Abstract
The goal of this study was to analyze the antileishmanial and antibacterial activity of Coffea arabica green seed biosynthesize silver nanoparticles (C. arabica AgNPs), as well as cytotoxicity and cytokine gene expression. UV–vis spectroscopy, FTIR, and FESEM methods used to examine the C. arabica AgNPs. MTT test was used to assess the antileishmanial and cytotoxicity effects. The gene expression level was assessed in NPs-treated J774 cells by qPCR. The synthesized C. arabica AgNPs were in the size range of 20–70 nm, through FESEM pictures. The IC50 values of the NPs were 65. 4 and 47.70 μg/mL against promastigotes and amastigotes of Leishmania major, but these values were 580.1 and 171.1 μg/mL for Glucantime® as the control drug. C. arabica AgNPs represented a significant increase in IL-12P40, as a Th1 cytokine, in comparison to Glucantime® at high concentrations (P < 0.01), whilst IL-10 expression level showed a significant reduction between NPs-treated and Glucantime®-treated macrophages at 250–1000 μg/mL concentrations (P < 0.001). Moreover, the NPs were cytotoxic on cancer cell lines of Hek293, MCF7, and A172 with the CC50 values of 437.2, 116.8, and 72.9 µg/mL, respectively. It showed a significant effect of these NPs against A172 (P < 0.001). Also, the lowest MIC values of the NPs were obtained for Bacillus subtilis and Staphylococcus aureus (204 µg/mL). According to the antileishmanial, anticancer, and antibacterial activity of these NPs, it can considered a bio-agent drug in the future in endemic countries.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nanobiotechnology is a cross-disciplinary field that joins the mix of nanoparticles (NPs) and related activities. NPs have been used as unique administrators for remedial purposes. Nanomaterials are essentially particles, generally 1–100 nm in size, with different shapes and morphologies (Bawa 2019). Attractive nanoparticles range in size from a few nanometers to tens of nanometers, making them comparable to or smaller than a quality (2 nm wide and 10–100 nm long), a protein (5–50 nm), a cell (10–100 nm), and an infection (20–450 nm). Surely, they can be collaborated with or tied to an organic element by covering with natural particles, so given that controllable method for 'labeling' or tending to it (Pankhurst et al. 2003).
Due to the sensible convention, eco-friendly, quick, non-pathogenic, and unique advanced approach of biosynthetic processes, the use of plants as reducing agents in silver nanoparticle mixtures has recently received attention. The utilization of biosynthesized metal NPs, for example, selenium, zinc oxide, magnesium, titanium, gold, copper, silver, palladium, platinum, and magnetite NPs has just been assessed against microorganisms as a proper alternative to conventional methods (Iravani et al. 2014). Coffea arabica green seeds silver nanoparticles (C. arabica AgNPs) are known to display mitigating, anticancer, antibacterial, antiviral, and against angiogenic movement (Jang 2015). The system of poisonousness of apoptosis incited by C. arabica AgNPs is all around affirmed in various cell lines (Yuan and Gurunathan 2017). The use of natural plant products to synthesize nanomaterials is becoming more popular due to its safety and pricing. Secondary plant metabolites such as flavonoids, ketones, aldehydes, terpenoids, amides, and carboxylic acid have been used as reducing agents in the bio-reduction reaction since its conception, due to the production of new metal nanoparticles (Sinha et al. 2018).
The caffeine-producing plant Coffea arabica (Rubiaceae) is a major cash crop in Cameroon, and a decoction of the leaves in water is used as an antimalarial treatment (Wahba et al. 2019). This is a big shrub with dark green oval leaves. It has four chromosomal arrays instead of two, which distinguishes it from other espresso species. The natural products are oval and grow in 7–9 months; they often contain two-level seeds (espresso beans), and when only one bean matures, it is referred to as a peaberry (Marcheafave et al. 2019; Silva et al. 2021). The availability and the administration of safe and effective drugs are critical in the treatment of infectious diseases. To increase the therapeutic index of infectious disease medicines and simplify their use, nanotechnology-based techniques have been the subject of extensive preclinical testing (Kirtane et al. 2021).
