Abstract
This study is focused on understanding the mechanism of neurobehavioral toxicity of lambda-cyhalothrin, a new generation type II synthetic pyrethroid in developing rats following their exposure from post-lactational day (PLD)22 to PLD49 and investigate whether neurobehavioral alterations are transient or persistent. Post-lactational exposure to lambda-cyhalothrin (1.0 or 3.0 mg/kg body weight, p.o.) affected grip strength and learning activity in rats on PLD50 and the persistent impairment of grip strength and learning was observed at 15 days after withdrawal of exposure on PLD65. A decrease in the binding of muscarinic–cholinergic receptors in frontocortical, hippocampal, and cerebellar membranes associated with decreased expression of choline acetyltransferase (ChAT) and acetylcholinesterase (AChE) in hippocampus was observed following exposure to lambda-cyhalothrin on PLD50 and PLD65. Exposure to lambda-cyhalothrin was also found to increase the expression of growth-associated protein-43 in hippocampus of rats on PLD50 and PLD65 as compared to controls. A significant increase in lipid peroxidation and protein carbonyl levels and decreased levels of reduced glutathione and activity of superoxide dismutase, catalase, and glutathione peroxidase in brain regions of lambda-cyhalothrin exposed rats were distinctly observed indicating increased oxidative stress. Inhibition of ChAT and AChE activity may cause down-regulation of muscarinic–cholinergic receptors consequently impairing learning activity in developing rats exposed to lambda-cyhalothrin. The data further indicate that long-term exposure to lambda-cyhalothrin at low doses may be detrimental and changes in selected behavioral and neurochemical end points may persist if exposure to lambda-cyhalothrin continues.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Use of synthetic pyrethroids has significantly increased as compared to organochlorine and organophosphorus insecticides due to their high bioefficacy, easy biodegradability, and photostability (Kale et al. 1999; Fetoui et al. 2008; Jurisic et al. 2010). It has been estimated that over 520 ton of active ingredients of synthetic pyrethroids are used annually all over the world in the vector control program alone (Kumar et al. 2009). Based on the differences in the structure and signs of acute toxicity, pyrethroids are classified into two classes—type I and type II (Narahashi 1996; Soderlund et al. 2002; Wolansky et al. 2006; Hossain and Richardson 2011). Type II pyrethroids unlike type I have α-cyano group attached to alcohol moiety. Besides, type I pyrethroids produce T syndrome associated with whole body tremors, hyperexcitation, convulsions, ataxia, etc. On the other hand, choreoathetosis, salivation, hyperactivity, and paralysis are the characteristic symptoms of CS syndrome, produced by type II pyrethroids (Narahashi 1996).
Lambda-cyhalothrin, a new generation type II synthetic pyrethroid, has extensive uses in controlling a wide range of insects and pests both in food and in non-food crops (Mathirajan et al. 2000; Kroeger et al. 2003; Fetoui et al. 2008, 2009; Seenivasan and Muraleedharan 2009). Use of lambda-cyhalothrin in public health program to control vectors particularly mosquitoes, cockroaches, flies, etc., and also in veterinary practices is quite common (Amweg et al. 2005; Spurlock and Lee 2008; Fetoui et al. 2008). Residues of lambda-cyhalothrin have been found to be present in vegetables and fruits (Amoah et al. 2006; Mohapatra and Ahuja 2010; Turgut et al. 2011), milk and blood of dairy cows (Bissacot and Vassilieff 1997), and also in cattle meat (Muhammad et al. 2010). Placental transfer of lambda-cyhalothrin has been observed in goats (Oliveira et al. 2000). Persistence of lambda-cyhalothrin on different indoor surfaces, especially while using it against malaria vectors in malaria epidemic-prone areas, has been reported (Mulambalah et al. 2010). Intense and continuous usage of pesticides may enhance their levels in the environment and thus increases the risk of human exposure including growing children. Due to high usage of lambda-cyhalothrin, non-target organisms including mammals and aquatic invertebrates have been reported to be extremely sensitive to its neurotoxic effects (Amweg et al. 2006; Gu et al. 2007; Lawler et al. 2007; Wang et al. 2007).
Of the various organs in the body, the brain is more sensitive to pesticides including synthetic pyrethroids. Understanding the mechanisms of neurotoxicity of pyrethroids therefore has been a subject of interest and reviewed by many investigators (Narahashi 1996; Bradberry et al. 2005; Shafer et al. 2005; Hossain and Richardson 2011). Prolonged opening of voltage sensitive sodium channels by disrupting their gating characteristics associated with depolarization and repetitive firing is the primary mechanism of neurotoxicity of pyrethroids (Narahashi 1996; Hossain and Richardson 2011). It has been found that type II pyrethroids hold the channel open for a longer time as compared to type I pyrethroids (Bradberry et al. 2005). Besides affecting the sodium channels, pyrethroids may also affect chloride channels, GABAA receptors, voltage-gated calcium channels, and mitochondrial electron transport chain (Narahashi 1996; Breckenridge et al. 2009). Increased oxidative stress in the brain of rats exposed to lambda-cyhalothrin has been reported (El-Demerdash 2007; Fetoui et al. 2008). Numerous studies have shown that exposure to cyhalothrin may affect the functioning of the central nervous system by modulating the neurotransmitters (Hossain et al. 2004, 2005, 2006; Nasuti et al. 2007; Wolansky and Harrill 2008). However, the impact of lambda-cyhalothrin on the brain cholinergic receptors which play an important role in modulating learning and memory and other behaviors is not understood. While exposure to lambda-cyhalothrin at low doses is quite likely to occur, most of these studies on cyhalothrin or lambda-cyhalothrin have been carried out at a high dose for a short period (Hossain et al. 2004, 2005, 2006; El-Demerdash 2007). Neurotoxicity of lambda-cyhalothrin in developing rats is not well understood. Exposure to lambda-cyhalothrin could occur during early period of brain development which starts pre-natally and continue till early post-lactational life. Further, developmental period is considered vulnerable to neurotoxic insults as it may have long-lasting neurobehavioral effects (Schroeder 2000; Julvez and Grandjean 2009; Miodovnik 2011; Landrigan and Miodovnik 2011).
In view of the increasing usage of lambda-cyhalothrin, the risk of human exposure is quite imminent. This study, has therefore, been carried out following post-lactational exposure of rats to lambda-cyhalothrin from PLD22 to PLD49 and the effect on brain cholinergic system and functional alterations associated with them have been studied on PLD50. Involvement of oxidative stress in the neurotoxicity of lambda-cyhalothrin has also been studied. To further find out whether these changes are transient or persistent, the effect on selected neurobehavioral end points was studied 15 days after withdrawal of lambda-cyhalothrin exposure.
Materials and Methods
Animals and Treatment
Female rats of Wistar strain (21 days old, PLD21) weighing around 24 ± 2 g, obtained from the central animal house of CSIR-Indian Institute of Toxicology Research (CSIR-IITR), Lucknow were used in the study. Rats were housed in an air-conditioned room at 25 ± 2°C with a 12-h light/dark cycle under standard hygiene conditions and had free access to pellet diet (Ashirwad Industries, Chandigarh, India) and water ad libitum. The experimental protocol was approved by the institutional animal ethics committee of CSIR-IITR, Lucknow, and all the experimental procedures were carried out in accordance with the guidelines laid down by the committee for the purpose of control and supervision of experiments on animals, Ministry of Environment and Forests (Government of India), New Delhi, India. Rats were divided into three groups and on PLD22, rats in two groups were administered lambda-cyhalothrin (Syngenta, India, 5% EC, suspended in corn oil) daily at either of the doses (1.0 or 3.0 mg/kg body weight, p.o.) till PLD49. The third group of rats was administered corn oil in an identical manner and served as control. Effect on behavioral parameters—grip strength and learning ability—was studied on PLD50. A separate set of rats was sacrificed on PLD50 for neurochemical and immunohistochemical assays. Brains were removed and processed for biochemical assay. To study whether neurobehavioral changes are transient or persistent, a set of rats was left to investigate behavioral, neurochemical, and immunohistochemical end points on PLD65.
