite standards [27].
Prostaglandin E2 Assay
PGE2 was measured by the method described by Wallace et al. [28] and determined by radioimmunoassay.
Acetylcholinesterase Activity
AChE activity was determined, according to Ellman et al. [29], with some modifications. Hydrolysis rates were measured at an ACh concentration of 0.8 mM in 1 mL assay solutions with 30 mM phosphate buffer, pH 7.5, and 1.0 mM DTNB at 25 °C. About 50 μL of rat hippocampus supernatant was added to the reaction mixture and preincubated for 3 min. The hydrolysis was monitored by the formation of the thiolate dianion of DTNB at 412 nm for 2–3 min (intervals of 30 s).
Protein Determination
Protein concentrations were measured by the method of Lowry et al. [30] or Bradford [31] using bovine serum albumin as standard.
Statistical Determination
Data were analyzed by the Student`s t test for unpaired samples. All analyses were performed using the Statistical Package for the Social Sciences (SPSS) software with a PC-compatible computer. Differences were considered statistically significant if p < 0.05.
Results
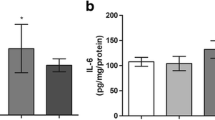
We first studied the effect of chronic Hcy administration on cytokine levels (TNF-α, IL-1β and IL-6) and chemokine CCL2 (MCP-1) in hippocampus of rats. Figure 1a shows that Hcy significantly increased the levels of TNF-α at 1 h [t(7) = 4.15; p < 0.01], but not at 12 h [t(7) = 0.80; p > 0.05] after chronic hyperhomocysteinemia. Figure 1b shows that IL-1β was increased at 1 h [t(7) = 5.47; p < 0.001] and 12 h [t(7) = 3.65; p < 0.01] after the last injection of Hcy injection, as compared to the control group. Similarly, IL-6 was increased at 1 h [t(7) = 4.30; p < 0.01] and 12 h [t(7) = 3.29; p < 0.05] after Hcy administration (Fig 1c). MCP-1 levels significantly increased at 1 h [t(7) = 3.55; p < 0.01], but not at 12 h [t(7) = 1.34; p > 0.05], after chronic hyperhomocysteinemia (Fig. 1d).
Effect of chronic administration of homocysteine on cytokine (TNF-α, IL-1β, IL-6) and chemokine CCL2 (MCP-1) levels in the hippocampus of rats. Results are expressed as mean ± SD for six animals per group. Different from control, *p < 0.05; **p < 0.01; ***p < 0.001 (Student’s t test). Hcy homocysteine, TNF-α tumor necrosis factor alpha, IL-1 β interleukin-1 beta, IL-6 interleukin-6, MCP-1 monocyte chemoattractant protein-1
The effect of chronic hyperhomocysteinemia on cytokines (TNF-α, IL-1β and IL-6) and chemokine CCL2 (MCP-1) levels was also investigated in serum of rats. Figure 2a shows that Hcy significantly increased TNF-α levels at 1 h [t(8) = 8.58; p < 0.001] and at 12 h [t(7) = 2.92; p < 0.05] after the last injection of this amino acid. Figure 2b shows that IL-1β was increased at 1 h [t(8) = 5.43; p < 0.001] and at 12 h [t(8) = 4.24; p < 0.01] after Hcy injection. Similarly, chronic hyperhomocysteinemia significantly increased IL-6 levels at 1 h [t(8) = 10.58; p < 0.001] and at 12 h [t(8) = 4.43; p < 0.01] (Fig. 2c); and MCP-1 levels at 1 h [t(8) = 6.11; p < 0.001] and at 12 h [t(8) = 3.56; p < 0.01] (Fig. 2d), as compared to the control group.
Effect of chronic administration of homocysteine on cytokine (TNF-α, IL-1β, IL-6) and chemokine CCL2 (MCP-1) levels in the serum of rats. Results are expressed as mean ± SD for six animals per group. Different from control, *p < 0.05; **p < 0.01; ***p < 0.001 (Student’s t test). Hcy homocysteine, TNF-α tumor necrosis factor alpha, IL-1 β interleukin-1 beta, IL-6 interleukin-6, MCP-1 monocyte chemoattractant protein-1
Next, nitrite levels were determined in hippocampus and serum of animals subjected to chronic Hcy administration. Figure 3a shows that Hcy significantly increased nitrite levels in hippocampus at 1 h [t(7) = 2.98; p < 0.05] and 12 h [t(6) = 2.63; p < 0.05], as compared to the control group. On the other hand, Fig. 3b shows that Hcy significantly decreased nitrite levels in serum at 1 h [t(8) = 2.32; p < 0.05], but did not alter this parameter at 12 h [t(6) = 0.91; p > 0.05] after chronic administration of this amino acid.