Leishmaniasis is a vector-borne disease caused by 22 human parasite species that is found in 98 nations, with the tropics and sub-tropics having the highest prevalence (Choi et al. 2021). To date, there are neither available vaccines nor safe and effective drugs against all forms of visceral, cutaneous, and mucocutaneous leishmaniasis. Biological proceedings are not feasible due to the complexity of the life cycle and the existence of various vectors and reservoir species (Choi et al. 2021). Chemotherapy of pentavalent antimonial compounds such as meglumine antimoniate (Glucantime®, MA) is currently the only universal control measure against the disease, but it is limited due to a variety of side effects, poor treatment adherence, and the development of resistance against the causative agents (Ponte-Sucre et al. 2017). Maltreatment of anti-infection agents may cause the spread of safe microbial strains, which thusly prompts a mind-boggling and difficult issue in medication. Consequently, it is basic to investigate new antimicrobial substances and supplant them with basic anti-toxins. Nanoparticles have pulled in researchers' inclinations because of their solid antimicrobial properties (Tariq et al. 2020). To produce nanoparticles, researchers have looked at several plant resources so far. One of the most widely consumed plant items is Coffea arabica seed. The bean of this plant was previously found to have high quantities of phenolic chemicals (Geremu et al. 2016), which can be utilized as a bioreduction to make NPs. Silver nanoparticles produced from dried roasted coffee seed in the form of a hydroalcoholic extract were tested for antibacterial activity (Dhand et al. 2016). To the best of our knowledge, no investigation has been conducted on the cytotoxicity and antileishmanial efficacy of AgNPs produced from green Coffea seeds. Biogenic combination of C. arabica with AgNPs, as non-harmful synthetic mixtures, was carried out in this study to create a safe and natural agent in order to assess antileishmanial, antibacterial and cytotoxicity effects.
Materials and methods
Plant sampling, identification, and extraction
Coffea arabica was purchased from a market in Kerman province, southeast of Iran, in July 2019. A voucher specimen of the plant submitted to the Herbarium Center of Kerman University of Medical Sciences' Department of Pharmacognosy (KF2321). The green Coffea seed mashed, weighed (300 g), and extracted by the maceration method with 70% ethanol (40 °C). The maceration process conducted three times with periodic stirring. Stirring used to balance the concentration of the fluid outside the grain of the powder. The pulp and macerate separated by using a filter. The maceration procedure repeated and filtered again. The extractive solutions were concentrated under reduced pressure for ethanol elimination, and then lyophilized, yielding dry extracts from the green seed.
Biosynthesis of silver nanoparticles
C. arabica AgNPs synthesized using AgNO3 as a metal base. 300L of silver nitrate (0.1%) and 100 µg/mL of green Coffea seeds residual (10 mL) combined for this purpose. The mixture was oven dried at 40 °C and rinsed many times with deionized water before being dried at 50 °C. The shade change monitored by using UV–visible spectroscopy.
Characterizations of silver nanoparticles
A UV–vis spectrophotometer (PerkinElmer, Germany) used to monitor the growth of silver nanoparticles at 300–600 nm. To determine the practical groups of the concentrate and characterize the unknown materials composition, the C. arabica AgNPs exposed to Fourier transform infrared spectroscopy (FTIR) analysis. Also, Field Emission-Scanning Electron Microscopy (FESEM) (Quanta 200, USA) in diameters of 500 and 10 nm was used to confirm the normal molecule size, shape, and appropriation of inserted silver nanoparticles.
Cell cultures and parasite growth conditions
The cell lines of Hek293, MCF7 (Human breast cancer), A172 (Human glioblastoma), and murine macrophage (J774-A1 cells, ECACC 91051511) provided by the Pasteur Institute of Tehran, Iran. Dulbecco's Modified Eagle's Medium (DMEM; Gibco, Invitrogen, U.S.A) used to culture cell lines, which contained 100 IU/ml penicillin, 100 µg/mL streptomycin (Gibco, U.S.A), 10% heat-inactivated FBS (Gibco, Invitrogen, Grand Island, NY, U.S.A.), and 1 mM sodium pyruvate at 37 °C in a CO2 incubator (5%). In addition, leishmaniasis Research Center of Kerman University of Medical Sciences provided promastigotes of Leishmania major (MRHO/IR/75/ ER), then sub-cultured in RPMI-1640 medium completed with 10% FBS and incubated at 25 ± 1 °C.