Although lambda-cyhalothrin has been found to be present in shallow ground water in the cotton-growing areas (Tariq et al. 2004), river sediments (Miranda et al. 2008), and persist in the top layer of the sandy loam soil in lysimeter studies (Tariq et al. 2006), there is no data of actual levels of lambda-cyhalothrin in the environment. High levels detected in the food materials and breast milk are the chief source of human exposure including developing children and infants (WHO 1990; Feo et al. 2012). Acute effects of cyhalothrin/lambda-cyhalothrin have been studied at doses ranging from 10 to 80 mg/kg body weight in experimental studies on rats (Hossain et al. 2004, 2005, 2006; Mate et al. 2010) while the effects of repeated exposure to lambda-cyhalothrin on genotoxicity, neurotoxicity, developmental/reproductive toxicity have been studied at doses from 0.8 to 61.2 mg/kg body weight in rats (Celik et al. 2003, 2005; Martinez-Larrañaga et al. 2003; Ratnasooriya et al. 2003; Fetoui et al. 2008). In this study, selection of doses (1.0 and 3.0 mg/kg body weight) which are 1/60th and 1/20th, respectively, of LD50 of lambda-cyhalothrin was based on the no observable adverse effect level, reported to be 2.5 mg/kg body weight in a 90-day oral study on rats (WHO 1990, Pesticide Tolerance 1998).
Behavioral Studies
Grip Strength
Forelimb grip strength was measured in the control and the treated rats using a computerized grip strength meter (TSE, Germany) following the standard procedure (Terry et al. 2003). Rats were gently held by the nape of the neck and base of the tail. The forelimbs of the rat were placed on the tension bar. Followed by this, the rat was pulled back gently until it released the bar and the reading was automatically recorded on the computer. Individual animals were exposed to five successive pulls by a person unaware of their treatment status. The mean of all the values was taken and processed for statistical analysis.
Learning Ability
The learning ability of rats was measured by assessing conditioned avoidance response (CAR) using a shuttle box apparatus (Techno, India) following the standard protocol (Moreira et al. 2001) with minor modification. Briefly, rats from the control and the lambda-cyhalothrin-treated groups were placed individually in one chamber of the shuttle box and habituated for 5 min. A conditioned stimulus was given in the form of a buzzer for 10 s followed by a buzzer and foot shock (0.5 mA) for 10 s. All the rats in the control and treated groups were subjected to 20 trials per day for 3 days. The intertrial interval was 1 min. Assessment of CAR was started on PLD48 and data of the final day on PLD50 has been included in the results. Similarly, to asses the effect of withdrawal of lambda-cyhalothrin exposure on the learning, assessment of CAR was started on PLD63 and the data of PLD65 has been included in the results. The CAR represents the cognitive ability of rats and was considered positive if the rats jumped to a shock-free chamber to avoid the foot shock and was calculated in terms of percentage out of 20 trials. Of the 20 trials per day, the total number of avoidances and the number of trials for the first avoidance were recorded for each rat. The percentage of CAR was calculated dividing the total number of avoidances by the total number of trials (20) and multiplied by 100 in each case.
Neurochemical Studies
For neurochemical studies, rats were sacrificed by cervical dislocation and the brains were removed rapidly and placed on ice. The brain was dissected into specific regions (frontal cortex, corpus striatum, hippocampus, and cerebellum) following the standard procedure (Glowinski and Iversen 1966). The brain regions were stored at −80°C for the assay of neurotransmitter receptors and the other set processed for the assay of oxidative stress parameters.
Assay of Acetylcholinesterase Activity in Selected Brain Regions
Activity of acetylcholinesterase was assayed following the method of Ellman et al. (1961) using acetylthiocholine iodide as a substrate and 5,5′-dithiobis-2 nitrobenzoic acid (DTNB) as the coloring agent. Briefly, the reaction mixture in a final volume of 1.0 ml contained phosphate buffer (0.1 M, pH 7.4), post-mitrochondrial fraction of brain regions (frontal cortex, hippocampus, and cerebellum) containing around 15–20 μg protein, acetylthiocholine iodide (ACTI), and DTNB (5 mM). The degradation of acetylthiocholine iodide was measured at 412 nm and the results are expressed as μmol ACTI hydrolysed/mg protein.
Assay of Muscarinic–Cholinergic Receptors in Selected Brain Regions
The assay of muscarinic–cholinergic receptors in frontal cortex, hippocampus, and cerebellum was carried out by the radioligand receptor binding assay following the standard procedure (Khanna et al. 1994). Briefly, crude synaptic membrane was prepared by homogenizing the brain regions (frontal cortex, hippocampus, and cerebellum) in 19 volumes of Tris–HCl buffer (5 mM, pH 7.4). The homogenate was centrifuged at 40,000×g for 15 min at 4°C. The sedimented pellet was washed twice by resuspending in homogenization buffer and recentrifuged at the same speed for 15 min at 4°C. Finally, the pellet was suspended in Tris–HCl buffer (40 mM, pH 7.4) and stored at −20°C for binding assays.
Binding incubations in a final volume of 1.0 ml were carried out in triplicate. For the assay of muscarinic–cholinergic receptors, 3H-QNB (42 Ci/mmol, Perkin Elmer, USA) was used as a radioligand. To assess the extent of non-specific binding, atropine sulfate (1 × 10−6 M) was used as a competitor. The reaction mixture in a final volume of 1 ml containing buffer (40 mM Tris–HCl, pH 7.4), together with membrane protein (300–400 μg) and 3H-QNB (1 × 10−9 M) radioligand was incubated for 15 min at 37°C in the presence or in the absence of atropine sulfate. At the end of incubation, contents of the binding tubes were immediately filtered on glass fiber discs (25 mm diameter, 1.0 μm pore size, Whatman GF/B) and washed twice rapidly with 5 ml chilled Tris–HCl buffer (40 mM, pH 7.4). Filters were dried and transferred into vials and scintillation mixture containing 2,5-diphenyl oxazole; 1,4-bis-5, phenyloxazolyl-benzene; naphthalene; toluene; methanol; and 1,4-dioxane added to it. The radioactivity was counted on β-scintillation counter (Packard, USA) at an efficiency of 30–40% for 3H to determine membrane bound radioactivity. The specific binding was calculated by subtracting the non-specific binding (in the presence of atropine sulfate) from the total binding (in the absence of atropine sulfate). Specific binding has been expressed as picomoles ligand bound/g protein. Scatchard analysis was carried out at varying concentrations of radioligands (generally 1/10 to 10 times of the affinity) to ascertain whether change in the binding is due to alteration in the affinity (K d) or number of receptor binding sites (B max).
Oxidative Stress
To assess the extent of oxidative stress following exposure to lambda-cyhalothrin, estimation of lipid peroxidation, protein carbonyl, and reduced glutathione levels and assay of activity of superoxide dismutase, catalase, and glutathione peroxidase was carried out in brain regions.
Assay of Lipid Peroxidation, Protein Carbonyl, and Reduced Glutathione Levels in Selected Brain Regions of Rats
As a measure of malondialdehyde (MDA) formation, levels of thiobarbituric acid reactive substances (TBARS) were estimated following the method of Ohkawa et al. (1979). Briefly, homogenate of brain regions in 0.1 M phosphate-buffered saline (10% w/v) was incubated with 8.1% sodium dodecyl sulfate (SDS, w/v) for 10 min at room temperature followed by the addition of 20% acetic acid. Thiobarbituric acid (TBA, 0.8%, w/v) was added in the reaction mixture after vortexing the contents of the tube. The tubes were kept in a boiling water bath for 1 h and the intensity of pink color (chromogen) formed during the reaction was read at 532 nm. The amount of TBARS was calculated using a molar extinction coefficient of 1.56 × 105 m cm−1.
Protein carbonyl content in brain regions was measured following the method of Levine et al. (1990) using 2,4-dinitrophenylhydrazine (DNPH) as a substrate. The difference in absorbance between the DNPH-treated and the HCl-treated samples was determined spectrophotometrically at 375 nm and the amount of carbonyl contents (C) was calculated using a molar extinction coefficient (ε) of 22.0 mM−1 cm−1 for aliphatic hydrazones.
Levels of reduced glutathione were measured in brain regions (frontal cortex, corpus striatum, hippocampus, and cerebellum) following the method of Hasan and Haider (1989). Briefly, 10% homogenate was deproteinized with an equal volume of trichloroacetic acid (TCA, 10%) and allowed to stand at 4°C for 1 h. The contents were centrifuged at 3,000×g for 15 min. The supernatant (0.5 ml) was added to 2 ml of Tris buffer (0.4 mM, pH 8.9) containing EDTA (0.02 M) followed by the addition of DTNB (0.01 M). The volume was made up to 3 ml by addition of 0.5 ml of distilled water and absorbance of yellow color read on a spectrophotometer at 412 nm. The results are expressed as μg GSH/g tissue.
