Abstract
Many studies have shown that deficits in olfactory and cognitive functions precede the classical motor symptoms seen in Parkinson’s disease (PD) and that olfactory testing may contribute to the early diagnosis of this disorder. Although the primary cause of PD is still unknown, epidemiological studies have revealed that its incidence is increased in consequence of exposure to certain environmental toxins. In this study, most of the impairments presented by C57BL/6 mice infused with a single intranasal (i.n.) administration of the proneurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) (1 mg/nostril) were similar to those observed during the early phase of PD, when a moderate loss of nigral dopamine neurons results in olfactory and memory deficits with no major motor impairments. Such infusion decreased the levels of the enzyme tyrosine hydroxylase in the olfactory bulb, striatum, and substantia nigra by means of apoptotic mechanisms, reducing dopamine concentration in different brain structures such as olfactory bulb, striatum, and prefrontal cortex, but not in the hippocampus. These findings reinforce the notion that the olfactory system represents a particularly sensitive route for the transport of neurotoxins into the central nervous system that may be related to the etiology of PD. These results also provide new insights in experimental models of PD, indicating that the i.n. administration of MPTP represents a valuable mouse model for the study of the early stages of PD and for testing new therapeutic strategies to restore sensorial and cognitive processes in PD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Parkinson’s disease (PD) is a neurodegenerative disorder that affects approximately 1% of the population older than 50 years (Duvoisin 1991). Classically, PD is considered to be a motor system disease, and its diagnosis is based upon the presence of a set of cardinal motor signs (e.g., rigidity, bradykinesia, tremor, and postural reflex disturbance). Unfortunately, the patients only fulfill these clinical criteria when 60–70% of the neurons of the substantia nigra (SN) are degenerated and the striatal dopamine (DA) content is reduced by 80% (Riederer and Wuketich 1976; Braak et al. 2004). Recent evidence suggests that areas in the central nervous system (CNS) processing olfactory information are affected at the early stages of PD, even before the development of its classical symptoms (Doty et al. 1988a; Muller et al. 2002; Braak et al. 2004). Consequently, olfactory dysfunction might be an early indicator of PD and the development of specific olfactory testing may represent an important tool in the clinical diagnosis of early stages of this disease (Doty et al. 1995).
Beyond the olfactory symptoms, in the early stages of PD, subtle cognitive impairments can be observed, consisting mainly of executive dysfunction with secondary visuospatial and mnemonic disturbances (Dubois and Pillon 1997; Bosboom et al. 2004). In about 20–40% of patients, these problems may eventually proceed to dementia, which constitutes an important risk factor for caregiver distress, decreased quality of life, and nursing home placement. Even non-demented PD patients have been reported to present visuospatial working memory deficits (Dubois and Pillon 1997; Stebbins et al. 1999; Lewis et al. 2003). Furthermore, although there are reports of declarative (or episodic) memory impairments in PD (Bondi and Kaszniak 1991), they are less severe in comparison to other neurodegenerative disorders such as Alzheimer’s disease (Bondi and Kaszniak 1991; Bosboom et al. 2004).
On the other hand, experimental models of PD have attempted to reproduce the pathogenic process and to involve areas of the brain pathologically affected in humans. Pathogenic modeling has been attempted using a range of toxins, as well as through the use of transgenic models of gene defects in familial PD and mutant rodent strains. However, there are still no accepted progressive models of PD that mimic the processes known to occur during cell death and that result in the motor deficits, pathology, biochemistry, and drug responsiveness as seen in humans (for recent review see Jenner 2008). Despite these limitations, over the past couple of decades, the proneurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) has become a widely used approach for modeling PD. In humans and non-human primates, MPTP causes a severe and irreversible PD-like syndrome (Langston et al. 1983). Although rodents are less sensitive to MPTP toxicity, largely because of the economic, logistic, and ethical constraints related to experimental research in primates, the MPTP mouse model has become the most commonly used animal model of PD (Schmidt and Ferger 2001).
A relatively unexplored route of penetration of neurotoxins is the nasal cavity. The nasal mucosa exhibit a large surface area, porous endothelial membrane, olfactory receptors are directly exposed to environment chemicals, distribution of olfactory receptors are contiguous with the CNS, high total blood flow, avoidance of first-pass metabolism, and a weak blood–brain barrier. Surprisingly, few studies (Dluzen and Kefalas 1996; Prediger et al. 2006; Rojo et al. 2006) have addressing the possibility that neurotoxins such as MPTP could damage the basal ganglia following intranasal (i.n.) absorption, despite the fact that olfactory function is disordered in PD (Doty et al. 1988a, 1995). In this context, Rojo et al. (2006) recently reported that C57BL/6 mice receiving daily i.n. inoculations with MPTP (60 mg/kg of body weight) for 30 days developed motor deficits that correlated with severe depletion of striatal DA levels and a loss of tyrosine hydroxylase (TH) and DA transporter staining in the SN and striatum. Moreover, despite the conspicuous insensitivity of rats to MPTP toxicity (Chiueh et al. 1984; Kalaria et al. 1987), we have recently demonstrated that a single i.n. infusion of MPTP in rats produces progressive signs of PD such as impairments in olfactory, cognitive, and motor functions (Prediger et al. 2006). Additionally, the i.n. administration of MPTP causes time-dependent loss of TH in the olfactory bulb and SN of rats, resulting in significant DA depletion in the olfactory bulb, prefrontal cortex, and striatum (Prediger et al. 2006) which are associated with alterations in the brain antioxidant status and lipid peroxidation (Franco et al. 2007), and apoptotic cell death mechanisms (Prediger et al. 2009).
To date, most studies performed with animal models of PD have focused on their ability to induce nigrostriatal dopaminergic pathway damage and motor alterations associated with advanced phases of PD. Because PD is accompanied by alterations in a variety of functions, including anxiety disorders (Schrag 2004), memory deficits (Dubois and Pillon 1997; Bosboom et al. 2004), and olfactory dysfunction (Doty et al. 1988a, 1995) evaluating whether the proposed animal models of PD alter any of these functions seems important. We therefore investigated the occurrence of olfactory, anxiety-related, cognitive, and motor alterations in C57BL/6 mice over a 20-day period after one bilateral i.n. administration of MPTP (1 mg/nostril). The potential of i.n. administration of MPTP to induce dopaminergic cells loss in mice was assessed by TH immunohistochemistry and Nissl staining in the olfactory bulb and SN. The functionality of dopaminergic terminals was evaluated by measurement of DA levels in different brain structures. Finally, caspase-3 immunoreactivity was analyzed in the SN to investigate the programmed cell death (apoptosis) as a pathogenic mechanism possibly involved in the neurodegeneration induced by i.n. administration of MPTP (Prediger et al. 2009).
Materials and Methods
Animals
Five to 6-month-old male C57BL/6 mice (Cente d’Elevage René Janvier, Le Genest St Ile, France), weighing 30–35 g at the beginning of the experiment, were chosen since they are more susceptible to the neurotoxic effects of MPTP (Sedelis et al. 2000). They were housed individually in cages, with free access to food and water, under controlled temperature (22 ± 2°C) and humidity (60–80°C) and a 12-h light/dark cycle (lights on 6:00 A.M.). All animal procedures were carried out in compliance with the European Directive No.: 86/609/EEC and the guidelines of the local Institutional Animal Care and Use Committee. All efforts were made to minimize the number of animals used and their suffering.
Intranasal Administration of MPTP
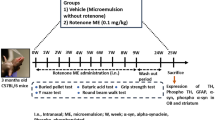
MPTP HCl (Sigma Chemical Co., USA) was administered by i.n. route according to the procedure described by Dluzen and Kefalas (1996) and recently modified in our laboratory (Prediger et al. 2006, 2009). Briefly, mice were lightly anaesthetized with isoflurane 0.96% (0.75 CAM; Abbot Laboratórios do Brasil Ltda., RJ, Brazil) using a vaporizer system (SurgiVet Inc., WI, USA) and a 7-mm piece of PE-10 tubing was inserted through the nostrils. As illustrated in Fig. 1a, the tubing was connected to a peristaltic pump set at a flow rate of 12.5 μl/min. The MPTP HCl was dissolved in 0.9% NaCl (saline) at a concentration of 20 mg/ml, after which it was infused for 4 min (1 mg/nostril). The control solution consisted of saline. Animals were given a 1-min interval to regain normal respiratory function and then this procedure was repeated with infusions administered through the contralateral nostrils.