Cytotoxic assay
To determine the effect of C. arabica AgNPs on the viability of Hek293, MCF-7, A172, and J774-A1 cell lines, an MTT assay was used. On a 96-well plate, 104 cells/well of each cell line plated and incubated for 24 h. The next day, the medium suctioned and 100 μl of various concentrations of the C. arabica AgNPs (1-500 μg/mL) poured into each well and brooded at 37 °C with 5% CO2 for a normal period of 72 h. After incubation, 20 μL of MTT solution [3-(4, 5-dimethyl-2-thiazolyl)- 2, 5-diphenyl-2H-tetrazolium bromide] (5 μg/mL) added into each well. After 3 h, the absorbance was estimated at 490 nm by the ELISA reader (BioTek- Elx 800). Cell practicality communicated as 100% for control (untreated cells). All samples performed in triplicates and the survival rate (%) calculated as the following (Saduqi et al. 2019): Survival rate (%) = (OD in treatment group/OD in control group) × 100.
The inhibitory concentration required for half cytotoxicity (CC50) value calculated utilizing the Probit test in the SPSS software (version 20).
Anti-promastigote assessment
The effect of silver nanoparticles on L. major promastigotes assessed with a colorimetric test WST-1 (4-[3-(4-iodophenyl)-2-(4-nitrophenyl)-2H-5-tetrazolio]-1,3-benzene disulfonate) (Roche-Mannheim, Germany) as described elsewhere (Saduqi et al. 2019). In a 96-well small-scale titer plate, 100 µL of logarithmic stage promastigotes (106 cells/mL) inserted. Each well received 100 µl of various doses of C. arabica AgNPs as well as MA as a reference medication (1-500 µg/mL) and incubated at 25 °C for 72 h. Then, 10 µL of WST-1 added to each well and incubated at 25 °C for 4 h. Promastigotes with no C. arabica AgNPs defined as an untreated control. Finally, the absorbance measured at 490 nm using an ELISA reader (BioTek-ELX800, USA). The Probit test of the SPSS software used to calculate the 50% inhibitory concentration (IC50 value).
Anti-amastigote assessment
Drug susceptibility of the intra-macrophage amastigotes was determined (Chang 1980). 1cm2 smear placed in the well of a 6-well slide (LabTek, NY, USA), then 200 µl J774A1 cells (106 cells/ml) added to the plate. After 2 h at 37 °C in 5% CO2, fixed phase pre-flagella form added to the macrophages (ratio 10: 1) and incubated for 24 h. Free parasites eliminated by washing with RPMI-1640 medium and infected cells treated with 100 µl C. arabica AgNPs and Glucantime® (1–500 µg/mL) at 37 °C and 5% CO2 for 72 h. Finally, the slides were dried, fixed with methanol, stained with Giemsa, and observed under a light microscope (Nikon, Japan). Furthermore, only macrophages and those containing amastigotes without any drugs used as negative and positive controls. The intracellular amastigotes of each concentration assessed by the mean number of amastigotes of 100 macrophages, which were implemented thrice (Sharifi et al. 2017, 2019). Finally, the IC50 values calculated by the probit test of SPSS software.
Evaluation of cytokines expression level
The expression levels of IL-12p40 (as the cytokine of the Th-1 pathway) and IL-10 (as the cytokine of the Th-2 pathway) evaluated in C. arabica AgNPs-treated and Glucantime®-treated macrophages by quantitative real-time PCR (qPCR) assays. The RNeasy Mini Kit (Cat. No. 74106, Qiagen, Germany) used to extract the total RNA from treated and untreated cells according to its protocol. For cDNA synthesize, RT reagent Kit (Takara, Japan) utilized by using 100 ng of extracted RNA in a thermal cycler. Finally, SYBR® Premix Ex TaqTM II (Takara, Japan) used for qPCR reaction in a total volume of 10 μl in the Rotor-Gene Q (Qiagen, Germany). Table 1 represented the target primers and reference gene sequences. The experiments conducted in triplicate.