Assay of Superoxide Dismutase, Catalase, and Glutathione Peroxidase Activity in Selected Brain Regions of Rats
Rat brain mitochondria were isolated following the procedure as described by Stahl et al. (1963). Briefly, the dissected brain regions were homogenized (10% w/v) in ice-cold buffer containing Tris–HCl (10 mM, pH 7.4), sucrose (320 mM), EDTA (5 mM), and BSA (0.1%). The homogenate was centrifuged at 1,000×g for 15 min at 4°C. The pellets were discarded and the supernatant was further centrifuged at 14,000×g for 15 min at 4°C. The supernatant, post-mitochondrial fraction was separated and preserved. Crude mitochondrial pellets were separated and washed with buffer and resuspended in Tris–HCl buffer (10 mM, pH 7.4) containing sucrose (0.44 M) and used for the assay of the activity of superoxide dismutase. Activity of superoxide dismutase was measured in the mitochondrial fraction using NADH as a substrate, following the method of Kakkar et al. (1984). The superoxide dismutase activity has been expressed in units/mg protein. One unit of the enzyme is the amount required to inhibit the rate of chromogen formation by 50%.
The activity of catalase in brain regions was measured spectrophotometrically in post-mitochondrial fraction using hydrogen peroxide (H2O2) as substrate following the method of Aebi (1984). The activity is expressed in μmol/mg protein.
The activity of glutathione peroxidase in brain regions was measured by the method of Flohe and Gunzler (1984). Briefly, 5% homogenate of brain regions (prepared in phosphate buffer, 0.1 M, pH 7.4) was centrifuged at 1,500×g for 10 min at 4°C. The supernatant was transferred in another tube and centrifuged at 10,000×g for 30 min at 4°C. The supernatant thus obtained was used for the assay of glutathione peroxidase activity. Reaction mixture in a final volume of 1 ml containing phosphate buffer (0.1 M, pH 7.4), reduced glutathione (2 mM), sodium azide (10 mM), H2O2 (1 mM), and enzyme preparation was incubated at 37°C for 15 min. The reaction was stopped by the addition of 0.5 ml TCA. The tubes were centrifuged at 1,500×g for 5 min for the protein to settle. Following this the supernatant was added into another tube containing 0.2 ml of phosphate buffer (0.1 M, pH 7.4) and 0.7 ml of DTNB (0.4 mg/ml). The reaction mixture was vortexed and absorbance was recorded at 420 nm. The values are expressed as nmol GSH oxidised/mg protein.
Western Blotting
Expression of choline acetyltransferase in hippocampus was assayed following the method of Jamal et al. (2007). Briefly, the brain regions were homogenized in RIPA buffer containing Tris–HCl (50 mM, pH 6.8), NaCl (150 mM), sodium deoxycholate (0.5%), SDS (0.1%), protease inhibitor, and triton-100X (1%) and centrifuged at 12,000×g for 15 min at 4°C to remove insoluble material. The pellets were discarded and supernatant further mixed with loading buffer containing Tris–HCl (60 mM, pH 6.8), SDS (2%), glycerol (10%), β-mercaptoethanol (5%), bromophenol blue (0.01%) and boiled for 5–7 min. The prepared samples (30 μg protein/lane) were electrophoresed on 12% SDS-PAGE, electroblotted on to nitrocellulose membranes (Millipore, USA) and blocked with blocking buffer (Western blocker solution™ Sigma, USA). After subsequent washing, the blots were incubated with primary antibody (Anti-ChAT, Sigma, USA, 1:2000) for 24 h at 4°C followed by incubation with horseradish peroxidase-linked secondary antibody (anti-mouse IgG, 1:4,000) at room temperature for 60 min. After the incubation, blots were washed and developed using an immobilon western chemiluminescent HRP substrate (Millipore, USA) following the recommended procedure. β-actin was probed as an internal control and used to confirm that an equal amount of protein was loaded in each lane. A digital gel image analysis system (VersaDoc, Model 1000, Bio Rad, Quantity 1) was used for semi-quantification of ChAT immunoreactivity.
Protein Estimation
Protein concentration in sample homogenates was measured following the method of Lowry et al. (1951) using bovine serum albumin (BSA) as the reference standard.
Immunohistochemistry
Immunohistochemical studies were carried out following the method of Goslin et al. (1990). Briefly, rats were anesthetized using ketamine/xylazine (37.5 mg/kg/5 mg/kg body weight, i.p.) and perfused with 150 ml of phosphate-buffered saline (PBS, 0.1 M, pH 7.4) followed by 250 ml of ice-cold 4% paraformaldehyde in PBS for fixation of tissues. Brains were removed and post-fixed in 10% paraformaldehyde in PBS and samples were kept in 10, 20, and 30% (w/v) sucrose in PBS. Serial coronal sections of 20-μm thickness were cut on a cryomicrotome (Microm HM 520, Labcon, Germany), incubated with primary [anti-choline acetyltransferase, anti-acetylcholinesterase, anti-growth-associated protein (anti-GAP)-43, Sigma, USA, 1:200] and secondary antibodies (biotinylated peroxidase linked, Sigma USA, 1:400) and processed as per protocol. The intensity of choline acetyltransferase, acetylcholinesterase, and GAP-43 positive neurons in the hippocampal region of the brain was determined using a computerized image analysis system (Leica Qwin 500 image analysis software) as described by Shingo et al. (2002).
Statistical Analysis
The data have been analyzed using one-way analysis of variance followed by Newman–Keuls test for multiple pair wise comparisons among various groups. All values have been expressed as mean ± SEM. Value up to p < 0.05 has been considered significant.
Results
No apparent signs and symptoms of toxicity were observed in rats during their exposure to lambda-cyhalothrin. Behavioral, neurochemical, and immunohistochemical studies in lambda-cyhalothrin exposed and control rats were carried out on PLD50 and PLD65 and the results are presented below,
Behavioral Studies
Post-lactational Exposure to Lambda-Cyhalothrin and Its Effect on the Grip Strength of Rats
Fore limb grip strength was monitored following exposure of rats from PLD22 to PLD49 to lambda-cyhalothrin (1.0 or 3.0 mg/kg body weight) to assess the effect on muscle weakness and results are presented in Fig. 1. A significant decrease in the forelimb grip strength (26%, p < 0.05) was observed in rats on PLD50 following exposure to lambda-cyhalothrin at the higher dose (3.0 mg/kg body weight) as compared to controls (Fig. 1A). The decrease (15%, p < 0.05) in the grip strength due to lambda-cyhalothrin exposure was found to persist for 15 days after withdrawal of exposure on PLD65 in comparison to respective controls. No significant change in grip strength was observed in rats exposed to lambda-cyhalothrin at the lower dose (1.0 mg/kg body weight) both on PLD50 and PLD65 as compared to respective controls (Fig. 1B).
Effect on grip strength following post-lactational exposure of rats to lambda-cyhalothrin. Rats were exposed to lambda-cyhalothrin (1.0 or 3.0 mg/kg body weight/day, p.o.) from PLD22 to PLD49. Effect on grip strength studied 28 days after exposure to lambda-cyhalothrin on PLD50 (at end of treatment) (A). To assess whether changes are transient or persistent, effect on grip strength was also studied 15 days after withdrawal of lambda-cyhalothrin exposure on PLD65 (B). Data have been analyzed by one-way analysis of variance followed by Newman–Keuls test. Values are mean ± SEM of five animals in each group. *Significantly differs from control group (p < 0.05)
Post-lactational Exposure to Lambda-Cyhalothrin and Its Effect on the Learning of Rats
A significant impairment in the learning (23%, p < 0.01; 43%, p < 0.001) was observed in rats exposed to lambda-cyhalothrin on PLD50 (Fig. 2A). The decrease in the learning was more marked in rats exposed to lambda-cyhalothrin at the higher dose and remained persistent (26%, p < 0.05) in these rats even 15 days after withdrawal of exposure on PLD65 (Fig. 2B). No significant effect in the learning was observed in rats exposed to lambda-cyhalothrin at the lower dose (1.0 mg/kg body weight) on PLD65 in comparison to respective controls (Fig. 2B).
Effect on CAR following post-lactational exposure of rats to lambda-cyhalothrin. Rats were exposed to lambda-cyhalothrin (1.0 or 3.0 mg/kg body weight/day, p.o.) from PLD22 to PLD49. Effect on CAR studied 28 days after exposure to lambda-cyhalothrin on PLD50 (at end of treatment) (A). To assess whether changes are transient or persistent, effect on CAR was also studied 15 days after withdrawal of lambda-cyhalothrin exposure on PLD65 (B). Data have been analyzed by one-way analysis of variance followed by Newman–Keuls test. Values are mean ± SEM of five animals in each group. *Significantly differs from control group (p < 0.05)
Neurochemical Studies
Post-lactational Exposure to Lambda-Cyhalothrin and Its Effect on the Acetylcholinesterase Activity in Selected Brain Regions of Rats
Exposure of rats to lambda-cyhalothrin caused a significant decrease in the activity of acetylcholinesterase, an enzyme involved in the metabolism of acetylcholine in frontal cortex (17%, p < 0.05; 38%, p < 0.001), hippocampus (24%, p < 0.05; 51%, p < 0.001), and cerebellum (15%, p > 0.05; 26%, p < 0.05) on PLD50 as compared to controls (Fig. 3). The decrease in the activity of acetylcholinesterase was more marked in rats exposed to lambda-cyhalothrin at the higher dose. Although a trend of recovery in the activity of acetylcholinesterase in lambda-cyhalothrin-treated rats was observed 15 days after withdrawal of exposure, it remained decreased in the frontal cortex (14%, p > 0.05; 25%, p > 0.05), hippocampus (17%, p > 0.05; 31%, p < 0.05), and cerebellum (9%, p > 0.05; 16%, p > 0.05) on PLD65 as compared to respective controls (Fig. 3).