Experimental Design
As summarized in Fig. 1b, MPTP was administered intranasally 5–18 days before the performance of behavioral experiments (olfactory discrimination, elevated plus-maze, social recognition, water maze, step-down, and activity chambers). Animals were killed 20 days after i.n. infusion of MPTP or vehicle for the measurement of TH immunohistochemistry in the olfactory bulb, SN, and striatum, and determination of Nissl staining and caspase-3 immunoreactivity in the SN or dosage of DA, 3,4-dihydroxyphenylacetic acid (DOPAC), homovallinic acid (HVA), noradrenaline (NA), 5-hydroxytryptamine (5-HT) in the olfactory bulb, striatum, prefrontal cortex, and hippocampus.
Behavioral Tests
Olfactory Discrimination
The olfactory discrimination ability of mice was assessed 5 days after i.n. MPTP administration with an olfactory discrimination test that had been previously evaluated in our laboratory (Prediger et al. 2005b, 2006). The task is based on the fact that rodents usually disclose preference for places impregnated with their own odor (familiar compartments), in spite of places with other non-familiar odors. Briefly, each mouse was placing for 5 min in a cage divided in two equal areas separated by an open door, where it could choose between one compartment with fresh sawdust (non-familiar compartment) and another with unchanged sawdust (familiar compartment) that the same mouse had occupied for 3 days before the test. Performance was monitored with a ViewPoint video-tracking system (Life Sciences, Montreal, Canada) and the following parameters were registered: time (s) and distance (cm) traveled in each compartment (familiar versus non-familiar) and total distance (cm) traveled.
Elevated Plus-Maze
Since up to 40% of patients with PD suffer from clinically significant anxiety (Schrag 2004) and anxiogenic-like responses have been reported in animal models of PD (Tadaiesky et al. 2008), we evaluated whether these emotional alterations may also occur in the present i.n. MPTP model. The elevated plus-maze was used on the basis of its documented ability to detect both anxiolytic- and anxiogenic-like drug effects in mice (Lister 1987). It was performed 7 days after i.n. MPTP administration. Briefly, the apparatus was made of wood covered with impermeable formica, and was placed 60 cm above the floor. The four arms were 18 cm long and 6 cm wide. Two opposite arms were surrounded by walls (6 cm high, closed arms), while the other two were devoid of enclosing walls (open arms). The four arms were connected by a central platform (6 × 6 cm2). The experiments were conducted in a sound-attenuated room under low-intensity light (12 lx). Each subject was placed in the centre of the maze facing a closed arm. The animals were monitored for a 5-min test period with a ViewPoint video-tracking system (Life Sciences, Montreal, Canada) and anxiogenic-like effects were defined as a decrease in the proportion of open-arm entries divided by the total number of arm entries, and the time spent on open arms relative to the total time spent on both arms. Whenever a mouse placed all four paws onto an arm, one entry was recorded. The total number of closed-arm entries was utilized as a measure of locomotor activity.
Social Recognition
Short-term social memory was assessed 12 days after i.n. MPTP administration with the social recognition task described by Dantzer et al. (1987) and previously evaluated in our laboratory (Prediger et al. 2005a, b; Xikota et al. 2008). The test was scored by the same rater in an observation room, where the mice had been habituated for at least 1 h before the beginning of the test. Juvenile C57BL/6 mice (Centre d’Elevage René Janvier, Le Genest St Ile, France) were kept in groups of 10 per cage and served as social stimuli for the adult mice. All juveniles were isolated in individual cages for 20 min prior to the beginning of the experiment. The social recognition task consisted of two successive presentations (5 min each), separated by a short period of time, where the juvenile mouse was placed in the home cage of the adult mouse and the time spent by the adult in investigating the juvenile (nosing, sniffing, grooming, or pawing) was recorded. At the end of the first presentation, the juvenile was removed and kept in an individual cage during the delay period and re-exposed to the same adult mouse after 30 min. In this paradigm, if the delay period is less than 40 min, the adult rodents display recognition of this juvenile, as indicated by a significant reduction in the social investigation time during the second presentation (Dantzer et al. 1987; Prediger et al. 2005a, b; Xikota et al. 2008). However, when the same juvenile is re-exposed after a longer time (more than 60 min) after the first presentation, the adult mouse no longer recognizes this juvenile, i.e., the social investigation time in the second presentation is similar to that observed during the first one. Thus, a 30-min interval between two presentations of the same conspecific juvenile was used to demonstrate possible MPTP-related deficits in the social recognition memory.
Water Maze
Mice were submitted 12–15 days after i.n. MPTP administration to a working memory version of the water maze task. Tests were performed in a circular swimming pool made of black painted fiberglass, 97 cm in diameter and 60 cm in height. For the tests, the tank was filled with water maintained at 23 ± 2°C. The target platform (10 × 10 cm2) was made of transparent Plexiglas and it was submerged 1–1.5 cm beneath the surface of the water. Starting points for animals were marked on the outside of the pool as north (N), south (S), east (E), and west (W). Four distant visual cues (55 × 55 cm2) were placed on the walls of the water maze room. They were all positioned with the lower edge 30 cm above the upper edge of the water tank and in the standard setting, the position of each symbol marked the midpoint of the perimeter of a quadrant (circle = NE quadrant, square = SE quadrant, cross = SW quadrant, and diamond = NW quadrant). The apparatus was located in a room with indirect incandescent illumination. A monitor and a video-recording system were installed in an adjacent room. The experiments were video-taped and the scores for latency of escape from the starting point to the platform and swimming speed were later measured through an image analyzer (CEFET, Curitiba, PR, Brazil).
Mice were submitted to a working memory version of the water maze using a protocol that was described previously (Prediger et al. 2006). This consisted of 4 training days, four consecutive trials per day, during which the animals were left in the tank facing the wall, then being allowed to swim freely to the submerged platform placed in the centre of one of the four imaginary quadrants of the tank. The initial position in which the animal was left in the tank was one of the four vertices of the imaginary quadrants of the tank, and this was varied among trials in a pseudo-random way. If a mouse did not find the platform during a period of 60 s, it was gently guided to it. The animal was allowed to remain on the platform for 30 s and then moved to the next initial position without leaving the tank it was removed immediately after completing the four subsequent daily trials. This procedure was used to ensure that the animals maintained the visuospatial information of the maze online during execution of the working memory task. On each subsequent training day the platform position was moved to the centre of another quadrant of the tank in a pseudo-random way.
Step-Down Inhibitory Avoidance
The step-down inhibitory avoidance apparatus is a 50 × 25 × 25 cm3 acrylic box, whose floor consisted of a grid of parallel stainless steel bars of 1 mm diameter spaced 1 cm apart. A 10 cm × 10 cm wide, 2 cm high, acrylic platform was placed in the center of the floor. The animals were placed on the platform and their latency to step-down on the grid with all four paws was measured with an automatic device. The animals were submitted to the inhibitory avoidance task 14–15 days after i.n. MPTP administration using a protocol similar to one described previously (Xikota et al. 2008). During the training session, immediately after stepping down on the grid, the animals received a 2.0-s, 0.3-mA scrambled foot shock. In order to evaluate short- and long-term memory, test sessions were performed 1.5 and 24 h after training, respectively. Retention test sessions were procedurally identical except that no foot shock was given and the step-down latency (maximum 180 s) was used as measure of retention.