Subsequently, the effect of chronic Hcy administration on prostaglandin E2 was evaluated in the hippocampus and serum of rats. Figure 4a shows that rats euthanized at 1 h [t(6) = 5.95; p < 0.001] and at 12 h [t(6) = 10.25; p < 0.001] after chronic hyperhomocysteinemia presented a significant increase in prostaglandin E2, as compared to the control group. In addition, Fig. 4b shows that Hcy injection increased prostaglandin E2 levels in serum of rats at 1 h [t(6) = 2.94; p < 0.05]; however, animals euthanized at 12 h [t(6) = 1.08; p > 0.05] after chronic hyperhomocysteinemia did not present alterations in this parameter.
Since, NF-κB regulate innate immune response, and it is activated rapidly in response to a wide range of stimuli, including pro-inflammatory cytokines, such as TNF-α and IL-1β [32], we investigated the effect of chronic hyperhomocysteinemia on immunocontent of cytosolic and nuclear fraction of NF-κB/p65 subunit. Figure 5a shows that chronic Hcy administration significantly increased the immunocontent of cytosolic [t(7) = 5.36; p < 0.001] and nuclear fraction of NF-kB/p65 subunit [t(8) = 3.15; p < 0.01] in the hippocampus of rats at 1 h after the last injection of this amino acid. Figure 5b shows that chronic hyperhomocysteinemia has no effect on the immunocontent cytosolic [t(7) = 0.242; p > 0.05] and nuclear NF-kB/p65 subunit [t(5) = 0.007; p > 0.05] at 12 h after hyperhomocysteinemia.
Effect of chronic hyperhomocysteinemia on cytosolic and nuclear immunocontent of NF-κB/p65 subunit at 1 h (a) and at 12 h (b) after homocysteine administration in hippocampus of rats. Bars represent the mean ± SD for 4–6 animals in each group. Different from control, **p < 0.01; ***p < 0.001 (Student’s t test). Hcy homocysteine, NF-κB nuclear factor-kappaB
Considering that AChE seem to be associated with inflammation we determined the effect of chronic Hcy administration on activity of AChE. Figure 6 shows that chronic hyperhomocysteinemia provoked an increase in AChE activity in the hippocampus of rats at 1 h [t(6) = 3.15; p < 0.05], but did not alter this enzyme at 12 h [t(7) = 0.53; p > 0.05] after the last injection.
Discussion
Hyperhomocysteinemia has been associated with vasculopathy in the peripheral and cerebral blood vessels [33, 34]. The mechanism by which hyperhomocysteinemia promotes endothelial dysfunction and subsequent vascular disease has recently been explored in the peripheral vessel system, but less extensively in the cerebral blood vessels [35–37].
Previous work using cell cultures has suggested the participation of inflammation in the pathogenesis of hyperhomocysteinemia [24, 38, 39], in the present study we initially investigated the effect of chronic hyperhomocysteinemia on cytokines in hippocampus and serum of rats. Results showed that chronic Hcy administration increases cytokines IL-1β and IL-6 in hippocampus of rats at 1 or 12 h after the last injection of this amino acid, whereas TNF-α and MCP-1 were increased only at 1 h after chronic hyperhomocysteinemia. Although the precise mechanisms of Hcy action on the inflammatory process are not fully understood, our findings suggest that the increase in cytokine levels could be closely related to the high brain and plasma levels of this amino acid, which achieve the peak as soon at 15 min after injection, returning to baseline levels after 12 h [25]. These data are in agreement with Su et al. [39], who reported that Hcy in vitro induces mRNA and protein expressions of the inflammatory cytokines TNF-α, IL-1β, IL-6, IL-8, and IL-12. Most importantly, there is now extensive evidence that neuroinflammation contributes to many acute and chronic degenerative disorders and, perhaps, some psychiatric diseases [16], and it is also possible that the cytokines profiles in severe hyperhomocysteinemia described here could contribute to the cognitive impairment that is frequently observed in this disease.