Effect on acetylcholinesterase activity following post-lactational exposure of rats to lambda-cyhalothrin. Rats were exposed to lambda-cyhalothrin (1.0 or 3.0 mg/kg body weight/day, p.o.) from PLD22 to PLD49. Effect on acetylcholinesterase activity studied 28 days after exposure on PLD50 (at end of treatment) (A). To assess whether changes are transient or persistent effect on acetylcholinesterase activity was also studied 15 days after withdrawal of lambda-cyhalothrin exposure on PLD65 (B). Data have been analyzed by one-way analysis of variance followed by Newman–Keuls test. Values are mean ± SEM of five animals in each group. *Significantly differs from control group (p < 0.05)
Post-lactational Exposure to Lambda-Cyhalothrin and Its Effect on the Muscarinic–Cholinergic Receptors in Selected Brain Regions of Rats
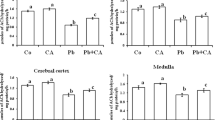
A decrease in the binding of 3H-QNB to frontocortical (24%, p > 0.05; 42%, p < 0.05), hippocampal (41%, p < 0.01; 44%, p < 0.01), and cerebellar (21%, p < 0.01; 54%, p < 0.001) membranes, known to label muscarinic–cholinergic receptors was observed in lambda-cyhalothrin-treated rats on PLD50 as compared to controls (Fig. 4). Scatchard analysis revealed that decrease in the binding of 3H-QNB to frontocortical, hippocampal, and cerebellar membranes was due to decreased number of receptor binding sites (Table 1). The decrease in the binding of 3H-QNB to hippocampal (12%, p > 0.05; 29%, p > 0.05) and cerebellar (11%, p > 0.05; 47%, p < 0.001) membranes was found to persist even after withdrawal of exposure while a trend of recovery was observed in the binding of 3H-QNB to frontocortical membranes in lambda-cyhalothrin-treated rats on PLD65 as compared to respective controls (Fig. 4).
Effect on muscarinic–cholinergic receptors following post-lactational exposure of rats to lambda-cyhalothrin. Rats were exposed to lambda-cyhalothrin (1.0 or 3.0 mg/kg body weight/day, p.o.) from PLD22 to PLD49. Effect on muscarinic–cholinergic receptors was studied 28 days after exposure to lambda-cyhalothrin on PLD50 (at end of treatment) (A). To assess whether changes are transient or persistent, effect on muscarinic–cholinergic receptors was also studied 15 days after withdrawal of lambda-cyhalothrin exposure on PLD65 (B). Data have been analyzed by one-way analysis of variance followed by Newman–Keuls test. Values are mean ± SEM of five animals in each group. *Significantly differs from control group (p < 0.05)
Oxidative Stress
Post-lactational Exposure to Lambda-Cyhalothrin and Its Effect on Lipid Peroxidation, Protein Carbonyl, and Reduced Glutathione Levels in Selected Brain Regions of Rats
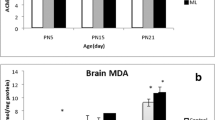
Levels of TBARS were increased in frontal cortex (15%, p > 0.05; 38%, p < 0.01), corpus striatum (80%, p < 0.05; 88%, p < 0.05), hippocampus (64%, p < 0.01; 73%, p < 0.01), and cerebellum (20%, p < 0.05; 33%, p < 0.01) of rats exposed to lambda-cyhalothrin on PLD50 as compared to controls (Fig. 5A). Increase in TBARS levels was more marked in rats exposed to lambda-cyhalothrin at the higher dose and suggests increased oxidative stress in brain. Further, the TBARS levels remained increased in frontal cortex (13%, p < 0.05; 21%, p < 0.05) and hippocampus (25%, p > 0.05; 18%, p > 0.05) and exhibited a trend of recovery in corpus striatum (6%, p > 0.05; 13%, p > 0.05) and cerebellum (4%, p > 0.05; 19%, p < 0.05), 15 days after withdrawal of exposure on PLD65 as compared to respective controls (Fig. 5B).
Effect on lipid peroxidation, protein carbonyl, and reduced glutathione levels in brain regions (frontal cortex, corpus striatum, hippocampus, and cerebellum) following post-lactational exposure of rats to lambda-cyhalothrin. Rats were exposed to lambda-cyhalothrin (1.0 or 3.0 mg/kg body weight/day, p.o.) from PLD22 to PLD49. Effect on lipid peroxidation, protein carbonyl levels, and reduced glutathione levels in brain regions was studied 28 days after exposure on PLD50 (at end of treatment) (A). To assess whether changes are transient or persistent, effect on lipid peroxidation, protein carbonyl, and reduced glutathione levels was also studied 15 days after withdrawal of lambda-cyhalothrin exposure on PLD65 (B). Data have been analyzed by one-way analysis of variance followed by Newman–Keuls test. Values are mean ± SEM of five animals in each group. *Significantly differs from control group (p < 0.05)
Protein carbonyl levels in frontal cortex (11%, p > 0.05; 48%, p < 0.05), corpus striatum (21%, p < 0.01; 86%, p < 0.01), hippocampus (23%, p > 0.05; 58%, p < 0.01), and cerebellum (59%, p < 0.01; 71%, p < 0.01) in lambda-cyhalothrin-treated rats were found to be increased on PLD50 as compared to controls (Fig. 5A). Increase in the levels of protein carbonyl was more in rats exposed to lambda-cyhalothrin at the higher dose. Levels of protein carbonyls remained increased in corpus striatum (18%, p < 0.01; 36%, p < 0.01) while a trend of recovery was observed in frontal cortex (2%, p > 0.05; 3%, p > 0.05), hippocampus (21%, p > 0.05; 30%, p < 0.05), and cerebellum (11%, p > 0.05; 21%, p > 0.05) in lambda-cyhalothrin-treated rats 15 days after withdrawal of exposure on PLD65 as compared to respective controls (Fig. 5B).
A decrease in the levels of reduced glutathione in frontal cortex (18%, p < 0.05; 21%, p < 0.05), corpus striatum (19%, p < 0.01; 11%, p < 0.01), hippocampus (7%, p > 0.05; 7%, p > 0.05), and cerebellum (9%, p < 0.05; 22%, p < 0.001) was observed in rats exposed to lambda-cyhalothrin (1.0 or 3.0 mg/kg body weight) from PLD22 to PLD49 on PLD50 as compared to controls (Fig. 5A). No significant change in the levels of reduced glutathione was observed in any of the brain regions in rats treated with lambda-cyhalothrin, 15 days after withdrawal of exposure on PLD65 in comparison to respective controls suggesting a trend of recovery (Fig. 5B).
Post-lactational Exposure to Lambda-Cyhalothrin and Its Effect on the Activity of Superoxide Dismutase, Catalase, and Glutathione Peroxidase in Selected Brain Regions of Rats
Activity of superoxide dismutase, an enzyme involved in the dismutation of superoxide radicals, was found to be decreased in frontal cortex (32%, p < 0.05; 39%, p < 0.05), corpus striatum (11%, p > 0.05; 44%, p < 0.05), hippocampus (44%, p < 0.05; 55%, p < 0.01), and cerebellum (55%, p < 0.01; 67%, p < 0.01) following post-lactational exposure of rats to lambda-cyhalothrin (1.0 or 3.0 mg/kg body weight) on PLD50 in comparison to controls (Fig. 6A). A trend of recovery in the activity of superoxide dismutase was observed in frontal cortex (1%, p > 0.05; 5%, p > 0.05), corpus striatum (11%, p > 0.05; 12%, p > 0.05), hippocampus (16%, p > 0.05; 16%, p > 0.05), and cerebellum (10, p > 0.05; 29%, p < 0.05) of lambda-cyhalothrin-treated rats 15 days after withdrawal of exposure as compared to respective controls (Fig. 6B).