Spontaneous Locomotor Activity
In order to assess possible effects of MPTP on locomotor activity, the animals were tested 18 days after i.n. MPTP administration in activity chambers. The activity chambers (20 × 20 × 20 cm3), with a steel grid floor, equipped with three parallel horizontal infrared beams positioned 3 cm above the floor and spaced evenly along the longitudinal axis, had a digital counter which recorded photocell beam interruptions. The items of data obtained were expressed as signals corresponding to spontaneous movements for 15 min.
Neurochemistry
Measurements of Monoamine Levels by High Performance Liquid Chromatography
For determining DA, DOPAC, HVA, NA, and 5-HT contents in brain, some mice were killed by decapitation 20 days after i.n. administration of MPTP or vehicle. Brains were removed immediately and the structures olfactory bulb, striatum, prefrontal cortex, and hippocampus were dissected, weighed, and sonicated for 5 s in 10 volumes (v/wt) of 0.1 N perchloric acid/0.05% disodium EDTA/0.05% sodium metabisulfite. DA, DOPAC, HVA, NA, and 5-HT were extracted, and 10 μl samples were injected onto a Beckman Ultrasphere 5 μm IP column (Beckman) (Hamon et al. 1988). Eluted DA, DOPAC, HVA, NA, and 5-HT were quantified electrochemically (at 0.65 V) and concentrations were calculated in nanograms per gram of tissue.
Immunohistochemistry
Two groups of animals were infused with MPTP, as previously described, and they were killed at 20 days after i.n. administration of MPTP or control. In the first group of animals (n = 6 animals per group) the anterior part of the brain was dissected for high performance liquid chromatography (HPLC) analysis and the posterior part of the brain, including the ventral mesencephalon, was fixed by immersion in 4% formaldehyde in 0.1 M phosphate buffer (pH 7.4) for 1 day and then placed in 0.1 M PBS containing 30% sucrose for 24 h before being frozen at −30°C in isopentane until further processing. This material was used to make a stereological analysis of TH-positive neurons and Nissl staining with thionin solution in the SN. The evaluation of TH-positive neurons in the SNpc was performed as previously reported (Douhou et al. 2002). Briefly, serial coronal 20-μm-thick sections were cut on a cryostat (Leica SM2000 R) and identified according to the mouse brain atlas of Franklin and Paxinos (1997). Sections between coordinates 1.26 and −0.08 mm with respect to the interaural axis contained the SN ventral tegmental area complex. One of every 10th section was used for immunostaining. Free-floating sections were incubated overnight at 4°C with anti-TH monoclonal antibody (1:300, catalog MAB318, Millipore/Chemicon International, Technology, Billerica, MA, USA) following 30-min preincubation in 0.1 M PBS containing 5% normal goat serum to block the non-specific binding and 0.15% Triton X-100. After incubation with primary antibodies, the sections were washed with PBS and incubated with the biotinylated secondary antibody (anti-rabbit IgG 1:250 for 2 h), then processed using an avidin–biotin–peroxidase complex (1:100, Vectastain, Elite ABC kit, Vector Laboratories, Burlingame, CA). Finally, the antigen–antibody complexes were visualized by reaction with 3,3′-diaminobenzidine (DAB) and H2O2 (0.5 mg/ml and 0.025%, respectively) in 25 mM TBS.
A second group of animals (n = 6 animals per group) were intracardially perfused with 4% of the fixative solution formaldehyde in 0.1 M phosphate buffer (pH 7.4). Brains were removed and postfixed 2 h in the same solution, and then placed in 0.1 M PBS containing 30% sucrose for 24 h, included in paraffin before being frozen at −30°C in isopentane until further processing. Immunohistochemistry detection of TH apoptotic cell death, immunoreactivity of TH in olfactory bulb and striatum were assessed on paraffin tissue sections (4 μm), using the polyclonal rabbit anti-caspase-3 (1:200; Cell Signaling Technology, Beverly, MA), and the same mouse anti-TH monoclonal antibody (1:300), respectively, as previously described (Tadaiesky et al. 2008). Following quenching of endogenous peroxidase with 3.0% hydrogen peroxide in methanol for 20 min, high temperature antigen retrieval was performed by immersion of the slides in a water bath at 95–98°C in 10 mM trisodium citrate buffer pH 6.0, for 45 min. After overnight incubation at 4°C with primary antibodies, the slides were washed with PBS and incubated with the appropriate biotinylated secondary antibody, and then processed using the Vectastain Elite ABC reagent (Vector Laboratories, Burlingame, CA, USA) according to the manufacturer’s instructions. The sections were washed in PBS, and the visualization was completed by using DAB (Dako Cytomation) in chromogen solution and counterstained with Harris’s hematoxylin. Tissues from control and MPTP treatments were placed on the same slide and processed under the same conditions. Caspase-3-cleaved stained cells in SNpc were determined on visual inspection with optical microscope (Eclipse 50I; Nikon, Melville, NY) at 400× magnification.
Analysis of TH Immunoreactivity
For quantification of TH-positive cells in SNpc, digital images were acquired by digital camera coupled to a Leitz microscope displaying the structure on a video monitor. Brain structures were identified according to the atlas of Franklin and Paxinos (1997). Settings for image acquisition were identical for control and MPTP tissues. We obtained six images SNpc for each animal. TH immunoreactivity was assessed using a computer-based image analyzer (Visioscan 2000, Biocom, les Ulis, France). For spatial mapping and counting of individually stained neurons, the sections were placed in the microscope field so that the midline of the brain was always oriented along the same line. Cells were counted on one side of the brain. The contour of the SNpc was first delineated manually at low magnification. Then, the stained neurons within the area were counted at higher magnification, marked on the digitized image of each section, and filed in the computer for archiving purposes. The number of TH-immunoreactive neurons was estimated by counting positive cells in six sections encompassing rostral, medial, and caudal levels of the SNpc. Values obtained were then plotted with respect to the cumulative distance between the sections and the surface area under this curve gave an estimate of the total number of neurons in the SNpc from its rostral to caudal extent, as previously described (Hirsch et al. 1992).
The number of TH-stained positive cells in the olfactory bulb was assessed at four levels. Four alternate 4 μm paraffin sections with an individual distance of ~100 μm of each section were obtained, and the number of TH-stained positive cells was examined microscopically at 400× magnification. The number of stained positive cells per field within the glomerular layer (GL) of each of the four sections was counted. The mean number of stained positive cells per field from four sections per animal was calculated for each treatment group. Quantitative optical density measurements of striatum nerve fibers TH positive were carried out using a computer-based image analysis system, NIH ImageJ 1.36b imaging software (NIH, Bethesda, MD, USA). We obtained three images of rostral striatal level, corresponding dorsolateral, dorsomedial, and ventrolateral regions, for each animal. Images of stained striatum sections were acquired using a Sight DS-5M-L1 digital camera (Nikon, Melville, NY, USA) connected to an Eclipse 50i light microscope (Nikon) at 400× magnification. Settings for image acquisition were identical for control and MPTP tissues. A threshold optical density that best discriminated staining from the background was obtained using the NIH ImageJ. Optical density values obtained in the images of the regions were averaged for each animal, and the results were expressed as optical density (O.D.) of TH immunostaining.
Statistical Analysis
Data for inhibitory avoidance task are shown as median (interquartile range) of step-down latencies. Comparisons of both training and test session step-down latencies between groups were performed with Wilcoxon non-parametric test and comparisons among groups by Kruskal–Wallis test using the Graph Pad Prism 4® GraphPad Software Inc. The rest of values are expressed as means ± S.E.M. (n equals the number of mice included in each analysis). Differences between groups in monoamine levels and TH staining were analyzed using unpaired Student’s t-test. Statistical analysis for the rest of the data was carried out using one- or two-way analysis of variance (ANOVA) with treatment and the number of trials (repeated measure) as the independent variables. Following significant ANOVAs, multiple post-hoc comparisons were performed using the Newman–Keuls test. The accepted level of significance for the tests was P ≤ 0.05. All tests were performed using the Statistica® software package (StatSoft Inc., Tulsa, OK, USA).