Minimum inhibitory concentrations (MIC) determination
The antibacterial effect of C. arabica AgNPs determined through the broth microdilution method. One mL of C. arabica AgNPs suspension added to the test cylinder and two-fold serial dilutions were done for coming to the last concentrations between 12.4 mg/mL and 3.1 mg/mL. Four microorganism species including Escherichia coli, Bacillus subtilis, Staphylococcus aureus, and methicillin-resistant S. aureus (MRSA) utilized in this investigation. 10 µl of the bacterial suspension (concentration to 0.5 McFarland usual) was added to each test tube and incubated at 37 °C for 24 h. The lowest concentration of C. arabica AgNPs that caused the lack of growth in bacteria indicated as MIC of silver NPs. Besides, Vancomycin, Imipenem, Cefixime, and Ciprofloxacin used as the control standard antibiotics.
Disk diffusion assay
All bacteria strains cultured on nutrient agar plates. Different concentrations of C. arabica AgNPs prepared in test tubes by diluting them with sterile water. Sterile blank antimicrobial disks immersed into prepared C. arabica AgNPs tubes and placed on the agar plates. In addition, the control standard antibiotic disks placed either. The plates incubated at 37 °C for one night and the inhibitory zone around the disks measured.
Statistical analysis
The data presented as mean ± SD. The differences between the test and control groups analyzed by ANOVA. Also, a t-test was used to assess the difference between the IC50 of the two groups. P < 0.05 as indicated significant. Prism 7.01 software (GraphPad Software, USA) was used to analysis data.
Results
Biosynthesis and characterization of silver nanoparticles
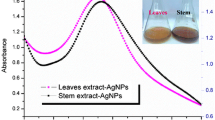
The synthesis of C. arabica AgNPs assessed by monitoring AgNO3 color change from colorless to yellow–brown and its absorption spectrum appeared at 450 nm. The reduction of Ag+ particles to silver particles upon contact with plant extract supernatants can lead to a color change from dull to bright yellow after reacting with Ag+ particles. The UV–vis results showed that the assimilation peak (λmax) of silver observed at 440 nm (Fig. 1). The frequencies ranging from 400 to 4000 cm−1 on the concentrate detected in Fig. 2. This shows the proximity of the IR homologs at 3356, 2923, 2852, 1654, 1516, 1383, 1126 (COC), 1072, 1050, and 834, 763, and 610 due to the NH2 cm−1 fluctuations. The absorption band at 3356 cm−1 is because of extending of the N–H band of amino gatherings or is demonstrative of present O–H bunches because of the nearness of alcohols, phenols, sugars, and so forth. The presence of a top at 2923 and 2852 cm−1 identified with that C–H, C=O, 1654 cm−1 (carbonyl gatherings), 1516 cm−1 (amide II), 1383 cm−1 (C=O), 1072, 1050 cm−1 (C–O–C) and 831, 763 and 610 cm−1 because of C–Cl gathering of alkyl halides. The filtering electron microscopy (FESEM) picture indicated the sporadic molded C. arabica AgNPs of 20–70 nm in size (Fig. 3).
Cytotoxic assay
The MTT results of different cell lines exposed to 1–500 µg/mL of C. arabica AgNPs represented the cytotoxic effect of these NPs on the cell lines of Hek293, MCF7, and A172 with the CC50 values of 437.2, 116.8 and 72.9 µg/mL, respectively. These values indicated the potent cytotoxic activity of these NPs towards these studied human cell lines. The inhibition percentage of these NPs on these cell lines have shown in Fig. 4. But, C. arabica AgNPs showed less cytotoxic effect against the J774-A1 cells, as the macrophage model, with the CC50 values of 742.4 µg/mL and Selectivity Index (SI) of 15.5 (Table 2).