Effect on the activity of superoxide dismutase, catalase, and glutathione peroxidase in brain regions (frontal cortex, corpus striatum, hippocampus, and cerebellum) following post-lactational exposure of rats to lambda-cyhalothrin. Rats were exposed to lambda-cyhalothrin (1.0 or 3.0 mg/kg body weight/day, p.o.) from PLD22 to PLD49. Effect on the activity of superoxide dismutase, catalase, and glutathione peroxidase in brain regions was studied 28 days after exposure on PLD50 (at end of treatment) (A). To assess whether changes are transient or persistent, effect on the activity of superoxide dismutase, catalase and glutathione peroxidase was also studied 15 days after withdrawal of lambda-cyhalothrin exposure on PLD65 (B). Data have been analyzed by one-way analysis of variance followed by Newman–Keuls test. Values are mean ± SEM of five animals in each group. *Significantly differs from control group (p < 0.05)
The activity of catalase was found to be decreased in frontal cortex (20%, p > 0.05; 35%, p < 0.05), corpus striatum (35%, p < 0.01; 72%, p < 0.001), hippocampus (32%, p < 0.001; 37%, p < 0.01), and cerebellum (18%, p > 0.05; 20%, p > 0.05) in rats exposed to lambda-cyhalothrin (1.0 or 3.0 mg/kg body weight) from PLD22 to PLD49 on PLD50 as compared to controls (Fig. 6A). The activity of catalase in these brain regions remained decreased in lambda-cyhalothrin-treated rats even after withdrawal of exposure for 15 days on PLD65 while changes were not significant as compared to respective controls (Fig. 6B).
A decrease in the activity of glutathione peroxidase was observed in frontal cortex (11%, p > 0.05; 28%, p < 0.01), corpus striatum (8%, p > 0.05; 21%, p < 0.01), hippocampus (11%, p < 0.05; 22%, p < 0.001), and cerebellum (11%, p < 0.05; 20%, p < 0.01) of rats following their exposure from PLD22 to PLD49 to lambda-cyhalothrin (1.0 or 3.0 mg/kg body weight) on PLD50 as compared to controls (Fig. 6A). The activity of glutathione peroxidase in lambda-cyhalothrin-treated rats exhibited a trend of recovery in hippocampus (1%, p > 0.05; 12%, p > 0.05), corpus striatum (1%, p > 0.05; 8%, p > 0.05), and cerebellum (1%, p > 0.05; 11%, p > 0.05) while it remained decreased in the frontal cortex (13%, p < 0.05; 13%, p < 0.05) 15 days after withdrawal of exposure on PLD65 in lambda-cyhalothrin-treated rats as compared to respective controls (Fig. 6B).
Western Blotting
Post-lactational Exposure to Lambda-Cyhalothrin and Its Effect on the Expression of Choline Acetyltransferase in Hippocampus
A significant decrease in the expression of ChAT protein in hippocampus (3.2 fold, p < 0.05 and 4.2 fold, p < 0.05) was observed in lambda-cyhalothrin exposed rats on PLD50 as compared to rats in the control group (Fig. 7). A trend of recovery was observed in the expression of ChAT protein (0.6-fold) in rats exposed to lambda-cyhalothrin at a low dose (1.0 mg/kg) while the expression of ChAT remained decreased (2.7-fold, p < 0.05) in those exposed to the higher dose (3.0 mg/kg) even after withdrawal of exposure on PLD65 as compared to respective controls (Fig. 7).
Effect on the expression of choline acetyltransferase protein in hippocampus following post-lactational exposure of rats to lambda-cyhalothrin. Rats were exposed to lambda-cyhalothrin (1.0 or 3.0 mg/kg body weight/day, p.o.) from PLD22 to PLD49. Effect on the expression of choline acetyltransferase protein was studied 28 days after exposure of lambda-cyhalothrin on PLD50 (at end of treatment) (A). To assess whether changes are transient or persistent, effect on the expression of choline acetyltransferase protein was also studied 15 days after withdrawal of lambda-cyhalothrin exposure on PLD65 (B). Data have been analyzed by one-way analysis of variance followed by Newman–Keuls test. Values are mean ± SEM of five animals in each group. *Significantly differs from control group (p < 0.05)
Immunohistochemical Studies
Post-lactational Exposure to Lambda-Cyhalothrin and Its Effect on the Expression of Choline Acetyltransferase, Acetylcholinesterase, and GAP-43 in Specific Brain Region of Rats
Post-lactational exposure to lambda-cyhalothrin in rats caused a significant decrease in the immunoreactivity of ChAT and AChE in hippocampal granular and pyramidal cells as compared to controls (Figs. 8, 9). Quantification of immunoreactivity revealed a decrease in percent area in ChAT (57%, p < 0.01; 77%, p < 0.001) and AChE (17%, p < 0.05; 58%, p < 0.01) expression, respectively, in lambda-cyhalothrin-treated rats on PLD50 as compared to controls (Figs. 8, 9). Although percent area in ChAT (27%, p < 0.05; 66%, p < 0.01) and AChE (14%, p > 0.05; 36%, p < 0.05) exhibited a trend of recovery, the expression remained decreased in the hippocampal sections in lambda-cyhalothrin treated rats at both the doses on PLD65 (Figs. 8, 9).
Photomicrographs of rat hippocampal sections illustrating effect on the choline acetyltransferase immunoreactivity following post-lactational exposure of rats to lambda-cyhalothrin. Rats were exposed to lambda-cyhalothrin (1.0 or 3.0 mg/kg body weight/day, p.o.) from PLD22 to PLD49. Effect on choline acetyltransferase immunoreactivity was studied 28 days after exposure to lambda-cyhalothrin on PLD50 (at end of treatment) (A). Exposed rats (b, c) showed diminished choline acetyltransferase immunoreactivity as compared to control (a). To assess whether changes are transient or persistent, effect on the choline acetyltransferase immunoreactivity was also studied 15 days after withdrawal of lambda-cyhalothrin exposure on PLD65 (B). A significant impairment in the expression of hippocampal choline acetyltransferase was evident 15 days after withdrawal of exposure in rats exposed to lambda-cyhalothrin at both the doses (e, f) as compared to control (d). Arrow indicates immunoreactivity for choline acetyltransferase. Scale bar 300 μm. *Significantly differs from control group (p < 0.05)
Photomicrographs of rat hippocampal sections illustrating effect on the acetylcholinesterase immunoreactivity following post-lactational exposure of rats to lambda-cyhalothrin. Rats were exposed to lambda-cyhalothrin (1.0 or 3.0 mg/kg body weight/day, p.o.) from PLD22 to PLD49. Effect on acetylcholinesterase immunoreactivity was studied 28 days after exposure to lambda-cyhalothrin on PLD50 (at end of treatment) (A). Exposed rats (b, c) showed diminished acetylcholinesterase immunoreactivity as compared to control (a). To assess whether changes are transient or persistent, effect on the acetylcholinesterase immunoreactivity was also studied 15 days after withdrawal of lambda-cyhalothrin exposure on PLD65 (B). A significant impairment was evident 15 days after withdrawal of exposure at the higher dose (f) when compared to respective control (d) whereas rats exposed at 1.0 mg/kg dose (e) showed restoration in acetylcholinesterase expression. Arrow indicates immunoreactivity for acetylcholinesterase. Scale bar 300 μm. *Significantly differs from control group (p < 0.05)
A significant increase in the expression of GAP-43 was observed in brain following post-lactational exposure of rats to lambda-cyhalothrin (1.0 or 3.0 mg/kg) for 28 days as compared to controls (Fig. 10). Quantification of immunoreactivity exhibited an increase in percent area in GAP-43 to several fold in hippocampal pyramidal granular cells treated with lambda-cyhalothrin at both the doses (Fig. 10). The changes were more marked in rats treated with lambda-cyhalothrin at the higher dose. The immunoreactivity of GAP-43 remained increased in rats treated with lambda-cyhalothrin at the higher dose even 15 days after withdrawal of exposure on PLD65 as compared to respective controls (Fig. 10).