Results
Effects of i.n. Administration of MPTP on the Olfactory Discrimination and Anxiety-Related Responses in Mice
The results for the effects of i.n. administration of MPTP (1 mg/nostril) on the olfactory discrimination ability of mice are illustrated in Fig. 2. One-way ANOVA revealed a significant effect for the treatment factor in the time [F(1,15) = 10.14; P ≤ 0.01] and distance traveled [F(1,15) = 3.38; P ≤ 0.05] in the familiar compartment. However, it indicated a non-significant effect for the treatment in the total distance traveled [F(1,15) = 0.23; P = 0.64].
The effects of intranasal administration of MPTP (1 mg/nostril) on odor discrimination ability of mice (n = 8–9 animals in each group). The animals were placed for 5 min in a cage, divided into two identical compartments, where it could choose between one compartment with fresh sawdust (non-familiar; black) and another with unchanged sawdust that the same mouse had occupied for 72 h before the test (familiar; white). Data are expressed as the mean ± S.E.M. of the a time (s) and b distance (cm) traveled in each compartment. *P ≤ 0.05 compared to the control-treated group (Newman–Keuls test)
Subsequent Newman–Keuls test indicated that mice infused with vehicle solution (i.n.) were able to discriminate between the familiar and the non-familiar compartments, spending much more time (Fig. 2a) and traveling high distances (Fig. 2b) in the familiar compartment. However, MPTP-infused mice presented an early disruption in olfactory discrimination ability verified at 5 days after treatment, spending similar time and traveling similar distance in the familiar and non-familiar compartments. These effects on the olfactory discrimination test are not related with motor impairments in MPTP-treated mice, since no alterations in total distance were observed (Fig. 2b).
In addition, the results of i.n. administration of MPTP in the behavioral parameters available in the elevated plus-maze are given in Table 1. One-way ANOVA revealed a non-significant effect for the treatment in the percentage of time spent on open arms [F(1,15) = 0.79; P = 0.39], in the open-arm entries [F(1,15) = 0.02; P = 0.88] and in the number of closed-arm entries [F(1,15) = 0.32; P = 0.58].
Effects of i.n. Administration of MPTP on Cognitive Performance in Mice
Social Recognition Test
Figure 3a summarizes the effect of i.n. infusion of MPTP (1 mg/nostril) on the short-term social recognition memory of mice. Twelve days after treatment, two-way ANOVA (treatment vs. repeated measures) followed by Newman–Keuls test indicated that control-treated mice spent less time investigating the juvenile mouse than in the first exposure. On the other hand, MPTP-treated mice spent as much time investigating the juvenile mouse during the second encounter as they did in the first exposure [F(1,15) = 121.56; P ≤ 0.0001], reflecting a clear impairment of the juvenile’s recognition ability.
The effects of intranasal administration of MPTP (1 mg/nostril) on the working memory of mice evaluated in the a social recognition and b water maze tasks (n = 8–9 animals in each group). Data are expressed as the mean ± S.E.M. of the: a investigation time (s) in the first (white) and second presentation (black) when the same juvenile was exposed for 5 min with an interval of 30 min; b latency (s) for escape to a submerged platform in a working memory version of the water maze. *P ≤ 0.05 compared to the respective trial of control-treated group (Newman–Keuls test)
Water Maze Test
Twelve to 15 days after i.n. administration of MPTP (1 mg/nostril), mice were tested in the working version of the water maze. At this time, animals did not present gross motor alterations that would otherwise confound the interpretation of cognitive impairment in memory tasks. The data presented in Fig. 3b show that all mice were able to learn the task, since their mean escape latency improved throughout the training trials. However, MPTP-infused animals spent more time to find the platform. Two-way ANOVA (treatment vs. repeated measures) revealed a significant effect for the main factors [treatment: F(1,54) = 8.04; P ≤ 0.01]; [repeated measures: F(3,162) = 12.53; P ≤ 0.0001], and for the interaction between these variables: [F(3,162) = 2.73; P ≤ 0.05]. Subsequent post-hoc comparisons indicated significant differences (P ≤ 0.05) between the MPTP and control groups for the 3nd and 4th training trials of the task (Fig. 3b).
Inhibitory Avoidance Test
The effects of the i.n. administration of MPTP (1 mg/nostril) on the short- and long-term memory of mice evaluated in the step-down inhibitory avoidance task are given in Fig. 4. The Kruskal–Wallis non-parametric test did not reveal any significant effects of the treatment on the step-down latencies during either the training session or long-term retention test session. However, it indicated a significant effect of the treatment (P ≤ 0.05) on the step-down latencies during the short-term retention test session. The Wilcoxon test indicated that the i.n. administration of MPTP significantly decreased the step-down latencies during the short-term retention test session (performed 1.5 h after the training session). These results suggest that the i.n. MPTP infusion treatment specifically disrupts the short-term retention of a step-down inhibitory avoidance task in mice (Fig. 4).
The effects of intranasal administration of MPTP (1 mg/nostril) on short-term (1.5 h) and long-term (24 h) retention of the step-down inhibitory avoidance memory in mice. Data are shown as median (interquartile ranges) of latencies to step-down in the training (white) and test (1.5 h: gray; 24 h: hatched) sessions (n = 7 animals in each group). *P ≤ 0.05 compared to the training session of the respective group (Wilcoxon test)
Effects of i.n. Administration of MPTP on Locomotor Activity in Mice
Figure 5 summarizes the effects of i.n. administration of MPTP (1 mg/nostril) on locomotor activity of mice evaluated in activity chambers. The exploratory behavior evaluated by number of crossings [F(1,15) = 0.16; P = 0.70] and rearing [F(1,15) = 0.02; P = 0.90] was not affected by MPTP administration 18 days after the treatment.
Analysis of Neurochemical Alterations Induce by i.n. Administration of MPTP in Mice
Monoamine Contents
With the purpose of determining the relationship between the behavioral alterations induced by i.n. administration of MPTP in mice and neurochemical alterations in dopaminergic, noradrenergic, and serotonergic neurotransmission, the levels of DA, DOPAC, HVA, NA, and 5-HT in the olfactory bulb, striatum, prefrontal cortex, and hippocampus were measured 20 days after MPTP treatment.
As illustrated in Fig. 6, unpaired Student’s t-test revealed that the i.n. infusion of MPTP (1 mg/nostril) promoted a significant DA depletion in the olfactory bulb (t = 3.10, P ≤ 0.01), striatum (t = 7.08, P ≤ 0.0001), and prefrontal cortex (t = 2.50, P ≤ 0.05) of mice. The levels of DA metabolites DOPAC and HVA were also significantly decreased in these brain structures (P < 0.05) (data not shown). On the other hand, the levels of DA and its metabolites were not altered by MPTP treatment in the hippocampus (t = 1.12, P = 0.29) (Fig. 6d).
The effects of intranasal administration of MPTP (1 mg/nostril) on dopamine (DA), noradrenaline (NA), and 5-hydroxytryptamine (5-HT) levels in a olfactory bulb, b striatum, c prefrontal cortex, and d hippocampus of mice. The values represent the mean ± S.E.M. monoamine levels (ng/g wet tissue) of 6–7 animals in each group. *P ≤ 0.05 compared to the control group (unpaired Student’s t-test)
Additionally, NA concentration was decreased in the olfactory bulb (t = 2.31, P ≤ 0.05), striatum (t = 2.50, P ≤ 0.05), and hippocampus (t = 3.53, P ≤ 0.01), whereas no significant alterations of NA were seen in prefrontal cortex (t = 0.46, P = 0.65). Moreover, no significant differences in the 5-HT levels were observed in any of the brain structures investigated (Fig. 6).