Anti-promastigote assay
The findings showed the critical antileishmanial action of these NPs against the promastigote phase of L. major in a dose-dependent manner (P < 0.05). Figure 5 represented a significant decrease in promastigote viability compared to Glucantime®, as the reference drug, especially at two concentrations of 250 and 500 μg/mL. Also, the IC50 values for C. arabica AgNPs and Glucantime® against promastigotes were 65.39 ± 0.05 and 580.1 ± 0.16 μg/mL, respectively; which showed lower IC50 compared to Glucantime® (P < 0.001) (Table 2).
Anti-amastigote assay
The results showed that C. arabica AgNPs significantly (P < 0.05) decreased the mean number of L. major amastigotes in J774-A1 cells in comparison to Glucantime® (Table 3). Moreover, these NPs indicated a 3.5-fold reduction in the IC50 value compared to the reference drug (47.7 ± 0.03 vs. 171.01 ± 0.01 μg/mL) (Table 2).
Gene expression
The expression of cytokines assessed in the C. arabica AgNPs and Glucantime® groups. IL12P40 gene expression increased in NP-treated macrophages, especially at the last three concentrations; while the expression level of IL-10 represented a significant decrease with increasing drug concentration, especially at concentrations of 250, 500 and 1000 μg/mL (P < 0.001) compared with Glucantime® (Fig. 6).
Antibacterial activity
The prepared C. arabica AgNPs represented a significant antibacterial effect against all four bacteria species by elevating concentration, especially at 750 and 1000 μg/mL, as S. aureus showed the highest inhibition zone (100 mm) (Fig. 7A). NPs at 1000 μg/mL caused the same inhibitory effect as Cefixime and a higher effect than Vancomycine against S. aureus. Also, at this concentration of NPs, more inhibition zone was identified against B. subtilis compared to all antibiotics. A more inhibitory effect observed against MRSA at 1000 μg/mL of NPs in comparison to two antibiotics of Ciprofloxacin and Cefixime (P < 0.01) (Fig. 7B). The C. arabica AgNPs showed the lowest MIC against B. Subtilis and S. aureus (204 μg/mL). These NPs indicated decreased MIC levels against B. Subtilis and S. aureus compared to the other antibiotics (Table 4).
Inhibition zone of different concentrations of C. arabica AgNPs (C. arabica silver nanoparticles) and CE (crude extract) (A), as well as IMP (Imipenem), CP (Ciprofloxacin), CFM (Cefixime) and V (Vancomycin) antibiotics (B) against E. coli, S. aureus, B. subtilis, and MRSA compared to the control. Error bars are SD (** P < 0.01 and *** P < 0.001)
Discussion
The proximity of a large variety of natural synthetic compounds such as starch, fat, proteins, catalysts and coenzymes, phenols flavonoids, terpenoids, alkaloids, gum, and others capable of delivering electrons for the reduction of Ag+ particles to Ag0 explains why C. arabica AgNPs are combined with natural components (Allahverdiyev et al. 2011). The dynamic fixing is liable for the decrease of Ag+ particles changes relying on living being/remove utilized. For nano-change of C. arabica AgNPs, electrons should be gotten from dehydrogenation of acids (ascorbic corrosive) and alcohols (catechol) in hydrophytes, keto to enol transformations (cyperaquinone, dietchequinone, remirin) in mesophytes or the two systems in xerophytes plants.
The decrease of Ag+ to Ag0 affirmed by the shading change of the response blend from colorless to brownish yellow. The absorption spectra of spherical C. arabica AgNPs have a maximum between 420 and 450 nm, according to the literature (Abdi et al. 2019). FTIR study demonstrates that likely the C–H, carboxyl (–C=O), hydroxyl (–OH), and (C–O–C) bunches in seed exudates are fundamentally associated with the decrease of Ag+ particles to Ag0 nanoparticles (Javed et al. 2020).
NPs such as C. arabica AgNPs contain special physicochemical properties such as size and shape (1–100 nm in diameter) and excellent surface-to-volume ratio resulting in higher chemical reactivity (Flores‐López et al. 2019). In this study, the FESEM image indicated the sporadic mold of C. arabica AgNPs of 20–70 nm in size.