Photomicrographs of rat hippocampal sections illustrating effect on the GAP immunoreactivity following post-lactational exposure of rats to lambda-cyhalothrin. Rats were exposed to lambda-cyhalothrin (1.0 or 3.0 mg/kg bodyweight/day, p.o.) from PLD22 to PLD49. Effect on GAP immunoreactivity was studied 28 days after exposure to lambda-cyhalothrin on PLD50 (at end of treatment) (A). Exposed rats (b, c) showed higher GAP immunoreactivity as compared to control (a). To assess whether changes are transient or persistent, effect on the GAP immunoreactivity was also studied 15 days after withdrawal of lambda-cyhalothrin exposure on PLD65 (B). A trend of recovery was evident 15 days after withdrawal of exposure in rats exposed to lambda-cyhalothrin at the lower dose (e) while significant change was observed in rats exposed at a higher dose (f) as compared to respective control (d). Arrow indicates immunoreactivity for GAP. Scale bar 300 μm. *Significantly differs from control group (p < 0.05)
Discussion
The primary site of action of pyrethroids is voltage sensitive sodium channels (Narahashi 1996; Shafer et al. 2005). It has been found that pyrethroids affect the gating characteristics of voltage sensitive sodium channels and disrupt the nerve functions both in insects and in mammals (Narahashi 1996; Hossain and Richardson 2011). Several other mechanisms including their action on voltage sensitive chloride channels associated with increased excitability in the CNS, GABAA receptors have been suggested in the neurotoxicity of pyrethroids (Narahashi 1996; Breckenridge et al. 2009). In experimental studies, modulation in the release of neurotransmitters following pyrethroid exposure largely by their action on voltage sensitive calcium channels has been reported (Soderlund et al. 2002; Shafer et al. 2005; Ray and Fry 2006; Brown et al. 2006; Nasuti et al. 2007). Differential effects on cholinergic neuro-transmission in the hippocampus of freely moving rats were observed both by type I and II classes of pyrethroids (Hossain et al. 2004). Cyhalothrin, a type II pyrethroid, inhibited the release of acetylcholine; however, deltamethrin another type II pyrethroid increased the release of acetylcholine from hippocampus. It was interesting that allethrin, a type I pyrethroid, had a dual effect since acetylcholine release from hippocampus was found to be increased at a low dose and decreased at a high dose. In another study, Hossain et al. (2005) found that choline acetyltransferase activity and high affinity choline uptake were differentially regulated by type I and II pyrethroids, while none of the pyrethroids affected the acetylcholinesterase activity. In this study, decrease in the binding of muscarinic–cholinergic receptors associated with decreased acetylcholinesterase activity in the frontal cortex, hippocampus, and cerebellum of lambda-cyhalothrin exposed rats indicate alterations in the cholinergic neurotransmission. Interestingly, decrease in the expression of acetylcholinesterase immunoreactivity also observed in lambda-cyhalothrin-treated rats is consistent with the decreased activity of brain acetylcholinesterase. Down-regulation of brain muscarinic–cholinergic receptors following chronic treatment with oxotremorine, a cholinergic agonist has been reported (Wehner and Upchurch 1989). As levels of acetylcholine could not be measured, it is difficult to comment whether alteration in acetylcholine levels is associated with decrease in the muscarinic–cholinergic receptors in brain regions. Since decrease in the muscarinic–cholinergic receptors has been found to be agonist induced and a key mechanism of adaptation (Hoskins and Ho 1992), alterations in acetylcholine levels in the brains of lambda-cyhalothrin exposed rats appear to be quite convincing. It is possible that the decreased activity of AChE may increase the acetylcholine levels associated with the down-regulation of muscarinic–cholinergic receptors. Further, decrease in the expression of ChAT, an enzyme responsible for the synthesis of acetylcholine in hippocampus indicates alteration in the cholinergic circuitry following exposure to lambda-cyhalothrin. The decrease in the expression of hippocampal ChAT protein and immunoreactivity and acetylcholinesterase activity could be due to the auto feedback control to compensate intracellular ACh homeostasis.
Involvement of free radicals in neurological and neurotoxicological disorders has been a subject of study (Andersen 2004; Halliwell 2006; Sayre et al. 2001, 2008). As the brain is the primary target of synthetic pyrethroids, incidences of pyrethroid-induced neurotoxicity have increased (Wolansky and Harrill 2008). Increased vulnerability of brain to toxic insults by free radicals is well recognized as the brain has high levels of polyunsaturated fatty acids. Increased oxygen consumption associated with low levels of antioxidant defense in the brain increases the risk of oxidative damage. Pyrethroids are more hydrophobic as compared to other classes of insecticides and thus biological membranes are their easy target (Michelangeli et al. 1990). Further, because of the presence of alpha-cyano moiety in lambda-cyhalothrin, there is release of cyanohydrins which are degraded to cyanide and aldehydes, the potential source of free radicals (Fetoui et al. 2008). Exposure of adult rats to lambda-cyhalothrin has been found to enhance oxidative stress in the brain and these effects were attenuated by vitamin C (Fetoui et al. 2008). However, these effects have been observed at a very high dose of lambda-cyhalothrin. Consistent with this, low level exposure to lambda-cyhalothrin decreased GSH levels in the brain in this study which could be due to either high utilization of GSH for conjugation or its involvement as an antioxidant in neutralizing the free radicals. Further, the decrease in the activity of superoxide dismutase, catalase, and glutathione peroxidase in the brain indicates impairment in the antioxidant defense. Decrease in the activity of superoxide dismutase, an enzyme involved in the dismutation of superoxide to hydrogen peroxide, could enhance the generation of superoxide radicals in the brain. At the same time, the decrease in the activity of catalase and glutathione peroxidase involved in the degradation of hydrogen peroxide to water and oxygen could be attributed to more of the hydrogen peroxide in the brain. Therefore, increased levels of superoxide and hydrogen peroxide may enhance peroxidation of lipids in membrane and could be linked to increased lipid peroxidation and protein carbonyl levels as observed in this study.
Role of GAP-43 (B-50 or neuromodulin) in modulating the growth of axon terminals and experience-dependent plasticity is well recognized (Benowitz and Routtenberg 1997). It has been found that GAP-43 phosphorylation site is a unique target and genetic over expression of GAP-43 increased learning and long-term potentiation in transgenic mice (Routtenberg et al. 2000). Expression of GAP-43 has been found to play a crucial and dual role in a classical study (Holahan et al. 2007). Moderate expression of GAP-43 in hippocampus enhanced memory, while excessive expression of GAP-43 produced neuroplasticity burden leading to degenerative and hypertrophic events culminating in memory dysfunctions in transgenic mice (Holahan et al. 2007). Interestingly, increased GAP-43 levels in hippocampus were found to affect the memory circuits in Alzheimer’s patients (Rekart et al. 2004). Impairment both in learning capacity and in memory associated with upregulation of GAP-43 mRNA in rat hippocampus has also been observed following chronic cerebral hypoperfusion concomitant with increased duration of occlusion of bilateral common carotid arteries (Liu et al. 2005). Further, increased expression of GAP-43 in hippocampus of developing rats pre-natally exposed to deltamethrin, a type II synthetic pyrethroid has been reported earlier (Aziz et al. 2001). Consistent with these reports, decrease in learning in lambda-cyhalothrin-treated rats as observed in this study could be due to excessive expression of GAP-43. Impairment in learning in lambda-cyhalothrin-treated rats could also be accounted due to other reasons. As the role of central cholinergic system is well recognized in learning, memory, and cognition, it is possible that the decrease in the binding of muscarinic–cholinergic receptors in hippocampus of lambda-cyhalothrin-treated rats may significantly affect the learning. Decrease in the expression of hippocampal AChE could also be associated with decreased learning response in lambda-cyhalothrin-treated rats as anti-cholinesterases have been found to affect learning (Farage-Elawar 1989; Sarin and Gill 1998). Age-related impairment in learning and memory has been attributed to oxidative protein damage in the brain (Forster et al. 1996). Liu et al. (2003) found that decline in learning and memory is associated with increased oxidative stress in the brain and chronic treatment with superoxide dismutase and catalase mimetics at low doses reversed the behavioral and biochemical changes in mice. The involvement of free radicals in the deterioration of motor activity and learning in aged mice was demonstrated and sub-chronic treatment with alpha-phenyl-tert-butyl nitrate, the free radical spin trapping agent was found to reverse the impairment (Fredriksson and Archer 1996). Impairment in learning in lambda-cyhalothrin-treated rats in this study could also be attributed to increased oxidative stress.
Further, impairment in grip strength following exposure to neurotoxicants, including pesticide has been reported (Shafer et al. 2005; Wolansky and Harrill 2008). In this study, the decrease in the grip strength was observed in rats exposed to lambda-cyhalothrin at the higher dose which persisted even after the withdrawal of exposure. The decrease in grip strength indicates muscle weakness and could be associated with cholinergic alterations.
The results indicate that inhibition of ChAT and AChE activity may cause down-regulation of brain muscarinic–cholinergic receptors consequently impairing learning activity in developing rats exposed to lambda-cyhalothrin. The data also indicates that brain cholinergic receptors are easy target of lambda-cyhalothrin. Enhanced oxidative stress in the brain appears to be an important reason for disruption of cholinergic functions following lambda-cyhalothrin exposure. The results further suggest that exposure to lambda-cyhalothrin at low doses during early period of life in rats may cause neurobehavioral modifications and such changes may persist in case of continued exposure to lambda-cyhalothrin.