Dopaminergic Damage
Figure 7 shows representative photomicrographs of TH immunohistochemistry in the olfactory bulb (Fig. 7a), ventral mesencephalom (Fig. 7c), and striatum (Fig. 7e) at 20 days after i.n. administration of MPTP or control. As can be seen, immunohistochemistry revealed a pronounced loss of TH-positive neurons in the olfactory bulb (55% loss) (Fig. 7b) and SNpc (65% loss) (Fig. 7d) of MPTP-treated mice. Moreover, optical density measurements demonstrated that the i.n. administration of MPTP induced a significant reduction of TH immunostaining in the striatum (55% lower) (Fig. 7f).
Representative figures of tyrosine hydroxylase (TH) immunostaining detection in the olfactory bulb, ventral mesencephalon and striatum at 20 days after intransal (i.n.) administration of MPTP or control a, c, and e, respectively. TH immunostaining showed that i.n. administration of MPTP induced a marked decrease in TH levels when compared to control group animals. Micrographs of a TH immunohistochemistry of olfactory bulb, EPL external plexiform layer, GL glomerular layer; c SN, SNpc compacta part of substantia nigra, SNr reticular part of substantia nigra, VTA ventral tegmental area; and e striatum, DM: dorsomedial striatum, DL dorsolateral striatum, VL ventrolateral striatum. The values represent the mean ± S.E.M. b TH-positive neurons per field (400× magnification) in olfactory bulb; d the total number of TH-positive neurons in the SNpc; and f the average of striatal TH optical density of 6 animals in each group. *P ≤ 0.05, **P ≤ 0.01, and ***P ≤ 0.001 compared to control group (unpaired Student’s t-test)
Additionally, histological analysis using Nissl staining revealed a cellular loss in the SN of MPTP-treated mice (Fig. 8a), indicating that the observed reduction of TH-positive cells is associated to dopamine neurons degeneration, thus discarding a possible TH downregulation in absence of neuronal death. Finally, immunohistochemistry revealed a marked increase in the number of caspase-3-cleaved positive cells in the SNpc of mice treated intranasally with MPTP (Fig. 8b, c), suggesting that apoptotic cell death plays a key role in the dopaminergic neurodegeneration observed in the present i.n. MPTP model.
Representative figures of Nissl staining with thionin and caspase-3-cleaved immunostaining detection in the substantia nigra pars compacta (SNpc) at 20 days after intransal (i.n.) administration of MPTP or control. a Micrographs of Nissl staining revealed the cellular loss in the SNpc of MPTP-infused mice, b caspase-3-cleaved immunostaining showed that i.n. administration of MPTP induced activation of caspase-3 signaling cascade of neuronal apoptosis, and c higher magnification of the caspase-3-cleaved positive neurons in the SNpc of MPTP-infused mice
Discussion
The present findings demonstrate that most of the impairments presented by mice treated with a single i.n. infusion of MPTP (1 mg/nostril) appear analogous to those observed during the early phase of PD, where sensory and memory deficits appears with no major motor alterations. Such infusion decreased the levels of the enzyme TH in the olfactory bulb, SN, and striatum by means of apoptotic mechanisms, resulting in a significant depletion of DA and its metabolites in different brain structures such as the olfactory bulb, striatum, and prefrontal, but not hippocampus. Moreover, a significant depletion of NA was also observed in the olfactory bulb and hippocampus.
Although the cause of PD is presently unknown, epidemiological and experimental studies suggest that exposure to several environmental agents, including agricultural chemicals, may contribute to its pathogenesis (Gorell et al. 1998). Sometimes such agents may enter the brain via the olfactory neuroepithelium, a concept termed the olfactory vector hypothesis (for review see Doty 2008). In accordance with this hypothesis, some studies have shown that i.n. infusion of dopaminergic neurotoxins such as 6-hydroxydopamine (6-OHDA) (Kawano and Margolis 1982) and MPTP (Dluzen and Kefalas 1996; Prediger et al. 2006, 2009; Rojo et al. 2006) can result in the invasion of these agents into the brains of rodents, sometimes severely damaging central brain structures. Interestingly, a mouse model of PD based on chronic i.n. infusion of MPTP was recently proposed by Rojo et al. (2006). After 30 days of repeated i.n. MPTP infusion (60 mg/kg/day) mice developed a significant reduction in ambulatory behavior that correlated with a drop of striatal DA to 20% and a drastic reduction in the TH immunoreactivity of striatum and SN (Rojo et al. 2006). Although the chronic i.n. inoculation of MPTP has undoubtedly contributed to a better understanding of many features of PD, including to asses the risk from environmental neurotoxins, it was focused on the ability of this model to induce nigrostriatal pathway damage and motor alterations associated with advanced phases of PD. As stated in the introduction, early preclinical stages of PD are accompanied by alterations in a variety of functions (including emotional, olfactory, and cognitive) and the management of the non-motor symptoms of PD remains a challenge. However, few studies have addressed consistently these preclinical symptoms in animal models of PD.
In this context, we have recently proposed a new experimental model of PD consisting of a single i.n. administration of MPTP in rats (Prediger et al. 2006, 2009). It has been suggested that the i.n. route bypasses the blood–brain barrier and avoids peripheral and brain capillaries metabolic effects capable of decreasing MPTP toxicity following systemic administration in rats (Kawano and Margolis 1982; Chiueh et al. 1984; Kalaria et al. 1987). Consistent with this suggestion, rats treated intranasally with MPTP suffered progressive impairments in olfactory, cognitive, and motor functions associated with time-dependent disruption of dopaminergic neurotransmission in different brain structures which appears to be correlated with different stages of the human PD (Prediger et al. 2006, 2009). Therefore, the present study further validates the proposed single i.n. administration of MPTP in mice.
Contrasting with our previous findings obtained in rats, the present single i.n. administration of MPTP did not alter the motor performance of mice evaluated in activity chambers at 18 days after treatment. Some explanations can be given for this apparent “inconsistency”. Firstly, it must be conceded that the later (21 days after i.n. MPTP administration) reduction in locomotor activity of MPTP-treated rats in the open field was unexpected, since only a moderate decrease (about 30%) in striatal DA was observed at this time. Indeed, rats infused bilaterally with MPTP directly into SNpc do not present gross motor alterations (Da Cunha et al. 2001, Miyoshi et al. 2002). Therefore, we can not rule out a possible habituation response of MPTP-treated rats in the open field since the animals were repeatedly tested (1, 7, 14, and 21 days after MPTP) in the same arena. In accordance with this view, the i.n. administration of MPTP did not alter the motor performance of rats when they were tested in the rotarod and grasping stretch tests (data not published). It is important to emphasize that the replication of clinical features of PD in MPTP rodent models seems to be dependent of many variables, such as species, strain, gender, age and/or the schedule of treatment utilized. In fact, the recovery of motor performance appears to be almost universal following acute and sub-acute MPTP models (Gerlach and Riederer 1996; Petroske et al. 2001). On the other hand, the chronic MPTP administration over several weeks by continuous infusion (Fornai et al. 2005), or in association with probenecid (Petroske et al. 2001; Schintu et al. 2009), was shown to induce a persistent degeneration of nigrostriatal neurons associated with long-lasting motor deficits in mice.
Approximately 90% of PD patients at early stage exhibit olfactory dysfunction unrelated to the use of anti-PD medications (e.g., l-DOPA, DA agonists, anticholinergic compounds) (Doty et al. 1988a) and according to the ‘Braak’s hypothesis,’ the olfactory bulb is among the first brain structures to exhibit PD-related pathology (Braak et al. 2004). Mimicking the clinical condition, the mice exhibited an early disruption in olfactory discrimination ability during the first days after MPTP treatment. Corroborating our findings, Schintu et al. (2009) have recently published a highlight study using a chronic intraperitoneal (i.p.) administration of MPTP (25 mg/kg) plus probenecid (250 mg/kg) in mice twice a week for 5 weeks and they demonstrated that olfactory dysfunction already appeared after the 1st injection, whereas motor impairments only appeared after the 3rd and worsened upon subsequent administrations. The present olfactory deficits induced by i.n. administration of MPTP were correlated with a significant reduction of both TH and DA levels in the olfactory bulb of mice. These results contrast with earlier studies that have failed to show significant changes in the olfactory bulb DA and TH protein levels following i.p. MPTP administration in mice (Dluzen 1992; Dluzen and Kefalas, 1996; Mitsumoto et al. 2005). The reduced susceptibility of mice olfactory bulb to MPTP toxicity has been associated to its reduced expression levels of dopamine transporter (Mitsumoto et al. 2005), which has been demonstrated to be essential for the toxic metabolite MPP+ uptake (Mayer et al. 1986) and the MPTP-induced dopaminergic neurodegeneration in mice (Bezard et al. 1999). Therefore, the dose of MPTP and/or the route of administration utilized in the current study may explain, in part, the discrepancy with previous reports.