Although some CNS cell types, including microglia, astrocytes and neurons, are able to secrete cytokines, studies support the involvement of peripherally-derived cells in contributing to brain inflammation and injury [40]. It has also been suggested that an associated blood–brain barrier dysfunction may occur whereby a leaky state promotes transendothelial migration of immune cells [40]. With regard to cytokines that are important modulators of inflammatory events [41], we have previously shown that acute Hcy administration increases the pro-inflammatory cytokines TNF-α, IL-1β, IL-6 and MCP-1 in brain and serum of rats [9].
In the present study, chronic Hcy administration increased TNF-α, IL-1β, IL-6 and MCP-1 in rat serum at 1 and 12 h after the last injection of this amino acid. It has recently been shown that Hcy may contribute to the progression of atherosclerosis, in part by enhancing vascular inflammation [23, 42]. Following Hcy-induced injury, endothelial cells are activated and are capable of producing various adhesion molecules and chemokines such as, VCAM-1, ICAM-1, E-selectin, P-selectin, β1-integrin, IL-8 which participate in inflammatory reactions in the arterial wall [24, 43–46]. Other potentially important inflammatory actions of Hcy include stimulation of TNF-α released by blood monocytes and their adhesion to endothelial cells [38, 39]. These results suggest that Hcy may contribute to the initiation and progression of vascular disease by promoting monocyte activation, resulting in the secretion of cytokines that might amplify the inflammatory response in the arterial wall.
Moreover, we also evaluated the effect of chronic hyperhomocysteinemia on nitrite levels. Hcy significantly increased nitrite levels in hippocampus of rats at 1 h and 12 h after the last Hcy injection. NO is produced by a group of enzymes called neuronal nitric oxide synthase (nNOS), inducible NOS (iNOS) and endothelial NOS (eNOS). These enzymes convert arginine into citrulline, producing NO in the process [47–49]. The activity of the NOS enzymes is subject to discreet and multiple interconnected mechanisms of regulation. NO regulates a diverse range of physiological and cellular processes, including endothelial cell migration, proliferation, extracellular matrix degradation, platelet function, angiogenesis and mitogenesis, which are all crucial for cardiovascular physiology [50, 51]. During inflammation, NO levels increase considerable, due to the induction of iNOS by cytokines [52]. In this context, Welch et al. [53] reported that Hcy induced NO synthesis in the vascular smooth muscle cells after NF-κβ-dependent transcriptional activation of iNOS. In accordance with our results, it has been reported that acute Hcy administration increases nitrite levels in rat brain [9].
Contrast data were obtained for rat serum; Hcy decreased nitrite levels at 1 h after chronic hyperhomocysteinemia, but did not alter this parameter at 12 h. Under normal conditions, NO has a role in the detoxification of Hcy through the formation of S-nitrosohomocysteine [54]. However, chronic exposure to Hcy increases the formation of superoxide anion (O2·−), which can react with NO to yield the potent oxidant, peroxynitrite (ONOO−) [55, 56]. In our study, nitrite levels was decreased in serum after chronic hyperhomocysteinemia, and we proposed that the bioavailability of NO may be reduced due to the generation of free radicals and lipid peroxidation caused by Hcy. In this context, Jiang et al. [57] showed that eNOS activity was significantly reduced by Hcy in endothelial cells of CBS null mice. Further research found that increased vascular oxidant stress in hyperhomocysteinemia not only leads to a decreased NO bioavailability, but also activates redox-sensitive signaling pathways that induce a pro-inflammatory state in the vessel wall [58, 59].
PGEs are members of the eicosanoid family and are not stored by cells; rather, they are synthesized from arachidonic acid via the actions of cyclooxygenase enzymes (COX), either constitutively or in response to cell-specific trauma, stimuli, or signaling molecules [60, 61]. The most abundant prostanoid in the human body is PGE2 [62], and has been considered the principal prostaglandin in acute inflammation, as well as in arthritic diseases such as rheumatoid arthritis [63] and osteoarthritis [64]. The effect of chronic Hcy administration on PGE2 in hippocampus and serum of rats was next investigated. Results showed that chronic Hcy administration increased PGE2 in the hippocampus at 1 and 12 h after injection. On the other hand, in serum we verified that Hcy increased PGE2 at 1 h after Hcy administration. It has also been reported that iNOS specifically binds to COX-2 and S-nitrosylates the enzyme on Cys526, resulting in an increased COX-2 catalytic activity and enhanced PGE2 production [65]. These effects of PGE2 in hippocampus could be responsible, at least in part, for the increase in the nitrite levels observed in our study.