References
Aebi H (1984) Catalase in vitro. In: Packer L (ed) Methods in enzymology, vol 105. Academic Press, New York, pp 121–126
Amoah P, Drechsel P, Abaidoo RC, Ntow WJ (2006) Pesticide and pathogen contamination of vegetables in Ghana’s urban markets. Arch Environ Contam Toxicol 50:1–6
Amweg EL, Weston DP, Ureda NM (2005) Use and toxicity of pyrethroid pesticides in the Central Valley, California, USA. Environ Toxicol Chem 24:966–972
Amweg EL, Weston DP, You J, Lydy MJ (2006) Pyrethroid insecticides and sediment toxicity in urban creeks from California and Tennessee. Environ Sci Technol 40:1700–1706
Andersen JK (2004) Oxidative stress in neurodegeneration: cause or consequence? Nat Med 10:S18–S25
Aziz MH, Agrawal AK, Adhami VM, Shukla Y, Seth PK (2001) Neurodevelopmental consequences of gestational exposure (GD14–GD20) to low dose deltamethrin in rats. Neurosci Lett 300:161–165
Benowitz LI, Routtenberg A (1997) GAP-43: an intrinsic determinant of neuronal development and plasticity. Trends Neurosci 20:84–91
Bissacot DZ, Vassilieff I (1997) Pyrethroid residues in milk and blood of dairy cows following single topical applications. Vet Hum Toxicol 39:6–8
Bradberry SM, Cage SA, Proudfoot AT, Vale JA (2005) Poisoning due to pyrethroids. Toxicol Rev 24:93–106
Breckenridge CB, Holden L, Sturgess N, Weiner M, Sheets L, Sargent D, Soderlund DM, Choi JS, Symington S, Clark JM, Burr S, Ray D (2009) Evidence for a separate mechanism of toxicity for the Type I and the Type II pyrethroid insecticides. Neurotoxicology 30S:S17–S31
Brown TP, Rumsby PC, Capelton AC, Rushton L, Levy LS (2006) Pesticides and Parkinson’s disease—is there a link? Environ Health Perspect 114:156–164
Celik A, Mazmanci B, Camlica Y, Askin A, Comelekoglu U (2003) Cytogenetic effects of lambda-cyhalothrin on Wistar rat bone marrow. Mutat Res 539:91–97
Celik A, Mazmanci B, Camlica Y, Comelekoglu U, Askin A (2005) Evaluation of cytogenetic effects of lambda-cyhalothrin on Wistar rat bone marrow by gavage administration. Ecotoxicol Environ Saf 61:128–133
El-Demerdash FM (2007) Lambda-cyhalothrin-induced changes in oxidative stress biomarkers in rabbit erythrocytes and alleviation effect of some antioxidants. Toxicol In Vitro 21:392–397
Ellman GL, Courtney KD, Anders V, Featherstone RM (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7:88–95
Farage-Elawar M (1989) Enzyme and behavioral changes in young chicks as a result of carbaryl treatment. J Toxicol Environ Health 26:119–131
Feo ML, Eljarrat E, Manaca MN, Dobaño C, Barcelo D, Sunyer J, Alonso PL, Menendez C, Grimalt JO (2012) Pyrethroid use-malaria control and individual applications by households for other pests and home garden use. Environ Int 38:67–72
Fetoui H, Garoui EM, Makni-ayadi F, Zeghal N (2008) Oxidative stress induced by lambda-cyhalothrin in rat erythrocytes and brain: attenuation by vitamin C. Environ Toxicol Pharmacol 26:225–231
Fetoui H, Garoui EM, Zegha IE (2009) Lambda-cyhalothrin-induced biochemical and histopathological changes in the liver of rats: ameliorative effect of ascorbic acid. Exp Toxicol Pathol 61:189–196
Flohe L, Gunzler WA (1984) Assays of glutathione peroxidase. Method Enzymol 105:114–121
Forster MJ, Dubey A, Dawson KM, Stutts WA, Lal H, Sohal RS (1996) Age-related losses of cognitive function and motor skills in mice are associated with oxidative protein damage in the brain. Proc Natl Acad Sci 93:4765–4769
Fredriksson A, Archer T (1996) Alpha-phenyl-tert-butyl-nitrone (PBN) reverses age-related maze learning performance and motor activity deficits in C57 BL/6 mice. Behav Pharmacol 7:245–253
Glowinski J, Iversen LL (1966) Regional studies of catecholamines in the rat brain. The disposition of 3H-norepinephrine, 3H-dopamine and 3H-dopa in various regions of the brain. J Neurochem 13:655–659
Goslin K, Schreyer DJ, Skene JHP, Banker G (1990) Changes in the distribution of GAP-43 during the development of neuronal polarity. J Neurosci 10:588–602
Gu BG, Wang HM, Chen WL, Cai DJ, Shan ZJ (2007) Risk assessment of lambda-cyhalothrin on aquatic organisms in paddy field in China. Regul Toxicol Pharmacol 48:69–74
Halliwell B (2006) Oxidative stress and neurodegeneration: where are we now? J Neurochem 97:1634–1658
Hasan M, Haider SS (1989) Acetyl homocysteine thiolactone protect against some neurotoxic effects of thallium. Neurotoxicology 10:257–262
Holahan MR, Honegger KS, Tabatadze N, Routtenberg A (2007) GAP-43 gene expression regulates information storage. Learn Mem 14:407–415
Hoskins B, Ho IK (1992) Tolerance to organophosphorus cholinesterase inhibition. In: Chambers JE, Levi PE (eds) Organophosphates: chemistry, fate, and effects. Academic Press, San Diego, pp 285–297
Hossain MM, Richardson JR (2011) Mechanism of pyrethroid pesticide-induced apoptosis: role of calpain and the ER stress pathway. Toxicol Sci 122:512–525
Hossain MM, Suzuki T, Sato I, Takewaki T, Suzuki K, Kobayashi H (2004) The modulatory effect of pyrethroids on acetylcholine release in the hippocampus of freely moving rats. Neurotoxicology 25:825–833
Hossain MM, Suzuki T, Sato I, Takewaki T, Suzuki K, Kobayashi H (2005) Neuromechanical effects of pyrethroids, allethrin, cyhalothrin and deltamethrin on the cholinergic processes in rat brain. Life Sci 77:795–807
Hossain MM, Suzuki T, Sato N, Sato I, Takewaki T, Suzuki K, Tachikawa E, Kobayashi H (2006) Differential effects of pyrethroid insecticides on extracellular dopamine in the striatum of freely moving rats. Toxicol Appl Pharmacol 217:25–34
Jamal M, Ameno K, Ameno S, Morishita J, Wang W, Kumihashi M, Ikuo U, Miki T, Ijiri I (2007) Changes in cholinergic function in the frontal cortex and hippocampus of rat exposed to ethanol and acetaldehyde. Neuroscience 144:232–238
Julvez J, Grandjean P (2009) Neurodevelopmental toxicity risks due to occupational exposure to industrial chemicals during pregnancy. Ind Health 47:459–468
Jurisic DA, Petrovic PA, Rajkovic VD, Nicin DS (2010) The application of lambda-cyhalothrin in tick controls. Exp Appl Acarol 52:101–109
Kakkar P, Das B, Viswanathan PN (1984) A modified spectrophotometric assay of superoxide dismutase. Indian J Biochem Biophys 21:130–132
Kale M, Rathore N, John S, Bhatnagar D (1999) Lipid peroxidative damage on pyrethroid exposure and alterations in antioxidant status in rat erythrocytes: a possible involvement of reactive oxygen species. Toxicol Lett 105:197–205
Khanna VK, Husain R, Seth PK (1994) Effect of protein malnutrition on the neurobehavioral toxicity of styrene in young rats. J Appl Toxicol 14:351–356
Kroeger A, Villegas E, Ordonez-Gonzalez J, Pavon E, Scorza JV (2003) Prevention of the transmission of chagas disease with pyrethroid-impregnated materials. Am J Trop Med Hyg 68:307–311
Kumar A, Rai DK, Sharma B, Pandey RS (2009) Lambda-cyhalothrin and cypermethrin induced in vivo alterations in the activity of acetylcholinesterase in a freshwater fish, Channa punctatus (Bloch). Pesticide Biochem Physiol 93:96–99
Landrigan PJ, Miodovnik A (2011) Children’s health and the environment: an overview. Mt Sinai J Med 78:1–10
Lawler SP, Dritz DA, Christiansen JA, Cornel AJ (2007) Effects of lambda-cyhalothrin on mosquito larvae and predatory aquatic insects. Pest Manag Sci 63:234–240
Levine RL, Garland D, Oliver CN, Amici A, Climent I, Lenz AG, Ahn B, Shaltiel S, Stadtman ER (1990) Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol 186:464–478