Our results are in accordance with several lines of evidence that strongly suggest the involvement of DA in olfactory processing. The olfactory bulb of mammals contains a large population of dopaminergic interneurons, principally periglomerular and external tufted cells, which are important for the odor information processing (Halasz and Shepherd 1983). Most data indicate that these dopaminergic cells constitute the entire DA content in the bulb, although there is a report of a minor projection from the ventral tegmental area (Gall et al. 1987). One systemic injection of a dopaminergic agonist can reduce odor detection in vivo (Doty and Risser 1989) and can abolish the odor-induced metabolic activation pattern in the olfactory bulb (Sallaz and Jourdan 1992). Together, these data indicate that DA might exert an inhibitory control on olfactory input.
Moreover, DA appears to be necessary for olfactory memory because its release increases during olfactory learning (Coopersmith et al. 1991), whereas DA receptor antagonists (Weldon et al. 1991; Prediger et al. 2004, 2005a) or treatments that reduce the dopaminergic neurotransmission such as MPTP (Dluzen and Kreutzberg 1993) and reserpine (Prediger et al. 2004, 2005a) inhibit olfactory memory. In accordance with these findings, in the present study, the mice infused with MPTP spent as much time investigating the familiar juvenile mouse during the second presentation as they did on the first encounter, suggesting and impairment in the ability to recognize the juvenile after a short period of time (30 min).
However, we cannot rule out the noradrenergic participation in the MPTP-related olfactory deficits of the present study. Consistent with previous findings (Dluzen 1992; Dluzen and Kefalas 1996), the present i.n. MPTP administration induced a marked NA depletion in the olfactory bulb of mice. Furthermore, the olfactory bulb of rodents receives a prominent noradrenergic input from the locus coeruleus (Shipley et al. 1985). Indeed, NA has been implicated as playing an important role in olfactory memory (Dluzen et al. 1998). That being said, however, 6-OHDA depletion of NA in the olfactory bulb has no demonstrable effect on the rat’s ability to detect odors (Doty et al. 1988b).
Anxiety disorders commonly occur in patients with PD, affecting approximately 40% of patients at early stages of the disease (Schrag 2004), although the pathophysiology of psychiatric symptoms in PD is not fully understood. Previous studies have suggested that the anxiogenic response elicited by 6-OHDA in rats could be related to dopaminergic depletion on the prefrontal cortex (Espejo 1997; Tadaiesky et al. 2008). Contrasting with these early findings, despite the marked DA depletion on the prefrontal cortex (about 60% of control levels) verified in the present study, no significant alteration in anxiety-related parameters evaluated in the elevated plus-maze was observed in MPTP-treated mice. Therefore, animal species, the dose of neurotoxin and/or the route of administration utilized in the current study may explain, in part, the discrepancy with previous reports.
Besides emotional deficits, PD seems to produce cognitive deficits, especially in working memory (Dubois and Pillon 1997; Stebbins et al. 1999; Lewis et al. 2003). Of high interest, the beneficial effects of the drugs currently available for the treatment of PD (such as l-DOPA) on improving the cognitive function affected in PD is controversial (Pillon et al. 1989; Poewe et al. 1991; Cooper et al. 1992; Growdon et al. 1998). Thus, the management of non-motor symptoms of PD remains a challenge.
Because the present i.n. administration of MPTP does not induce gross motor alterations that would preclude assessment of memory function, we also investigated what kinds of memory are affected in these animals. Reinforcing the results obtained in the social recognition paradigm, a single i.n. administration of MPTP (1 mg/nostril) significantly decreased the step-down latencies during the short-term retention test session (performed 1.5 h after training session). MPTP-treated mice were also significantly impaired in their ability to locate the platform in the working-memory version of the water maze. Taken together, these findings indicate a disruption in the mouse’s working memory consecutive to i.n. administration of MPTP, consistent with the cognitive deficits observed in PD patients. Consistent with the present data, we have recently demonstrated that rats treated with i.n. infusion of MPTP present significant working memory impairments when tested in the water maze task (Prediger et al. 2006).
Indeed, there is compelling evidence that the prefrontal cortex plays a critical role in working memory (Passingham and Sakai 2004). Even though most of the dopaminergic afferents to the prefrontal cortex arise from the ventral tegmental area, some of them come from the central area of the SN (Albanese and Bentivoglio 1982). Thus, the prefrontal cortex processes working memory information through cortico-basal parallel loops that are also modulated by dopaminergic projections from the SN (Alexander et al. 1986). For this reason, the depletion of DA observed in the prefrontal cortex consecutive to the i.n. administration of MPTP in mice could explain their impairment in performing the working memory tests. Furthermore, the pattern of DA depletion in the prefrontal cortex observed in the present study is in agreement with our previous data utilizing i.n. infusion of MPTP in rats (Prediger et al. 2006) and resembles the alterations observed in the brain of early-stage PD patients (Zgaljardic et al. 2003; Bruck et al. 2005).
Consistent with previous evidence that lesions of the nigrostriatal pathway failed to alter DA hippocampal levels in rats (Miyoshi et al. 2002; Prediger et al. 2006; Tadaiesky et al. 2008), the DA content in the mouse’s hippocampus was not altered after i.n. administration of MPTP, suggesting that this area is not involved on the behavioral impairments found herein. However, one may consider that DA hippocampal levels are very low; therefore, we cannot exclude the possibility that the HPLC analysis was unable to detect small differences in DA levels in this area.
The cellular mechanisms underlying PD-related neurodegeneration are not well-understood. Nonetheless, considerable evidence indicates that programmed cell death plays a key role in the neurodegeneration observed in both PD patients and MPTP-treated animals (for review see Mattson 2000). MPTP-induced oxidative stress activates a series of cellular factors, which subsequently initiate apoptotic cell death. Among them, c-Jun N-terminal kinase (JNK) signaling cascade and caspase activation are recognized as key mediators in MPTP-induced neuronal apoptosis (Saporito et al. 1999; Kaul et al. 2003). The previous findings from our group using the single i.n. administration of MPTP in rats indicated that it causes oxidative stress (Franco et al. 2007) and a sustained activation of JNK in the olfactory bulb and SN leading to caspase-3 activation (Prediger et al. 2009). In the present study, we further demonstrate a pronounced number of caspase-3-cleaved-positive cells in the SNpc of mice treated intranasally with MPTP, reinforcing the notion of the involvement of apoptotic cell death mechanisms on dopaminergic neurodegeneration observed in the i.n. MPTP model.
In conclusion, our findings reinforce the notion that the olfactory system represents a particularly sensitive route for the transport of neurotoxins into the CNS that may be related to the etiology of PD. These results also suggest that the i.n. administration of MPTP represents a valuable mouse model for the study of the early stages of PD and for testing new therapeutic strategies to restore sensorial and cognitive processes in PD. However, our PD mouse model does not fully mimic human PD. Thus, even though the observed sequence of olfactory and cognitive impairments is similar to the sequence of analogous changes seen in PD, the time frame of development of these problems is shorter. Finally, because of its demonstrated toxicity to humans, the use of MPTP among researchers is a serious concern (see Przedborski et al. 2001). Thus, our i.n. MPTP model of PD would seem preferable to other rodent models of PD in which high concentrations of MPTP are needed to induce alterations in CNS.