Additionally, we evaluated the immunocontent of cytosolic and nuclear NF-κB/p65 subunit in hippocampus of rats subjected to chronic Hcy administration. We demonstrated that chronic hyperhomocysteinemia significantly increased the immunocontent of cytosolic and nuclear NF-κB/p65 subunit at 1 h, but did not observe any alteration in the immunocontent of NF-κB/p65 subunit at 12 h after chronic administration of this amino acid. Increased vascular oxidative stress in hyperhomocysteinemia has been shown to activate pro-inflammatory signaling pathways in endothelial cells, including the NF-κβ pathway [66]. In this context, Hcy has been shown to stimulate ICAM-1 and TNF-α expression in endothelial cells, mediated by the activation of NF-κβ, via a mitogen-activated protein kinase (MAPK) pathway [67]. NF-κβ may also be activated through a protein kinase C signaling mechanism, which seems to be stimulated by Hcy [42]. In addition, reports showed that Hcy-induced IL-6 gene expression occurs through the activation of NF-κβ [68]. Furthermore, recent studies have demonstrated the involvement of Hcy actions linked to oxidative stress [69, 70], and which NF-κβ is a transcriptional factor whose activation by signaling pathways is correlated with elevated ROS levels [71]. In this context, Matté et al. [69] showed that chronic Hcy administration increased DNA damage, as evaluated by the comet assay, and disrupted antioxidant defenses (enzymatic and non-enzymatic) in parietal cortex and blood/plasma. It has been previously demonstrated that acute Hcy administration decreases catalase activity (CAT) in rat hippocampus and that vitamins E and C completely prevent this effect, indicating that the participation of oxidative stress is probably involved in the actions of Hcy [72]. In fact, NF-κβ may be a point of convergence by which different agents cause inflammatory activation in hyperhomocysteinemia.
ACh is rapidly hydrolyzed by AChE in neural synapses and the motor endplate. Considering the inflammatory suppressive effect of ACh, it is conceivable that AChE activity is an intrinsic regulator of inflammation [73]. Indeed, peritoneal injection of AChE inhibitors reduce serum pro-inflammatory cytokine levels and improve survival in a murine model of sepsis [74]; intravenous AChE inhibitors reduce IL-1β in brain and blood and decrease serum AChE activity in mice [75]; and basal AChE activity in the circulation is inversely related to serum IL-6 levels induced by endotoxin in humans [76]. Based on these data, we also investigated the effect of chronic Hcy administration on AChE activity. We observed that the activity of this enzyme was increased in hippocampus at 1 h after the last injection of Hcy. Considering the role of ACh in inhibiting the release of pro-inflammatory cytokines [73, 75], we might speculate that this increase in AChE activity may cause an impairment of ACh ability in regulating the inflammatory processes, which would explain, at least in part, the alterations in cytokine levels described above.
In summary, we showed that chronic hyperhomocysteinemia induced immune activation by increasing cytokines (TNF-α, IL-1β, IL-6), chemokine CCL2 (MCP-1), nitrite and PGE2 levels, immunocontent of NF-κB/p65 subunit and AChE activity. Collectively, our results provide an additional insight into the inflammatory mechanisms of Hcy, and may contribute, at least in part, to explain the complex factors involved in the cerebrovascular and vascular dysfunction exhibited by hyperhomocysteinemic patients.