Liu R, Liu IY, Bi X, Thompson RF, Doctrow SR, Malfroy B, Baudry M (2003) Reversal of age-related learning deficits and brain oxidative stress in mice with superoxide dismutase/catalase mimetics. Proc Natl Acad Sci 100:8526–8531
Liu HX, Zhang JJ, Zheng P, Zhang Y (2005) Altered expression of MAP-2, GAP-43, and synaptophysin in the hippocampus of rats with chronic cerebral hypoperfusion correlates with cognitive impairment. Brain Res Mol Brain Res 139:169–177
Lowry OH, Rosenbrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the folin phenol reagent. J Biol Chem 192:265–275
Martinez-Larrañaga MR, Anadón A, Martínez MA, Martínez M, Castellano VJ, Díaz MJ (2003) 5-HT loss in rat brain by type II pyrethroid insecticides. Toxicol Ind Health 19:147–155
Mate MS, Ghosh RC, Mondal S, Karmakar DB (2010) Effect of lambda cyhalothrin on rats: an acute toxicity study. J Indian Soc Toxicol 6:25–28
Mathirajan VG, Natarajan K, Kuttalam S, Chandrasekaran S, Regupathy A (2000) Efficacy of lambda cyhalothrin (Karate 5 EC) against brinjal shoot and fruit borer (Leucinodes orbonalis Guen.). J Pestic Res 12:117–119
Michelangeli F, Robson MJ, East JM, Lee AG (1990) The conformation of pyrethroids bound to lipid bilayers. Biochem Biophys Acta 1028:49–57
Miodovnik A (2011) Environmental neurotoxicants and developing brain. Mt Sinai J Med 78:58–77
Miranda K, Cunha ML, Dores EF, Calheiros DF (2008) Pesticide residues in river sediments from the Pantanal Wetland, Brazil. J Environ Sci Health B 43:717–722
Mohapatra S, Ahuja AK (2010) Persistence and dissipation of lambda-cyhalothrin in/on mango (Mangifera indica). Indian J Agric Sci 80:306–308
Moreira EG, Vassilieff I, Vassilieff VS (2001) Developmental lead exposure: behavioural, alterations in the short and long-term. Neurotoxicol Teratol 23:489–495
Muhammad F, Akhtar M, Rahman ZU, Farooq HU, Khaliq T, Anwar MI (2010) Multi-residue determination of pesticides in the meat of cattle in Faisalabad-Pakistan. Egypt Acad J Biol Sci 2:19–28
Mulambalah CS, Siamba DN, Ngeiywa MM, Vulule JM (2010) Evaluation of lambda-cyhalothrin persistence on different indoor surfaces in a malaria epidemic-prone area in Kenya. J Biol Sci 5:258–263
Narahashi T (1996) Neuronal ion channels as the target sites of insecticides. Pharmacol Toxicol 78:1–14
Nasuti C, Gabbianelli R, Falcioni M, Stefano A, Sozio P, Cantalamessa F (2007) Dopaminergic system modulation, behavioral changes, and oxidative stress after neonatal administration of pyrethroids. Toxicology 229:194–205
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358
Oliveira C, Vassilieff VS, Vassilieff I (2000) Residues and placental transfer of lambda-cyhalothrin in goats. J Vet Res I 4:146–152
Pesticide Tolerances (1998) Lambda-cyhalothrin. Fed Regist 63:7291–7299
Ratnasooriya WD, Ratnayake SSK, Jayatunga YNA (2003) Effects of IconR, a pyrethroid insecticide on early pregnancy of rats. Hum Exp Toxicol 22:523–533
Ray DE, Fry JR (2006) Reassessment of the neurotoxicity of pyrethroid insecticides. Pharmacol Ther 111:174–193
Rekart JL, Quinn B, Mesulam MM, Routtenberg A (2004) Subfield-specific increase in brain growth protein in post-mortem hippocampus of Alzheimer’s patients. Neuroscience 126:579–584
Routtenberg A, Cantallops I, Zaffuto S, Serrano P, Namgung U (2000) Enhanced learning after genetic overexpression of a brain growth protein. Proc Natl Acad Sci USA 97:7657–7662
Sarin S, Gill KD (1998) Biochemical and behavioral deficits in adult rat following chronic dichlorvos exposure. Pharmacol Biochem Behav 59:1081–1086
Sayre LM, Smith MA, Perry G (2001) Chemistry and biochemistry of oxidative stress in neurodegenerative disease. Curr Med Chem 8:721–738
Sayre LM, Perry G, Smith MA (2008) Oxidative stress and neurotoxicity. Chem Res Toxicol 21:172–188
Schroeder SR (2000) Mental retardation and developmental disabilities influenced by environmental neurotoxic insults. Environ Health Perspect 3:395–399
Seenivasan S, Muraleedharan NN (2009) Residues of lambda-cyhalothrin in tea. Food Chem Toxicol 47:502–505
Shafer TJ, Meyer DA, Crofton KM (2005) Developmental neurotoxicity of pyrethroid insecticides: Critical review and future research needs. Environ Health Perspect 113:123–136
Shingo T, Date I, Yoshida H, Ohmoto T (2002) Neuroprotective and restorative effects of intrastriatal grafting of encapsulated GDNF-producing cells in a rat model of Parkinson’s disease. J Neurosci Res 69:946–954
Soderlund DM, Clark JM, Sheets LP, Mullin LS, Piccirillo VJ, Sargent D, Stevens JT, Weiner ML (2002) Mechanism of pyrethroid neurotoxicity: implication for cumulative risk assessment. Toxicology 171:3–59
Spurlock F, Lee M (2008) Synthetic pyrethroid use patterns, properties and environmental effects. American Chemical Society, Washington, DC
Stahl Wl, Smith JC, Napolitano LM, Basford RE (1963) Brain mitochondria. I. Isolation of bovine brain mitochondria. J Cell Biol 19:293–307
Tariq MI, Afzal S, Hussain I (2004) Pesticides in shallow groundwater of Bahawalnagar, Muzafargarh, D.G. Khan and Rajan Pur districts of Punjab, Pakistan. Environ Int 30:71–79
Tariq MI, Afzal S, Hussain I (2006) Degradation and persistence of cotton pesticides in sandy loam soils from Punjab, Pakistan. Environ Res 100:184–196
Terry AV, Stone JD, Buccafusco JJ, Sickles DW, Sood A, Prendergast MA (2003) Repeated exposure to subthreshhold doses of chlorpyrifos in rats: hippocampal damage, impaired axonal transport and deficits in spatial learning. J Pharmacol Exp Ther 305:375–384
Turgut C, Ornek H, Cutright TJ (2011) Determination of pesticide residues in Turkey’s table grapes: the effect of integrated pest management, organic farming, and conventional farming. Environ Monit Assess 173:315–323
Wang W, Cai DJ, Shan ZJ, Chen WL, Poletika N, Gao XW (2007) Comparison of the acute toxicity for gamma-cyhalothrin and lambda-cyhalothrin to zebra fish and shrimp. Regul Toxicol Pharmacol 47:184–188
Wehner JM, Upchurch M (1989) The effects of chronic oxotremorine treatment on spatial learning and tolerance development in mice. Pharmacol Biochem Behav 32:543–551
Wolansky MJ, Harrill JA (2008) Neurobehavioral toxicology of pyrethroid insecticides in adult animals: a critical review. Neurotoxicol Teratol 30:55–78
Wolansky M, Gennings C, Crofton K (2006) Relative potencies for acute effects of pyrethroids on motor function in rats. Toxicol Sci 89:271–277
World Health Organisation (1990) Cyhalothrin, Environmental Health Criteria, 99; Geneva, Switzerland
Acknowledgments
The authors thank the Director, CSIR-Indian Institute of Toxicology Research, Lucknow for his keen interest in the present study. The authors appreciate Dr. Pramod Kumar for extending unconditional technical assistance. The authors also thank Professor (Ms). Madhu Mehrotra, Head, Department of English and Modern European Languages, University of Lucknow, Lucknow and Mr. B. D. Bhattacharji, Senior Principal Scientist, CSIR-IITR, Lucknow for painstakingly going through the manuscript and making the necessary changes in the language. The financial support by Indian Council of Medical Research, New Delhi for carrying out the study is acknowledged. The CSIR-IITR Communication No. is 2960.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ansari, R.W., Shukla, R.K., Yadav, R.S. et al. Cholinergic Dysfunctions and Enhanced Oxidative Stress in the Neurobehavioral Toxicity of Lambda-Cyhalothrin in Developing Rats. Neurotox Res 22, 292–309 (2012). https://doi.org/10.1007/s12640-012-9313-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12640-012-9313-z