References
Albanese A, Bentivoglio M (1982) The organization of dopaminergic and nondopaminergic mesencephalocortical neurons in the rat. Brain Res 238:421–425
Alexander GE, DeLong MR, Strick PL (1986) Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci 9:357–381
Bezard E, Gross CE, Fournier MC, Dovero S, Bloch B, Jaber M (1999) Absence of MPTP-induced neuronal death in mice lacking the dopamine transporter. Exp Neurol 155:268–273
Bondi MW, Kaszniak AW (1991) Implicit and explicit memory in Alzheimer’s disease and Parkinson’s disease. J Clin Exp Neuropsychol 13:339–358
Bosboom JL, Stoffers D, Wolters ECh (2004) Cognitive dysfunction and dementia in Parkinson’s disease. J Neural Transm 111:1303–1315
Braak H, Ghebremedhin E, Rub U, Bratzke H, Del Tredici K (2004) Stages in the development of Parkinson’s disease-related pathology. Cell Tissue Res 318:121–134
Bruck A, Aalto S, Nurmi E, Bergman J, Rinne JO (2005) Cortical 6-[18F]fluoro-l-dopa uptake and frontal cognitive functions in early Parkinson’s disease. Neurobiol Aging 26:891–898
Chiueh C, Markey SP, Burns RS, Johannessen JN, Pert A, Kopin IJ (1984) Neurochemical and behavioral effects of systemic and intranigral administration of N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine in the rat. Eur J Pharmacol 100:189–194
Cooper JA, Sagar HJ, Doherty SM, Jordan N, Tidswell P, Sullivan EV (1992) Different effects of dopaminergic and anticholinergic therapies on cognitive and motor function in Parkinson’s disease. A follow-up study of untreated patients. Brain 115:1701–1725
Coopersmith R, Weihmuller FB, Kirstein CL, Marshall JF, Leon M (1991) Extracellular dopamine increase in neonatal olfactory bulb during odor preference training. Brain Res 564:149–153
Da Cunha C, Gevaerd MS, Vital MA, Miyoshi E, Andreatini R, Silveira R, Takahashi RN, Canteras NS (2001) Memory disruption in rats with nigral lesions induced by MPTP: a model for early Parkinson’s disease amnesia. Behav Brain Res 124:9–18
Dantzer R, Bluthe RM, Koob GF, Le Moal M (1987) Modulation of social memory in male rats by neurohypophyseal peptides. Psychopharmacology 91:363–368
Dluzen DE (1992) 1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) reduces norepinephrine concentrations in the olfactory bulbs of male mice. Brain Res 586:144–147
Dluzen DE, Kefalas G (1996) The effects of intranasal infusion of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) upon catecholamine concentrations within olfactory bulbs and corpus striatum of male mice. Brain Res 741:215–219
Dluzen DE, Kreutzberg JD (1993) 1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) disrupts social memory/recognition processes in the male mouse. Brain Res 609:98–102
Dluzen DE, Muraoka S, Landgraf R (1998) Olfactory bulb norepinephrine depletion abolishes vasopressin and oxytocin preservation of social recognition responses in rats. Neurosci Lett 254:161–164
Doty RL (2008) The olfactory vector hypothesis of neurodegenerative disease: is it viable? Ann Neurol 63:7–15
Doty RL, Risser JM (1989) Influence of the D2 dopamine receptor agonist quinpirole on the odor detection performance of rat before and after spiperone administration. Psychopharmacology 98:310–315
Doty RL, Deems D, Stellar S (1988a) Olfactory dysfunction in Parkinson’s disease: a general deficit unrelated to neurologic signs, disease state, or disease duration. Neurology 38:1237–1244
Doty RL, Fergusson-Segall M, Lucki I, Kreider M (1988b) Effects of intrabulbar injections of 6-hydroxydopamine on ethyl acetate odor detection in castrate and non-castrate male rats. Brain Res 444:95–103
Doty RL, Bromley SM, Stern MB (1995) Olfactory testing as an aid in the diagnosis of Parkinson’s disease: development of optimal discrimination criteria. Neurodegeneration 4:93–97
Douhou A, Debeir T, Murer MG, Do L, Dufour N, Blanchard V, Moussaoui S, Bohme GA, Agid Y, Raisman-Vozari R (2002) Effect of chronic treatment with riluzole on the nigrostriatal dopaminergic system in weaver mutant mice. Exp Neurol 176:247–253
Dubois B, Pillon B (1997) Cognitive deficits in Parkinson’s disease. J Neurol 244:2–8
Duvoisin RC (1991) Parkinson’s disease, 3rd edn. Raven Press, New York
Espejo EF (1997) Selective dopamine depletion within the medial prefrontal cortex induces anxiogenic-like effects in rats placed on the elevated plus maze. Brain Res 762:281–284
Fornai F, Schlüter OM, Lenzi P, Gesi M, Ruffoli R, Ferrucci M, Lazzeri G, Busceti CL, Pontarelli F, Battaglia G, Pellegrini A, Nicoletti F, Ruggieri S, Paparelli A, Südhof TC (2005) Parkinson-like syndrome induced by continuous MPTP infusion: convergent roles of the ubiquitin-proteasome system and alpha-synuclein. Proc Natl Acad Sci USA 102:3413–3418
Franco J, Prediger RD, Pandolfo P, Takahashi RN, Farina M, Dafre AL (2007) Antioxidant responses and lipid peroxidation following intranasal 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) administration in rats: increased susceptibility of olfactory bulb. Life Sci 80:1906–1914
Franklin KBJ, Paxinos G (1997) The mouse brain in stereotaxic coordinates. Academic Press, San Diego
Gall CM, Hendry SH, Seroogy KB, Jones EG, Haycock JW (1987) Evidence of coexistence of GABA and dopamine in neurons of the rat olfactory bulb. J Comp Neurol 266:307–318
Gerlach M, Riederer P (1996) Animal models of Parkinson’s disease: an empirical comparison with the phenomenology of the disease in man. J Neural Transm 103:987–1041
Gorell JM, Johnson CC, Rybicki BA, Peterson EL, Richardson RJ (1998) The risk of Parkinson’s disease with exposure to pesticides, farming, well water, and rural living. Neurology 50:1346–1350
Growdon JH, Kieburtz K, McDermott MP, Panisset M, Friedman JH (1998) Levodopa improves motor function without impairing cognition in mild non-demented Parkinson’s disease patients. Parkinson Study Group. Neurology 50:1327–1331
Halasz N, Shepherd GM (1983) Neurochemistry of the vertebrate olfactory bulb. Neuroscience 10:759
Hamon M, Fattaccini CM, Adrien J, Gallissot MC, Martin P, Gozlan H (1988) Alterations of central serotonin and dopamine turnover in rats treated with ipsapirone and other 5-hydroxytryptmaine 1A agonists with potential anxiolytic properties. J Pharmacol Exp Ther 246:745–752
Hirsch EC, Lejeune O, Colliot G, Corkidi G, Tajani M (1992) Computer methods in nuclei cartography. Methods Neurosci 10:62–79
Jenner P (2008) Functional models of Parkinson’s disease: a valuable tool in the development of novel therapies. Ann Neurol Suppl 2:S16–S29
Kalaria RN, Mitchell MJ, Harik SI (1987) Correlation of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine neurotoxicity with blood-brain barrier monoamine-oxidase activity. Proc Natl Acad Sci USA 84:3521–3525
Kaul S, Kanthasamy A, Kitazawa M, Anantharam V, Kanthasamy AG (2003) Caspase-3 dependent proteolyytic activation of protein kinase C delta mediates and regulates 1-methyl-4-phenylpyridinium (MPP+)-induced apoptotic cell death in dopaminergic cells: relevance to oxidative stress in dopaminergic degeneration. Eur J Neurosci 18:1387–1401
Kawano T, Margolis FL (1982) Transsynaptic regulation of olfactory bulb catecholamines in mice and rats. J Neurochem 39:342–348