References
Mudd SH, Levy HL, Skovby F (2001) Disorders of transsulfuration. In: Scriver CR, Beaudet AL, Sly WS, Valle D (eds) The metabolic and molecular bases of inherited disease. McGraw-Hill, New York, pp 1279–1327
Mattson MP, Kruman II, Duan W (2002) Folic acid and homocysteine in age-related disease. Ageing Res Rev 1:95–111
Sachdev P (2004) Homocysteine and neuropsychiatric disorders. Rev Bras Psiquiatr 26:50–56
Regland B (2005) Schizophrenia and single-carbon metabolism. Prog Neuropsychopharmacol Biol Psychiatry 29:1124–1132
Welch GN, Loscalzo J (1998) Homocysteine and atherothrombosis. N Engl J Med 338:1042–1050
Diaz-Arrastia R (2000) Homocysteine and neurologic disease. Arch Neurol 57:1422–1427
Zou CG, Banerjee R (2005) Homocysteine and redox signaling. Antioxid Redox Signal 7:547–559
Tyagi N, Gillespie W, Vacek JC et al (2009) Activation of GABA-A receptor ameliorates homocysteine-induced MMP-9 by ERK pathway. J Cell Physiol 220:257–266
da Cunha AA, Ferreira AG, Wyse AT (2010) Increased inflammatory markers in brain and blood of rats subjected to acute homocysteine administration. Metab Brain Dis 25:199–206
Das UN (2006) Clinical laboratory tools to diagnose inflammation. Adv Clin Chem 41:189–229
Wang T, Zhang X, Li JJ (2002) The role of NF-κB in the regulation of cell stress responses. Int Immunopharmacol 2:1509–1520
Hawiger J (2001) Innate immunity and inflammation: a transcriptional paradigm. Immunol Res 23:99–109
Csiszar A, Wang M, Lakatta EG et al (2008) Inflammation and endothelial dysfunction during aging: role of NF-kappaB. J Appl Physiol 105:1333–1341
Dallot E, Méhats C, Oger S et al (2005) A role for PKCzeta in the LPS-induced translocation NF-κappaB p65 subunit in cultured myometrial cells. Biochimie 87:513–521
Ghirnikar RS, Lee YL, Eng LF (1998) Inflammation in traumatic brain injury: role of cytokines and chemokines. Neurochem Res 23:329–340
Lucas SM, Rothwell NJ, Gibson RM (2006) The role of inflammation in CNS injury and disease. Br J Pharmacol 147:S232–S240
Das UN (2007) Acetylcholinesterase and butyrylcholinesterase as possible markers of low-grade systemic inflammation. Med Sci Monit 13:RA214–RA221
Andriantsitohaina R, Surprenant A (1992) Acetylcholine released from guinea-pig submucosal neurones dilates arterioles by releasing nitric oxide from endothelium. J Physiol 453:493–502
Ross R (1999) Atherosclerosis-an inflammatory disease. N Engl J Med 340:115–126
Weiss N, Keller C, Hoffmann U et al (2002) Endothelial dysfunction and atherothrombosis in mild hyperhomocysteinemia. Vasc Med 7:227–239
Gori AM, Corsi AM, Fedi S et al (2005) A proinflammatory state is associated with hyperhomocysteinemia in the elderly. Am J Clin Nutr 82:335–341
Keating AK, Freehauf C, Jiang H et al (2011) Constitutive induction of pro-inflammatory and chemotactic cytokines in cystathionine beta-synthase deficient homocystinuria. Mol Genet Metab 103:330–337
Sung FL, Slow YL, Wang G et al (2001) Homocysteine stimulates the expression of monocyte chemoattractant protein-1 in endothelial cells leading to enhanced monocyte chemotaxis. Mol Cell Biochem 216:121–128
Poddar R, Sivasubramanian N, DiBello PM et al (2001) Homocysteine induces expression and secretion of monocyte chemoattractant protein-1 and interleukin-8 in human aortic endothelial cells: implications for vascular disease. Circulation 103:2717–2723
Streck EL, Matté C, Vieira PS et al (2002) Reduction of Na+, K+-ATPase activity in hippocampus of rats subjected to chemically induced hyperhomocysteinemia. Neurochem Res 27:1593–1598
Zanotto-Filho A, Gelain DP, Schröder R et al (2009) The NFkappaB-mediated control of RS and JNK signaling in vitamin A-treated cells: duration of JNK-AP-1 pathway activation may determine cell death or proliferation. Biochem Pharmacol 77:1291–1301
Green LC, Wagner DA, Glogowski J et al (1982) Analysis of nitrate, nitrite and [15 N]nitrate in biological fluids. Anal Biochem 126:131–138
Wallace JL, Morris GP, Beck PL et al (1988) Effects of sucralfate on gastric prostaglandin and leukotriene synthesis: relationship to protective actions. Can J Physiol Pharmacol 66:666–670