For decades, leishmaniasis overlooked, leading to serious health problems such as death, scarring, stigma, and severe depression (Bailey et al. 2019). As a result, creative ways for developing more effective and less harmful nanomedicine to treat and manage Leishmania parasites are high on the priority list for research (Ismail et al. 2019). One of the effective ways is to combine nanometals with bioactive chemicals to create a dynamic anti-leishmaniasis agent with good biocompatibility (Akbari et al. 2017). According to the findings, the IC50 values of C. arabica AgNPs was 65.4 and 47.7 μg/mL against promastigote and amastigote stages of L. major, whereas these values were 580.1 and 171.1 μg/mL for Glucantime® as control drug. Also, these NPs induced a significant reduction in the mean number of amastigotes in J774-A1 cells in comparison to Glucantime®, especially at the last two concentrations. Besides, IL-12P40 and IL-10 genes expression showed increased and decreased levels, respectively, in C. arabica AgNPs-treated macrophages compared to Glucantime®-treated cells, especially at 250, 500, and 1000 μg/mL concentrations.
The synergetic action of both silver nanoparticles and bioactive phytochemicals produced from C. arabica green seed residues linked to the surface of C. arabica AgNPs could explain the high anticancer activity of these biogenic NPs (Farah et al. 2016). In addition, nanoformulation of organic chemicals can cause higher stability and cell penetration, leading to reduced stress, greater cytotoxicity, and cell death (Kajani et al. 2016). Various studies have established the cytotoxic effects of compounds biosynthesized using different plant extracts in contradiction to different cancer cell lines (Ogur 2014; Romeilah 2016). In our results, these NPs had the cytotoxic activity on all three cancer cell lines, with the best inhibitory effect on A172 cells (92%).
Silver nanoparticles are widely employed in medicine, food storage, textile coatings, public health, and environmental applications due to their antibacterial qualities (Gao et al. 2015). Some mechanisms for C. arabica AgNPs' antibacterial action have been proposed. Silver ions produced from these NPs thought to increase their bactericidal efficacy by inhibiting DNA replication, bacteria growth, respiration, and ATP synthesis, ultimately leading to cell death (Feng et al. 2000; Sondi and Salopek-Sondi 2004; Morones et al. 2005). C. arabica AgNPs are able to penetrate the bacterial cell wall and get into the cytoplasm (Alsammarraie et al. 2018). According to the obtained results, it seems that C. arabica AgNPs immediately crossed S. aureus, B. subtilis, and E. coli cell walls, but the MRSA cell wall showed resistance to low concentrations of the NPs. Also, more inhibition zone was identified against B. subtilis in NPs-treated compared to the antibiotic-treated bacteria. Moreover, C. arabica AgNPs showed the lowest MIC against B. Subtilis and S. aureus (204 μg/mL) compared to the other antibiotics.
Conclusions
Visual confirmation of Ag-NPs synthesis obtained by watching the color change in the solution. To our knowledge, this is the first manuscript that demonstrated notable antileishmanial activity of synthesized C. arabica AgNPs against the promastigotes and amastigotes of L. major in a dose-dependent manner as well as the significant cytotoxic effect on studied cell lines. These prepared NPs displayed a remarkable bactericidal activity; so, it can considered as a bio-agent drug in the future. Because clinical evidence is currently limited, more research is need.
Data availability
All data used to support the findings of this study are included in the article.