Langston JW, Ballard P, Tetrud JW, Irwin I (1983) Chronic Parkinsonism in humans due to a product of meperidine-analog synthesis. Science 219:979–980
Lewis SJG, Dove A, Robbins TW, Barker RA, Owen AM (2003) Cognitive impairments in early Parkinson’s disease are accompanied by reductions in activity in frontostriatal neural circuitry. J Neurosci 23:6351–6356
Lister RG (1987) The use of a plus-maze to measure anxiety in the mouse. Psychopharmacology 92:180–185
Mattson MP (2000) Apoptosis in neurodegenerative disorders. Nat Rev Mol Cell Biol 1:120–129
Mayer RA, Kindt MV, Heikkila RE (1986) Prevention of the nigrostriatal toxicity of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine by inhibitors of 3,4-dihydroxyphenylethylamine transport. J Neurochem 47:1073–1079
Mitsumoto Y, Mori A, Ohashi S, Nakai M, Moriizumi T (2005) Differential effects of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine in the olfactory bulb and the striatum in mice. Neurosci Res 51:111–115
Miyoshi E, Wietzikoski S, Camplessei M, Silveira R, Takahashi RN, Da Cunha C (2002) Impaired learning in a spatial working memory version and in a cued version of the water maze in rats with MPTP-induced mesencephalic dopaminergic lesions. Brain Res Bull 58:41–47
Muller A, Reichmann H, Livermore A, Hummel T (2002) Olfactory function in idiopathic Parkinson’s disease (IPD): results from cross-sectional studies in IPD patients and long-term follow-up of de-novo IPD patients. J Neural Transm 109:805–811
Passingham D, Sakai K (2004) The prefrontal cortex and working memory: physiology and brain imaging. Curr Opin Neurobiol 14:163–168
Petroske E, Meredith GE, Callen S, Totterdell S, Lau YS (2001) Mouse model of Parkinsonism: a comparison between subacute MPTP and chronic MPTP/probenecid treatment. Neuroscience 106:589–601
Pillon B, Dubois B, Bonnet AM, Esteguy M, Guimaraes J, Vigouret JM, Lhermitte F, Agid Y (1989) Cognitive slowing in Parkinson’s disease fails to respond to levodopa treatment: the 15-objects test. Neurology 39:762–768
Poewe W, Berger W, Benke T, Schelosky L (1991) High-speed memory scanning in Parkinson’s disease: adverse effects of levodopa. Ann Neurol 29:670–673
Prediger RD, Batista LC, Miyoshi E, Takahashi RN (2004) Facilitation of short-term social memory by ethanol in rats is mediated by dopaminergic receptors. Behav Brain Res 153:149–157
Prediger RD, Batista LC, Takahashi RN (2005a) Caffeine reverses age-related deficits in olfactory discrimination and social recognition memory in rats. Involvement of adenosine A1 and A2A receptors. Neurobiol Aging 26:957–964
Prediger RD, Da Cunha C, Takahashi RN (2005b) Antagonistic interaction between adenosine A2A and dopamine D2 receptors modulates the social recognition memory in reserpine-treated rats. Behav Pharmacol 16:209–218
Prediger RD, Batista LC, Medeiros R, Pandolfo P, Florio JC, Takahashi RN (2006) The risk is in the air: intranasal administration of MPTP to rats reproducing clinical features of Parkinson’s disease. Exp Neurol 202:391–403
Prediger RD, Rial D, Medeiros R, Figueiredo CP, Doty RL, Takahashi RN (2009) Risk is in the air: an intranasal MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine) rat model of Parkinson’s disease. Ann N Y Acad Sci. doi:10.1111/j.1749-6632.2009.03885.x)
Przedborski S, Jackson-Lewis V, Naini AB, Jakowec M, Petzinger G, Miller R, Akran M (2001) The parkinsonian toxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP): a technical review of its utility and safety. J Neurochem 76:1265–1274
Riederer P, Wuketich S (1976) Time course of nigrostriatal degeneration in parkinson’s disease. A detailed study of influential factors in human brain amine analysis. J Neural Transm 38:277–301
Rojo AI, Montero C, Salazar M, Close RM, Fernandez-Ruiz J, Sanchez-Gonzalez MA, de Sagarra MR, Jackson-Lewis V, Cavada C, Cuadrado A (2006) Persistent penetration of MPTP through the nasal route induces Parkinson’s disease in mice. Eur J Neurosci 24:1874–1884
Sallaz M, Jourdan F (1992) Apomorphine disrupts odour-induced patterns of glomerular activation in the olfactory bulb. Neuroreport 3:833–836
Saporito MS, Brown EM, Miller MS, Carswell S (1999) CEP-1347/KT-7515, an inhibitor of c-jun N-terminal kinase activation, attenuates the 1-methyl-4-phenyl tetrahydropyridine-mediated loss of nigrostriatal dopaminergic neurons in vivo. J Pharmacol Exp Ther 288:421–427
Schintu N, Frau L, Ibba M, Garau A, Carboni E, Carta AR (2009) Progressive dopaminergic degeneration in the chronic MPTPp mouse model of Parkinson’s disease. Neurotox Res 16:127–139
Schmidt N, Ferger B (2001) Neurochemical findings in the MPTP model of Parkinson’s disease. J Neural Transm 108:1263–1282
Schrag A (2004) Psychiatric aspects of Parkinson’s disease: an update. J Neurol 251:795–804
Sedelis M, Hofele K, Auburger GW, Morgan S, Huston JP, Schwarting RK (2000) MPTP susceptibility in the mouse: behavioral, nurochemical, and histological analysis of gender and strain differences. Behav Genet 30:171–182
Shipley MT, Halloran FJ, de la Torre J (1985) Surprisingly rich projection from locus coeruleus to the olfactory bulb in the rat. Brain Res 329:294–299
Stebbins GT, Gabrieli JD, Masciari F, Monti L, Goetz CG (1999) Delayed recognition memory in Parkinson’s disease: a role for working memory? Neuropsychologia 37:503–510
Tadaiesky MT, Dombrowski PA, Figueiredo CP, Cargnin-Ferreira E, Da Cunha C, Takahashi RN (2008) Emotional, cognitive and neurochemical alterations in a premotor stage model of Parkinson’s disease. Neuroscience 156:830–840
Weldon DA, Travis ML, Kennedy DA (1991) Posttraining D1 receptor blockade impairs odor conditioning in neonatal rats. Behav Neurosci 105:450–458
Xikota JC, Rial D, Ruthes D, Pereira R, Figueiredo CP, Prediger RD, Walz R (2008) Mild cognitive deficits associated to neocortical microgyria in mice with genetic deletion of cellular prion protein. Brain Res 1241:148–156
Zgaljardic DJ, Borod JC, Foldi NS, Mattis P (2003) A review of the cognitive and behavioral sequelae of Parkinson’s disease: relationship to frontostriatal circuitry. Cogn Behav Neurol 16:193–210
Acknowledgments
This work was supported by grants from the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), the Coordenacão de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), the Programa de Apoio aos Núcleos de Excelência (PRONEX), and the Fundação de Apoio a Pesquisa do Estado de Santa Catarina (FAPESC), all of Brazil. ASA Jr is supported by scholarship from CAPES-Brazil. AERM is supported by CONACYT (México cod.76101, 93485). RDSP and EDB are supported by research fellowships from CNPq-Brazil. RDSP, EDB, and RRV are supported by CAPES-COFECUB (France/Brazil; 491/2005) and FAPESP-INSERM (08/55092-9).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Prediger, R.D.S., Aguiar, A.S., Rojas-Mayorquin, A.E. et al. Single Intranasal Administration of 1-Methyl-4-Phenyl-1,2,3,6-Tetrahydropyridine in C57BL/6 Mice Models Early Preclinical Phase of Parkinson’s Disease. Neurotox Res 17, 114–129 (2010). https://doi.org/10.1007/s12640-009-9087-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12640-009-9087-0