Ellman GL, Courtney KD, Andres V Jr (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7:88–95
Lowry OH, Rosebrough NJ, Farr AL et al (1951) Protein measurement with the folin phenol reagent. J Biol Chem 193:265–275
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Li Q, Verma IM (2002) NF-kB regulation in the immune system. Nat Rev Immunol 2:725–734
Clarke R, Daly L, Robinson K et al (1991) Hyperhomocysteinemia: an independent risk factor for vascular disease. N Engl J Med 324:1149–1155
Hankey GJ, Eikelboom JW (1999) Homocysteine and vascular disease. Lancet 354:407–413
Cook JW, Taylor LM, Orloff SL et al (2002) Homocysteine and arterial disease. Experimental mechanisms. Vasc Pharmacol 38:293–300
Nappo F, De Rosa N, Marfella R, De Lucia D et al (1999) Impairment of endothelial functions by acute hyperhomocysteinemia and reversal by antioxidant vitamins. JAMA 281:2113–2118
Woo KS, Qiao M, Chook P et al (2002) Homocysteine, endothelial dysfunction, and coronary artery disease: emerging strategy for secondary prevention. J Card Surg 17:432–435
Dalal S, Parkin SM, Homer-Vanniasinkam S et al (2003) Effect of homocysteine on cytokine production by human endothelial cells and monocytes. Ann Clin Biochem 40:534–541
Su SJ, Huang LW, Pai LS et al (2005) Homocysteine at pathophysiologic concentrations activates human monocyte and induces cytokine expression and inhibits macrophage migration inhibitory factor expression. Nutrition 21:994–1002
Giulian D, Chen J, Ingeman JE et al (1989) The role of mononuclear phagocytes in wound healing after traumatic injury to adult mammalian brain. J Neurosci 9:4416–4429
Clinton SK, Libby P (1992) Cytokines and growth factors in atherogenesis. Arch Pathol Lab Med 116:1292–1300
Wang G, Siow YL, Karmin O (2000) Homocysteine stimulates nuclear factor kB activity and monocyte chemoattractant protein-1 expression in vascular smooth muscle cells: a possible role for protein kinase C. Biochem J 352:817–826
Hofmann MA, Lalla E, Lu Y et al (2001) Hyperhomocysteinemia enhances vascular inflammation and accelerates atherosclerosis in a murine model. J Clin Invest 107:675–683
Wang G, Woo CW, Sung FL et al (2002) Increased monocyte adhesion to aortic endothelium in rats with hyperhomocysteinemia: role of chemokine and adhesion molecules. Arterioscler Thromb Vasc Biol 22:1777–1783
Postea O, Krotz F, Henger A et al (2006) Stereospecific and redox-sensitive increase in monocyte adhesion to endothelial cells by homocysteine. Arterioscler Thromb Vasc Biol 26:508–513
Rongioletti M, Baldassini M, Papa F et al (2005) Homocysteinemia is inversely correlated with platelet count and directly correlated with sE- and sP-selectin levels in females homozygous for C677T methylenetetrahydrofolate reductase. Platelets 16:185–190
Alderton WK, Cooper CE, Knowles RG (2001) Nitric oxide synthases: structure, function and inhibition. Biochem J 357:593–615
Rekka EA, Chrysselis MC (2002) Nitric oxide in atherosclerosis. Mini Rev Med Chem 2:585–593
Nathan C, Xie QW (1994) Nitric oxide synthase: roles, tolls and controls. Cell 78:915–918
Cooke JP, Losordo DW (2002) Nitric oxide and angiogenesis. Circulation 105:2133–2135
Naseem KM (2005) The role of nitric oxide in cardiovascular diseases. Mol Aspect Med 26:33–65
Pacher P, Beckman JS, Liaudet L (2007) Nitric oxide and peroxynitrite in health and disease. Physiol Rev 87:315–424
Welch GN, Upchurch GR Jr, Farivar RS et al (1998) Homocysteine-induced nitric oxide production in vascular smooth-muscle cells by NF-κβ-dependent transcriptional activation of Nod 2. Proc Assoc Am Physicians 110:22–31
Stamler JS, Osborne JA, Jaraki O et al (1993) Adverse vascular effects of homocysteine are modulated by endothelium-derived relaxing factor and related oxides of nitrogen. J Clin Invest 91:308–318
Zhang X, Li H, Jin H et al (2000) Effects of homocysteine on endothelial nitric oxide production. Am J Physiol Renal Physiol 279:F671–678
Tyagi N, Moshal KS, Ovechkin AV et al (2005) Mitochondrial mechanism of oxidative stress and systemic hypertension in hyperhomocysteinemia. J Cell Biochem 96:665–671