References
Abdi V, Sourinejad I, Yousefzadi M, Ghasemi Z (2019) Biosynthesis of silver nanoparticles from the mangrove Rhizophora mucronata: its characterization and antibacterial potential. Iran J Sci Technol Trans A Sci 43:2163–2171
Akbari M, Oryan A, Hatam G (2017) Application of nanotechnology in treatment of leishmaniasis: a review. Acta Trop 172:86–90
Allahverdiyev AM, Abamor ES, Bagirova M, Rafailovich M (2011) Antimicrobial effects of TiO2 and Ag2O nanoparticles against drug-resistant bacteria and leishmania parasites. Future Microbiol 6:933–940
Alsammarraie FK, Wang W, Zhou P, Mustapha A, Lin M (2018) Green synthesis of silver nanoparticles using turmeric extracts and investigation of their antibacterial activities. Colloids Surf, B 171:398–405
Bailey F, Mondragon-Shem K, Haines LR, Olabi A, Alorfi A, Ruiz-Postigo JA, Alvar J, Hotez P, Adams ER, Vélez ID (2019) Cutaneous leishmaniasis and co-morbid major depressive disorder: a systematic review with burden estimates. PLoS Negl Trop Dis 13:e0007092
Bawa R (2019) Current immune aspects of biologics and nanodrugs: an overview. Immune Asp Biopharm Nanomed. pp 1–82
Chang K (1980) Human cutaneous lieshmania in a mouse macrophage line: propagation and isolation of intracellular parasites. Science 209:1240–1242
Choi HL, Jain S, Postigo JAR, Borisch B, Dagne DA (2021) The global procurement landscape of leishmaniasis medicines. PLoS Negl Trop Dis 15:e0009181
Dhand V, Soumya L, Bharadwaj S, Chakra S, Bhatt D, Sreedhar B (2016) Green synthesis of silver nanoparticles using Coffea arabica seed extract and its antibacterial activity. Mater Sci Eng, C 58:36–43
Farah MA, Ali MA, Chen S-M, Li Y, Al-Hemaid FM, Abou-Tarboush FM, Al-Anazi KM, Lee J (2016) Silver nanoparticles synthesized from Adenium obesum leaf extract induced DNA damage, apoptosis and autophagy via generation of reactive oxygen species. Colloids Surf, B 141:158–169
Feng QL, Wu J, Chen G, Cui F, Kim T, Kim J (2000) A mechanistic study of the antibacterial effect of silver ions on Escherichia coli and Staphylococcus aureus. J Biomed Mater Res 52:662–668
Flores-López LZ, Espinoza-Gómez H, Somanathan R (2019) Silver nanoparticles: electron transfer, reactive oxygen species, oxidative stress, beneficial and toxicological effects. Mini review. J Appl Toxicol 39:16–26
Gao X, Yourick JJ, Topping VD, Black T, Olejnik N, Keltner Z, Sprando RL (2015) Toxicogenomic study in rat thymus of F1 generation offspring following maternal exposure to silver ion. Toxicol Rep 2:341–350
Geremu M, Tola YB, Sualeh A (2016) Extraction and determination of total polyphenols and antioxidant capacity of red coffee (Coffea arabica L.) pulp of wet processing plants. Chem Biol Technol Agric 3:1–6
Iravani S, Korbekandi H, Mirmohammadi SV, Mekanik H (2014) Plants in nanoparticle synthesis. Rev Adv Sci Eng 3:261–274
Ismail HH, Hasoon SA, Saheb EJ (2019) The anti-Leishmaniasis activity of green synthesis silver oxide nanoparticles. Ann Trop Med Public Health 22:28–38
Jang H (2015) Protective effects of phenolic acid derivatives of coffee and Indian gooseberry extracts on retinal degeneration
Javed B, Nadhman A, Mashwani Z-U-R (2020) Phytosynthesis of Ag nanoparticles from Mentha longifolia: their structural evaluation and therapeutic potential against HCT116 colon cancer, Leishmanial and bacterial cells. Appl Nanosci 10:3503–3515
Kajani AA, Zarkesh-Esfahani SH, Bordbar A-K, Khosropour AR, Razmjou A, Kardi M (2016) Anticancer effects of silver nanoparticles encapsulated by Taxus baccata extracts. J Mol Liq 223:549–556
Kirtane AR, Verma M, Karandikar P, Furin J, Langer R, Traverso G (2021) Nanotechnology approaches for global infectious diseases. Nat Nanotechnol 16:369–384
Marcheafave GG, Tormena CD, Pauli ED, Rakocevic M, Bruns RE, Scarminio IS (2019) Experimental mixture design solvent effects on pigment extraction and antioxidant activity from Coffea arabica L. leaves. Microchem J 146:713–721