Jiang X, Yang F, Tan H et al (2005) Hyperhomocystinemia impairs endothelial function and eNOS activity via PKC activation. Arterioscler Thromb Vasc Biol 25:2515–2521
Woo CW, Cheung F, Chan VW et al (2003) Homocysteine stimulates inducible nitric oxide synthase expression in macrophages: antagonizing effect of ginkgolides and bilobalide. Mol Cell Biochem 243:37–47
Postea O, Krotz F, Henger A et al (2006) Stereospecific and redox-sensitive increase in monocyte adhesion to endothelial cells by homocysteine. Arterioscler Thromb Vasc Biol 26:508–513
Berenbaum F (2000) Proinflammatory cytokines, prostaglandins, and the chodrocyte: mechanisms of intracellular activation. Jt Bone Spine 67:561–564
Funk CD (2001) Prostaglandins and leukotrienes: advances in eicosanoid biology. Science 294:1871–1875
Serhan CN, Levy B (2003) Success of prostaglandin E2 in structure-function is a challenge for structure-based therapeutics. Proc Natl Acad Sci USA 100:8609–8611
Bombardier S, Cattani P, Ciabattoni G et al (1981) The synovial prostaglandin system in chronic inflammatory arthritis: differential effects of steroidal and non-steroidal anti-inflammatory drugs. Br J Pharmacol 73:893–901
Amin AR, Attur M, Patel RN et al (1996) Superinduction of cyclooxygenase-2 activity in human osteoarthritisaffected cartilage: influence of nitric oxide. J Clin Invest 99:1231–1237
Kim SF, Huri DA, Snyder SH (2005) Inducible nitric oxide synthase binds, S-nitrosylates, and activates cyclooxygenase-2. Science 310:1966–1970
Au-Yeung KK, Woo CW, Sung FL et al (2004) Hyperhomocysteinemia activates nuclear factor-kappaβ in endothelial cells via oxidative stress. Circ Res 94:28–36
Bai YP, Liu YH, Chen J et al (2007) Rosiglitazone attenuates NF-kappaB-dependent ICAM-1 and TNF-alpha production caused by homocysteine via inhibiting ERK1/2/p38MAPK activation. Biochem Biophys Res Commun 360:20–26
Zhang L, Jin M, Hu XS et al (2006) Homocysteine stimulates nuclear factor kappaB activity and interleukin-6 expression in rat vascular smooth muscle cells. Cell Biol Int 30:592–597
Matté C, Mackedanz V, Stefanello FM et al (2009) Chronic hyperhomocysteinemia alters antioxidant defenses and increases DNA damage in brain and blood of rats: protective effect of folic acid. Neurochem Int 54:7–13
Streck EL, Vieira PS, Wannmacher CM et al (2003) In vitro effect of homocysteine on some parameters of oxidative stress in rat hippocampus. Metab Brain Dis 18:147–154
Li N, Karin M (1999) Is NF-kappaB the sensor of oxidative stress? FASEB J 13:1137–1143
Wyse AT, Zugno AI, Streck EL et al (2002) Inhibition of Na+, K+-ATPase activity in hippocampus of rats subjected to acute administration of homocysteine is prevented by vitamins E and C treatment. Neurochem Res 27:1685–1689
Borovikova LV, Ivanova S, Zhang M et al (2000) Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature 405:458–462
Hofer S, Eisenbach C, Lukic IK et al (2008) Pharmacologic cholinesterase inhibition improves survival in experimental sepsis. Crit Care Med 36:404–408
Pollak Y, Gilboa A, Ben-Menachem O et al (2005) Acetylcholinesterase inhibitors reduce brain and blood interleukin-1beta production. Ann Neurol 57:741–745
Ofek K, Krabbe KS, Evron T et al (2007) Cholinergic status modulations in human volunteers under acute inflammation. J Mol Med 85:1239–1251
Acknowledgments
We thank Fernando de Queiróz Cunha, Fernando Spiller and Giuliana Bertozi for their collaboration and technical assistance in prostaglandin E2 assay and Laboratório Nobel RIE Ltda. This work was supported in part by grants from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq–Brazil) and Fundação de Amparo à Pesquisa do Estado do Rio Grande do Sul (FAPERGS, RS, Brazil).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
da Cunha, A.A., Ferreira, A.G.K., Loureiro, S.O. et al. Chronic Hyperhomocysteinemia Increases Inflammatory Markers in Hippocampus and Serum of Rats. Neurochem Res 37, 1660–1669 (2012). https://doi.org/10.1007/s11064-012-0769-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-012-0769-2