Morones JR, Elechiguerra JL, Camacho A, Holt K, Kouri JB, Ramírez JT, Yacaman MJ (2005) The bactericidal effect of silver nanoparticles. Nanotechnology 16:2346
Ogur R (2014) Studies with Myrtus communis L.: anticancer properties. J Intercultural Ethnopharmacol 3:135
Pankhurst QA, Connolly J, Jones SK, Dobson J (2003) Applications of magnetic nanoparticles in biomedicine. J Phys D Appl Phys 36:R167
Ponte-Sucre A, Gamarro F, Dujardin J-C, Barrett MP, López-Vélez R, García-Hernández R, Pountain AW, Mwenechanya R, Papadopoulou B (2017) Drug resistance and treatment failure in leishmaniasis: a 21st century challenge. PLoS Negl Trop Dis 11:e0006052
Romeilah R (2016) Chemical compositions, antioxidant, anticancer activities and biological effects of Myrtus communis L. and Origanum vulgare essential oils. Asian J Biochem 11:104–117
Saduqi M, Sharifi I, Babaei Z, Keyhani A, Mostafavi M, Parizi MH, Ghasemian M, Bamorovat M, Sharifi F, Aflatoonian MR (2019) Anti-leishmanial and immunomodulatory effects of Epigallo-catechin 3-O-Gallate on Leishmania tropica: apoptosis and gene expression profiling. Iran J Parasitol 14:521–533
Sharifi F, Sharififar F, Sharifi I, Alijani HQ, Khatami M (2017) Cytotoxicity, leishmanicidal, and antioxidant activity of biosynthesised zinc sulphide nanoparticles using Phoenix dactylifera. IET Nanobiotechnol 12:264–269
Sharifi F, Sharifi I, Keyhani A, Asadi-Khanuki A, Sharififar F, Pournamdari M (2019) Leishmanicidal, cytotoxic and apoptotic effects of Gossypium hirsutum bulb extract and its separated fractions on Leishmania major. J Vector Borne Dis 56:330
Silva MDO, Honfoga JNB, Medeiros LLD, Madruga MS, Bezerra TKA (2021) Obtaining bioactive compounds from the coffee husk (Coffea arabica L.) using different extraction methods. Molecules 26:46
Sinha SK, Zanoni G, Maiti D (2018) Natural product synthesis by C−H activation. Asian J Organ Chem 7:1178–1192
Sondi I, Salopek-Sondi B (2004) Silver nanoparticles as antimicrobial agent: a case study on E. coli as a model for Gram-negative bacteria. J Colloid Interface Sci 275:177–182
Tariq SL, Ali HM, Akram MA, Janjua MM, Ahmadlouydarab M (2020) Nanoparticles enhanced phase change materials (NePCMs)-A recent review. Appl Therm Eng 176:115305
Wahba AE, El-Sayed AK, El-Falal AA, Soliman EM (2019) New antimalarial lanostane triterpenes from a new isolate of Egyptian Ganoderma species. Med Chem Res 28:2246–2251
Yuan Y-G, Gurunathan S (2017) Combination of graphene oxide–silver nanoparticle nanocomposites and cisplatin enhances apoptosis and autophagy in human cervical cancer cells. Int J Nanomed 12:6537
Acknowledgements
We would like to thank the Herbal and Traditional Medicines and Tropical and Infectious Diseases research centers for their immense assistance in performing this research.
Funding
This investigation was supported by the Herbal and Traditional Medicines Research Center (grant number 96001000) and approved by the ethical committee of Kerman University of Medical Sciences (Ethics code of IR.KMU.REC.1396.2380). The funder had no role in the study design, data collection, data analysis, and manuscript preparation.
Author information
Authors and Affiliations
Contributions
FS: Conceptualization, Data curation, Methodology, Writing- Original draft. NM: Methodology, Formal analysis, Investigation. RTO: Investigation, Formal analysis, Writing - Review & Editing. IS: Supervision, Validation, Review & Editing. MD: Software, Validation. SS: Investigation, Software, Methodology. FS: Funding acquisition, Writing - Review & Editing. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sharifi, F., Mohamadi, N., Tavakoli Oliaee, R. et al. The potential effect of silver nanoparticles synthesized with Coffea arabica green seeds on Leishmania major proliferation, cytotoxicity activity, and cytokines expression level. J Parasit Dis 47, 131–139 (2023). https://doi.org/10.1007/s12639-022-01549-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12639-022-01549-4